Published online Oct 28, 2012. doi: 10.3748/wjg.v18.i40.5793
Revised: July 27, 2012
Accepted: July 29, 2012
Published online: October 28, 2012
AIM: To observe the efficacy of peg-interferon in the treatment of hepatitis delta virus (HDV) and to identify the factors that would be predictive of the sustained viral response (SVR).
METHODS: This prospective study was conducted in Medical Unit IV of the Liaquat University of Medical and Health Sciences Hospital Jamshoro from June 2008 to September 2011. This study cohort included all patients of either sex who presented during this time with hepatitis B surface antigen positivity, hepatitis B virus DNA > 20 000 IU/mL, serum glutamic pyruvic transaminase (SGPT) > 2(upper limit of normal), HDV-RNA positivity with fibrosis stage ≥ 2. Informed consent was obtained from each of these individuals. Patients were diagnosed with hepatitis D on the basis of detectable viral antibodies and the presence of HDV-RNA in their serum. A liver biopsy was performed in all cases and fibrosis staging was performed in accordance with the METAVIR scoring system. All eligible patients were administered peg-interferon at a weekly dosage of 1.5 μg/kg body weight for 48 wk. HDV-RNA was assayed at the end of this treatment period and again at 24 wk later. A biochemical response was determined by a normalization of SGPT at the end of the treatment or during follow up. The end of treatment response was defined by a HDV-RNA negative status. A sustained virological response was defined by undetectable serum HDV-RNA at six months after the end of treatment.
RESULTS: Among the 277 patients enrolled in our present study, 238 completed a course of peg-interferon therapy of which 180 (75.6%) were male and 58 (24.4%) female. Biochemical responses were achieved in 122/238 (51.3%) patients. End of treatment responses were achieved in 71/238 (29.8%) cases. A SVR was achieved in 70 of these patients (29.4%). A strong association was found between the SVR and the end of treatment responses (P = 0.001), biochemical responses (P = 0.001) and the degree of fibrosis (P = 0.002).
CONCLUSION: Peg-interferon therapy can induce remission in nearly one third of patients harboring HDV.
- Citation: Samiullah S, Bikharam D, Nasreen. Treatment of chronic hepatitis delta virus with peg-interferon and factors that predict sustained viral response. World J Gastroenterol 2012; 18(40): 5793-5798
- URL: https://www.wjgnet.com/1007-9327/full/v18/i40/5793.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i40.5793
Hepatitis delta virus (HDV) is a defective RNA virus that requires hepatitis B virus (HBV) to achieve replication. In long-term carriers of HBV, HDV infection can result in acute fulminant hepatitis or severe chronic hepatitis often progressing to cirrhosis and hepatocellular carcinoma[1]. Nearly 15-30 million people worldwide are infected with HDV[2]. Hepatitis D is dependent on hepatitis B for its prevalence. In Pakistan, the prevalence of hepatitis B is about 2.5% but in certain areas of Sindh province is much higher, in the range of 4.5% to 6.4%, with consequent increases in the prevalence of hepatitis D[3]. These areas are collectively referred to as the delta belt.
The introduction of a vaccination program for HBV, blood testing including that of pregnant women, the application of safety measures against blood born infections, the use of disposable syringes, and an increased public awareness of hepatitis B has markedly reduced the prevalence of this disease in many countries. However, hepatitis B is still a major health problem in several countries including Pakistan where HDV was found in 16.6% of hepatitis B carriers[4,5]. Indeed, a recently published study has detected HDV in 36.6% to 69.5% of hepatitis B patients in different parts of Pakistan[6].
The treatment options for HDV have remained disappointing over many years. Oral agents such as ribavirin, lamivudine, and famciclovir have been found to be ineffective against HDV[7]. The effectiveness of currently available and seemingly more potent oral antiviral drugs against hepatitis B such as adefovir, dipivoxil, entecavir, telbivudine and tenofovir is difficult to assess due to a lack of adequate clinical data[8]. The only medication which has proved effective to date against HDV infection is interferon. Up to 70% of affected patients can achieve biochemical responses using this drug, among which significant numbers achieve a virological response[9]. The efficacy of interferon based treatments is dose related, as a 12 mo treatment at 9 MU was found to be more effective than lower dose regimens. Unfortunately because of the dose-related side effects of standard interferon, patient compliance has tended to be very poor[10]. Recently however, peg-interferon, a product of the conjugation of interferon with polyethylene glycol has replaced standard interferon in clinical use because its prolonged half life allows for a single weekly dose to be administered. There are few previous studies of peg-interferon variant to date and it is difficult to draw any conclusions regarding its efficacy[11]. An evaluation of this drug in a large number of patients is therefore required and is also necessary to assess the factors that are predictive of a viral response in the treated patients.
The aim of our current study was to observe the efficacy of peg-interferon in the treatment of HDV and to evaluate the factors that are predictive of an sustained viral response (SVR) in the treated patients.
This prospective study was conducted in Medical Unit IV of the Liaquat University of Medical and Health Sciences Hospital, Jamshoro, from June 2008 to September 2011. The patient cohort comprised consecutive cases of either sex who were hepatitis B surface antigen (HBsAg) positive, had HBV DNA > 20 000 IU/mL, a serum glutamic pyruvic transaminase (SGPT) > 2 (upper limit of normal), and were HDV-RNA positive with a fibrosis stage ≥ 2. Informed consent was obtained from each patient and the study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. All of the subject patients were received from Hepatitis Prevention and Control Program, Sindh Chief Minister’s initiative.
We did not include patients below 18 or above 65 years age; with concomitant hepatitis C virus infection; fibrosis stage < 2 on liver biopsy; or with decompensated cirrhosis of the liver, i.e., history of ascites, variceal bleeding or hepatic encephalopathy. Also excluded were pregnant women or nursing mothers and cases of metabolic liver disease such as Wilson disease or hemochromatosis. Leukopenia (< 2500/mm3), neutropenia (< 1000/mm3), hemoglobin < 10 g/dL, and the presence of other severe diseases (cardiomyopathy, diabetes mellitus, arterial hypertension, neoplasia, neurologic diseases, depression and/or psychiatric disorders were additional exclusion criteria.
Patients were diagnosed with hepatitis D on the basis of antibodies and HDV-RNA in the serum. Patients in our cohort were also positive for HBsAg and had hepatitis B DNA levels > 20 000 IU/mL. Routine serological tests such as complete blood counts, liver function test, prothrombin time, SGPT, serum albumin, anti hepatitis D antibodies, HBsAg and an ultrasound of the abdomen were performed by the research laboratory of the Liaquat university of Medical and Health Sciences. Hepatitis D RNA and HBV-DNA tests were performed by the molecular laboratory of Liaquat university of Medical and Health Sciences. Liver biopsies were performed for all patients upon obtaining written consent and having fully explained the procedure to each individual. The biopsy procedure was performed in each case under ultrasound guidance with a 14 gauge tru-cut biopsy needle. The biopsy sample was considered adequate if it was greater than 10 mm in size and contained more than 5 portal tracts[12]. Biopsy samples were sent to a pathologist who had no knowledge of the clinical status of the patient. The staging of fibrosis was performed in accordance with the METAVIR scoring system as follows: F0, no fibrosis; F1, portal fibrosis without septa; F2, few septa; F3, numerous septa without cirrhosis; or F4, cirrhosis[13].
All eligible patients were administered peg-interferon weekly at 1.5 μg/kg body weight for 48 wk as part of the Hepatitis Prevention and Control Program, Sindh Chief Minister’s initiative. This program was initiated by the Government of Sindh province in Pakistan and provides free peg-interferon-base treatments to all affected patients. This program covers a population of 55.24 million in the Sindh province of Pakistan.
Patients were followed up every month to obtain complete blood picture and SGPT. All patients were evaluated for possible complications of interferon therapy. HDV-RNA was assayed at the end of treatment and at 24th wk later. A biochemical response was confirmed if there was a normalization of the SGPT levels at the end of treatment or during follow up. An end of treatment response was defined as a HDV-RNA negative result at the end of treatment. An SVR was defined as undetectable HDV-RNA in the serum at six months after the end of treatment. A non responder was defined as a patient with persistent detectable HDV-RNA at the end of treatment[14].
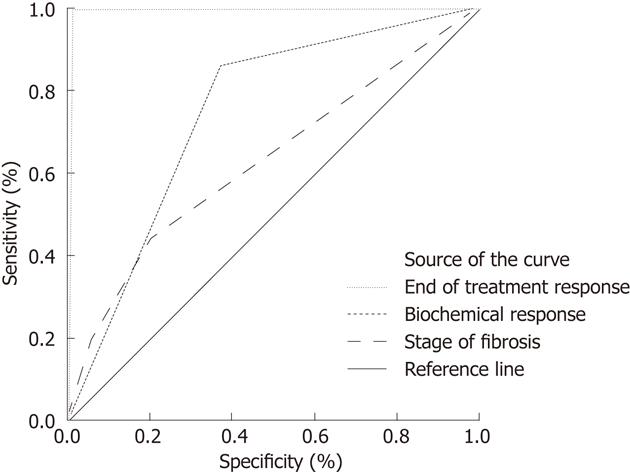
Continuous variables such as age, SGPT and platelet count were computed as the mean ± SD. Categorical variables such as sex, end of treatment response, biochemical response, SVR and stage of fibrosis were expressed as a percentage. Receiver operating characteristic (ROC) curve comparisons were performed between the end of treatment response, biochemical response, stage of fibrosis with SVR by calculating the area under the curve and 95% confidence intervals (CIs) as described by Hanley et al[15]. A P value of 0.05 was considered statistically significant. All data were processed on SPSS Version 16.
Of the 277 patients enrolled in this study, 238 completed the course of interferon therapy. Among the remaining 39 patients, 31 were excluded from the study because of minimal (stage 0 and 1) fibrosis and eight patients were dropped because of interferon-induced complications early in study period. Of the remaining 238 patients who were analyzed, 180 (75.6%) were male and 58 (24.4%) female. The mean age was 29.6 ± 8.5 years, hemoglobin (g/dL) was 11.49 ± 2.45, platelet count (103/mm3) was 145.6 ± 14.3, and SGPT (IU/L) was 134.18 ± 14.3. Hepatitis B DNA level (IU/mL) 155.1 ± 70.1. Fibrosis stage 2 was present in 172 (72.3%), stage 3 in 44 (18.5%) and stage 4 in 22 (9.2%) patients. A biochemical response was achieved in 122/238 (51.3%) patients and not in 116 (48.7%) cases. End of treatment responses were achieved in 71/238(29.8%) cases and not in 167 (70.2%) patients. SVR was achieved in 70 (29.4%) patients.
The baseline characteristics of the patients in the study cohort are listed in Table 1. A strong association was found between SVR and end of treatment response (P = 0.001) as 70/71 (98.5%) patients who obtained an end of treatment response also achieved an SVR. The association between an SVR and the stage of fibrosis was also significant (P = 0.002) as 39/172 (22.6%) patients with fibrosis stage 2, 18/44 (40.9%) patients with fibrosis stage 3 and 13/22 (59%) patients with fibrosis stage 4 achieved SVR, and with the biochemical response (P = 0.001) as 60/122 (49.1%) of such patients also achieved SVR. Table 2 shows relation of end of treatment response, biochemical response and stage of fibrosis with SVR. ROC curve comparing the end of treatment response, biochemical response, stage of fibrosis and SVR is shown in Figure 1. The end of treatment response was found to be predictive of an SVR with a sensitivity of 98%, specificity of 94%, positive predictive value (PPV) of 94.45, and negative predictive value (NPV) of 98% (P = 0.001). A biochemical response was also predictive of an SVR with a sensitivity of 85.7%, specificity of 63.2%, PPV of 69.9% and NPV of 81.5% (P = 0.001). The degree of fibrosis predicted an SVR with a sensitivity of 44.3%, specificity of 46%, PPV of 45% and NPV of 45.3% (P = 0.002).
| Continuous variables (mean ± SD) | |
| Age (yr) | 29 ± 8.5 |
| Hemoglobin (g/dL) | 11.49 ± 2.45 |
| The mean platelet count (103/mm3) | 145.6 ± 14.3 |
| SGPT (IU/L) | 134.18 ± 14.3 |
| Hepatitis B DNA level (IU/mL) | 155 × 103± 70.1 × 103 |
| Categorical variable, frequency (%) | |
| Sex | |
| Male | 180 (75.6) |
| Female | 58 (24.4) |
| Stage of fibrosis | |
| Stage 2 | 172 (72.3) |
| Stage 3 | 44 (18.5) |
| Stage 4 | 22 (9.2) |
| Biochemical response | 122 (51.3) |
| End of treatment | 71 (29.8) |
| Sustained viral response | 70 (29.4) |
| Variable | SVR achieved | SVR not achieved | P value |
| BR | 60/122 (49.1) | 62/122 (50.9) | 0.001 |
| ETR | 70/71 (98.5) | 1/71 (1.5) | 0.001 |
| Stage of fibrosis | 0.002 | ||
| Stage 2 | 39/172 (22.6) | 133/172 (77.3) | |
| Stage 3 | 18/44 (40.9) | 26/44 (59.09) | |
| Stage 4 | 13/22 (59) | 9/22 (40.9) |
This is the largest prospective study yet reported on the efficacy of peg-interferon monotherapy treatment for hepatitis D. The 238 patients included in the study cohort was far larger than the population analyzed in the previous hep-net-international delta hepatitis intervention trial, a multicenter trial comprising 90 patients in three arms. In our current prospective study, a biochemical response was achieved in 51.3%, end of treatment in 29.8% and SVR in 29.4% of the 238 patients with hepatitis D after 48 wk of peg-interferon therapy. Niro et al[16] previously studied 16 patients receiving peg-interferon for 72 wk and found biochemical response in 6 (37.5%) patients with 5 patients showing an end of treatment response and maintaining an SVR. In another earlier study by Wedemeyer et al[17] conducted on 29 patients receiving peg-interferon for 48 wk, a biochemical response was found in 8 (24%) cases and 9 (28%) patients showed an end of treatment response. When the therapy was extended to 72 wk, 13 (45%) patients achieved a complete biochemical response and 9 (31%) had an end of treatment response and SVR of 23% at 48 wk was improved to 28% at 72 wk. Erhardt et al[18] have reported that among 12 patients treated using peg-interferon, biochemical normalization occurred in 3 (25%) patients and an SVR in 2 (17%) patients. Castelnau et al[19] in their study of 12 patients treated for 12 mo with peg-interferon observed complete biochemical response in 8 (57%) patients, and an end of treatment response in 8 (57%) cases of whom 6 (43%) obtained an SVR. The discrepancies between the results of these various studies are likely due to the small number of patients analyzed.
In our present study, strong predictors of an SVR were an end of treatment response, biochemical response and the stage of fibrosis. We observed a strong association between the end of treatment response (P = 0.001) and an SVR as 70/71 (98.5%) patients achieved both responses. Indeed, an end of treatment response was predictive of an SVR with a PPV of 94.45% and an NPV of 98% (P = 0.001). Niro et al[16] also confirmed the importance of an end of treatment response as the 3/16 (19%) of their patients who achieved end of treatment response increased to 4/16 (25%) at the 24 wk follow up point. Wedemeyer et al[17] observed an end of treatment response in 9/29 (31%) of the patients in their cohort after 72 wk of treatment, out of which 5 patients maintained clearance of HDV-RNA at the end of follow up. However, the outcomes of the remaining four patients was unknown, making the end of treatment response an important predictor of SVR or end of follow up response.
Erhardt et al[18], in a study of 10 patients, found end of treatment response to peg-interferon in 2/10 (17%) cases that were maintained after 24 wk of follow up. Castelnau et al[19], in a study of 14 hepatitis D patients, report an SVR in all 8 (57%) patients who achieved an end of treatment virological response.
Based on our current findings, the biochemical response is an important predictor of an SVR as 60/70 (85.7%) (P = 0.001) of our hepatitis D patients achieved both responses. Niro et al[16] previously reported a biochemical response in 6 patients out of which 3 achieved SVR during a follow up of 11 patients for 72 wk. According to the study of Wedemeyer et al [17], 7 hepatitis D patients achieved SVR out of 13 (53.8%) patients who achieved a biochemical response after 72 wk of peg-interferon treatment. Castelnau et al[19] have reported that 75% of hepatitis D patients who achieved an end of treatment response were HDV-RNA negative six months after of the discontinuation of their treatment.
In our current analyses, the response to treatment increased with the degree of fibrosis (P = 0.002) as 39/172 (22.6%) patients with fibrosis stage 2, 18/44 (40.9%) patients with fibrosis stage 3 and 13/22 (59%) patients with fibrosis stage 4 achieved SVR. Reports on hepatitis D are sparse at present but the two publications recently presented at the 62nd Annual Meeting of the American Association for the Study of Liver Disease (2011) in San Francisco on hepatitis B has shed some new light on the importance of the stage of fibrosis for the treatment response outcome. Marcellin et al[20] treated 641 hepatitis B patients with tenofovir for five years and performed paired biopsies in 348 of these cases, one at baseline and a second at 5 years after the start of treatment. These authors found an overall histological improvement in 98% of their patients, which was more marked in patients with advanced fibrosis. In another study by Gane et al[21], paired biopsies were performed in 72 Asian patients with hepatitis B receiving tenofovir treatment for five years. A histological regression of fibrosis of ≥ 1 point decrease in the Ishak score was noted in 42/72 (58.3%) patients at five years which was more marked in 6/18 patients with liver cirrhosis. These two observations and our present findings indicate that the response to treatment will increase as the degree of fibrosis increases. This result will be of definite benefit in deciding the time of treatment.
Niro et al[16] in his study performed a baseline biopsy in 11 of 16 hepatitis D patients, of which 6 patients consented for paired biopsy after 72 wk of peg-interferon treatment. These authors reported an improvement of more than 1 point in Necro inflammatory activity as well as fibrosis in 4 out of 6 patients. Erhardt et al[18] report in their study of 12 patients that 11 allowed a baseline biopsy and 7 a followed biopsy after six months of treatment. An improvement in the stage of fibrosis was observed in responders as well as in non-responders. In contrast to previous studies however, no improvement was found in cases of cirrhosis of the liver.
In conclusion, peg-interferon therapy can induce remission in nearly one third of patients infected with HDV. Patients who achieve a biochemical response or an end of treatment response with a high stage fibrosis on liver histology, also achieved SVR.
We are thankful to Dr. Majeed Chutto, Program Manager Hepatitis Prevention and Control Program Sindh Chief Minister’s initiative for providing peg-interferon based treatment to patients. We are thankful to Dr. Mehtab Abbasi, Dr. Zareen, Dr. Faizan, Dr. Manan for assisting during the liver biopsy and follow up of the patients. We appreciate the efforts of Mr. Ali Jan, Assistant Computer Operator for record keeping.
Nearly 150 million people worldwide are infected with hepatitis delta virus (HDV). Hepatitis D is dependent on hepatitis B for its prevalence. In Pakistan, the prevalence of hepatitis B is about 2.5% but in certain areas of Sindh province is much higher, in the range of 4.5% to 6.4%, with consequent increases in the prevalence of hepatitis D.
The treatment options for HDV have remained disappointing over many years. Recently however, peg-interferon, a product of the conjugation of interferon with polyethylene glycol has replaced standard interferon in clinical use because its prolonged half life allows for a single weekly dose to be administered. Although the previous studies has shown sustained viral response (SVR) in substantial number of patients but the number of patient studied were so small that it was very difficult to draw any conclusion.
The above mentioned studies are on such small scale that’s it is difficult to draw any conclusion. An evaluation of this drug in a large number of patients is therefore required and is also necessary to assess the factors that are predictive of a viral response in the treated patients. This is the largest prospective study yet reported on the efficacy of peg-interferon monotherapy treatment for hepatitis D. The 238 patients included in the study cohort was far larger than the population analyzed in the previous hep-net-international delta hepatitis intervention trial, a multicenter trial comprising 90 patients in three arms. In the authors’ current prospective study, a biochemical response was achieved in 51.3%, end of treatment in 29.8% and SVR in 29.4% of the 238 patients with hepatitis D after 48 wk of peg-interferon therapy.
The present study has been conducted on larger scale. One of very important observation seen was better response of patients with more aggressive disease. This will further open the debate as this finding is in contrast to what was previously being observed that milder disease respond better. The present study will help with selecting the patients in future at proper time as well as those who can better response to treatment.
This is a large study of 277 patients with delta virus infection. A total of 238 patients completed a full course of 48 wk of treatment with pegylated interferon. The SVR rate was 29.4%. The data is interesting.
| 1. | Rizzetto M. Hepatitis D: virology, clinical and epidemiological aspects. Acta Gastroenterol Belg. 2000;63:221-224. [PubMed] |
| 3. | Ali SA, Donahue RM, Qureshi H, Vermund SH. Hepatitis B and hepatitis C in Pakistan: prevalence and risk factors. Int J Infect Dis. 2009;13:9-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 4. | Wedemeyer H, Manns MP. Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nat Rev Gastroenterol Hepatol. 2010;7:31-40. [PubMed] |
| 5. | Mumtaz K, Hamid SS, Adil S, Afaq A, Islam M, Abid S, Shah HA, Jafri W. Epidemiology and clinical pattern of hepatitis delta virus infection in Pakistan. J Gastroenterol Hepatol. 2005;20:1503-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Abbas Z, Jafri W, Raza S. Hepatitis D: Scenario in the Asia-Pacific region. World J Gastroenterol. 2010;16:554-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 85] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Wedemeyer H, Böker KH, Pethig K, Petzold DR, Flemming P, Tillmann HL, Vollmar J, Bastürk M, Goldmann E, Griffin KE. Famciclovir treatment of chronic hepatitis B in heart transplant recipients: a prospective trial. Transplantation. 1999;68:1503-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Yurdaydin C, Bozkaya H, Onder FO, Sentürk H, Karaaslan H, Akdoğan M, Cetinkaya H, Erden E, Erkan-Esin O, Yalçin K. Treatment of chronic delta hepatitis with lamivudine vs lamivudine + interferon vs interferon. J Viral Hepat. 2008;15:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Farci P, Mandas A, Coiana A, Lai ME, Desmet V, Van Eyken P, Gibo Y, Caruso L, Scaccabarozzi S, Criscuolo D. Treatment of chronic hepatitis D with interferon alfa-2a. N Engl J Med. 1994;330:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 219] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Kage M, Shimamatu K, Nakashima E, Kojiro M, Inoue O, Yano M. Long-term evolution of fibrosis from chronic hepatitis to cirrhosis in patients with hepatitis C: morphometric analysis of repeated biopsies. Hepatology. 1997;25:1028-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Tekin R, Ayaz C, Celen M. Efficiency of Treatment of Long Period Pegylated interferon in Delta Hepatitis. Anatol J Clin Invest. 2010;4:206-209. |
| 12. | Cholongitas E, Senzolo M, Standish R, Marelli L, Quaglia A, Patch D, Dhillon AP, Burroughs AK. A systematic review of the quality of liver biopsy specimens. Am J Clin Pathol. 2006;125:710-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 13. | The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994;20:15-20. [PubMed] |
| 14. | Niro GA, Smedile A, Ippolito AM, Ciancio A, Fontana R, Olivero A, Valvano MR, Abate ML, Gioffreda D, Caviglia GP. Outcome of chronic delta hepatitis in Italy: a long-term cohort study. J Hepatol. 2010;53:834-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839-843. [PubMed] |
| 16. | Niro GA, Ciancio A, Gaeta GB, Smedile A, Marrone A, Olivero A, Stanzione M, David E, Brancaccio G, Fontana R. Pegylated interferon alpha-2b as monotherapy or in combination with ribavirin in chronic hepatitis delta. Hepatology. 2006;44:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Wedemeyer H, Yurdaydìn C, Dalekos GN, Erhardt A, Çakaloğlu Y, Değertekin H, Gürel S, Zeuzem S, Zachou K, Bozkaya H. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med. 2011;364:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 369] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 18. | Erhardt A, Gerlich W, Starke C, Wend U, Donner A, Sagir A, Heintges T, Häussinger D. Treatment of chronic hepatitis delta with pegylated interferon-alpha2b. Liver Int. 2006;26:805-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Castelnau C, Le Gal F, Ripault MP, Gordien E, Martinot-Peignoux M, Boyer N, Pham BN, Maylin S, Bedossa P, Dény P. Efficacy of peginterferon alpha-2b in chronic hepatitis delta: relevance of quantitative RT-PCR for follow-up. Hepatology. 2006;44:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Marcellin P, But M, Gane EJ. Five years of Treatment with Tenofovir DF (TDF) for Chronic Hepatitis B (CHB) Infection is Associated with Sustained Viral Suppression and Significant Regression of Histological Fibrosis and Cirrhosis. 62nd AASLD;. 2011;Nov 4-8; San Francisco, CA. |
| 21. | Gane EJ, Marcellin P, Sievert W. Five years of Treatment with Tenofovir DF (TDF) for Chronic Hepatitis B (CHB) Infection in Asian Patients is Associated with Sustained Viral Suppression and Significant Regression of Histological Fibrosis and Cirrhosis. 62nd AASLD;. 2011;Nov 4-8; San Francisco, CA. |
Peer reviewers: Kentaro Yoshika, Associate Professor, Division of Gastroenterology, Department of I, Fujita Health University School of Medicine, 1-98 Dengakugakubo, Kutsukade, Toyoake 470-1190, Japan; Jeff Butterworth, MB, FRCP, Department of Gastroenterology, Shrewsbury and Telford Hospital NHS Trust, Mytton Oak Road, Shrewsbury, Shropshire SY3 8XQ, United Kingdom; BS Anand, Professor, Digestive Diseases Section (111D), VA Medical Center, 2002 Holcombe Blvd., Houston, TX 77030, United States
S- Editor Lv S L- Editor A E- Editor Xiong L