Published online Oct 28, 2012. doi: 10.3748/wjg.v18.i40.5779
Revised: August 23, 2012
Accepted: August 25, 2012
Published online: October 28, 2012
AIM: To perform a meta-analysis of observational studies to further elucidate the relationship between oral bisphosphonate use and gastrointestinal cancer risk.
METHODS: Systematic literature search was conducted in MEDLINE, EMBASE, and the Cochrane Library to identify studies through January 2011. Search terms were “bisphosphonates” or trade names of the drugs, and “observational studies” or “cohort studies” or “case-control studies”. Two evaluators reviewed and selected articles on the basis of predetermined selection criteria as followed: (1) observational studies (case-control or cohort studies) on bisphosphonate use; (2) with at least 2 years of follow-up; and (3) reported data on the incidence of cancer diagnosis. The DerSimonian and Laird random effects model were used to calculate the pooled relative risk (RR) with 95% confidence interval (CI). Two-by-two contingency table was used to calculate the outcomes not suitable for meta-analysis. Subgroup meta-analyses were conducted for the type of cancer (esophageal, gastric and colorectal cancers). Sensitivity analyses were performed to examine the effect sizes when only studies with long-term follow-up (mean 5 years; subgroup 3 years) were included.
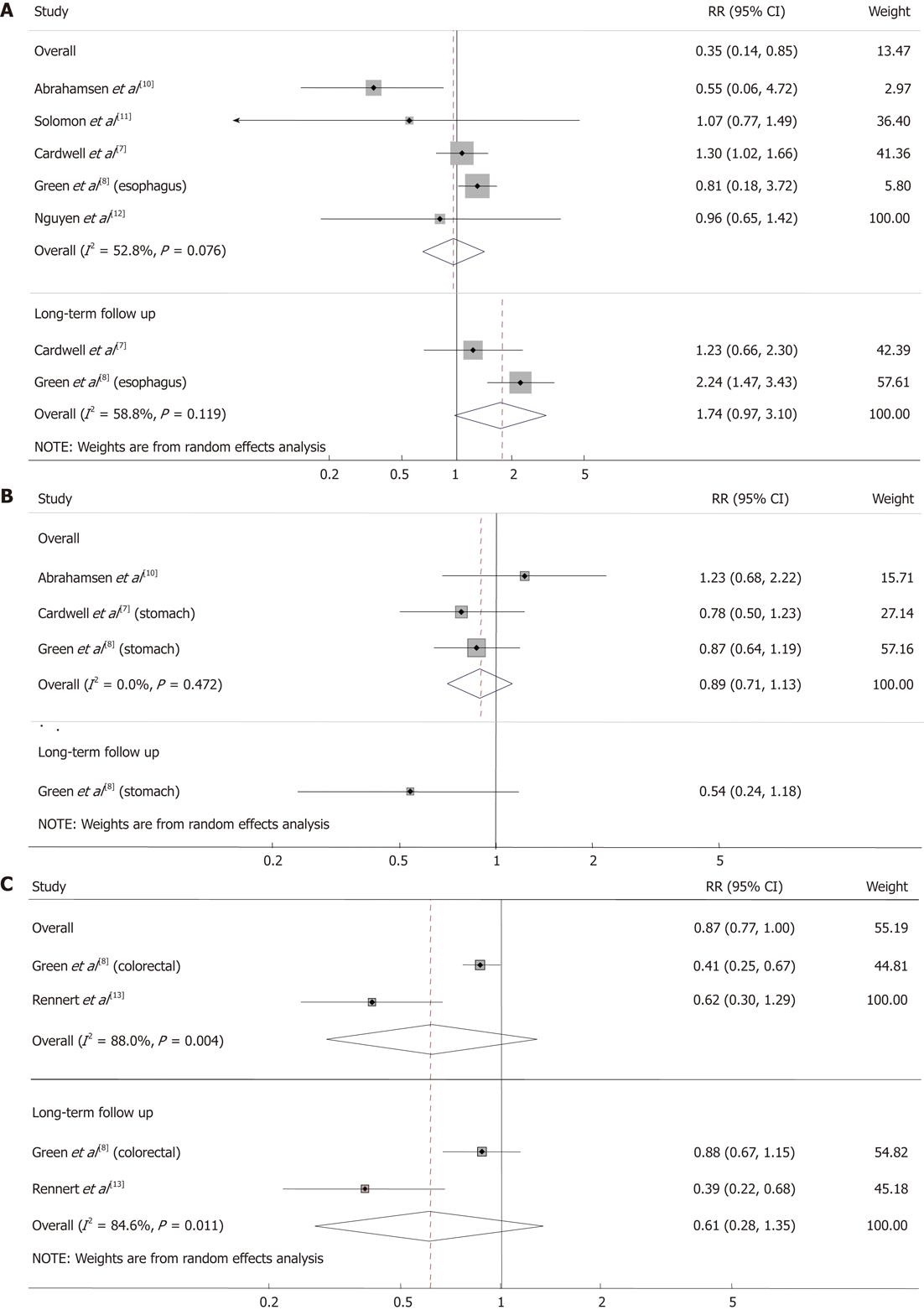
RESULTS: Of 740 screened articles, 3 cohort studies and 3 case-control studies were included in the analyses. At first, 4 cohort studies and 3 case-control studies were selected for the analyses but one cohort study was excluded because the cancer outcomes were not categorized by type of gastrointestinal cancer. More than 124 686 subjects participated in the 3 cohort studies. The mean follow-up time in all of the cohort studies combined was approximately 3.88 years. The 3 case-control studies reported 3070 esophageal cancer cases and 15 417 controls, 2018 gastric cancer cases and 10 007 controls, and 11 574 colorectal cancer cases and 53 955 controls. The percentage of study participants who used bisphosphonate was 2.8% among the cases and 2.9% among the controls. The meta-analysis of all the studies found no significant association between bisphosphonate use and gastrointestinal cancer. Also no statistically significant association was found in a meta-analysis of long-term follow-up studies. There was no negative association between bisphosphonate use and the incidence of esophageal cancer in the overall analysis (RR 0.96, 95% CI: 0.65-1.42, I2 = 52.8%, P = 0.076) and no statistically significant association with long-term follow-up (RR 1.74, 95% CI: 0.97-3.10, I2 = 58.8%, P = 0.119). No negative association was found in the studies reporting the risk of gastric cancer (RR 0.89, 95% CI: 0.71-1.13, I2 = 0.0%, P = 0.472). In case of colorectal cancer, there was no association between colorectal cancer and bisphosphonate use (RR 0.62, 95% CI: 0.30-1.29, I2 = 88.0%, P = 0.004) and also in the analysis with long-term follow-up (RR 0.61, 95% CI: 0.28-1.35, I2 = 84.6%, P = 0.011).
CONCLUSION: Oral bisphosphonate use had no significant effect on gastrointestinal cancer risk. However, this finding should be validated in randomized controlled trials with long-term follow-up.
- Citation: Oh YH, Yoon C, Park SM. Bisphosphonate use and gastrointestinal tract cancer risk: Meta-analysis of observational studies. World J Gastroenterol 2012; 18(40): 5779-5788
- URL: https://www.wjgnet.com/1007-9327/full/v18/i40/5779.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i40.5779
Bisphosphonates are commonly used in postmenopausal women with osteoporosis. In vitro and in vivo studies have suggested that bisphosphonate has anticancer properties[1]: promoting apoptosis[2], inhibiting tumor cell adhesion and invasion[3], inhibiting angiogenesis[4], altering tumor-associated macrophage function[4], and enhancing immune surveillance[1,5]. However, it was recently reported that the Food and Drug Administration received reports of 23 cases of esophageal cancer in the United States and another 31 cases in Europe and Japan, all occurring from 1995 through 2008 among patients using oral bisphosphonates[6]. Since then, there have been several cohort studies and case-control studies to elucidate the association between the use of bisphosphonate and the risk of gastrointestinal tract cancer. However, results of the observational studies are inconsistent[7,8].
As yet, there have been no randomized controlled trials demonstrating a causal relationship between bisphosphonate use and gastrointestinal track cancer. Moreover, no meta-analysis has been performed despite the inconsistent results of observational studies. Therefore, in the present study, we aimed to investigate the association between the use of oral bisphosphonate and the risk of gastrointestinal cancer via meta-analysis of cohort studies and case-control studies.
We conducted a systematic literature search of MEDLINE, 1977 April 2011; EMBASE, 1971 April 2011; and the Cochrane Database of Systematic Reviews in the Cochrane Library, 1973 April 2011. We identified observational studies of bisphosphonate use whose primary or secondary outcomes included gastrointestinal tract cancer. The search terms were "bisphosphonates" or trade names of the drugs, and "observational studies" or "cohort studies" or "case-control studies" (Table 1). All the searches were restricted to human studies. In addition, a manual review of references from primary and review articles was performed to locate any additional relevant studies. All the potentially relevant articles were independently reviewed by 2 investigators (Oh YH and Yoon C). Disagreements between evaluators were resolved by discussion or consultation with a third author (Park SM).
| Search strategy for MEDLINE | Search strategy for EMBASE | Search strategy for Cochrane reviews of the Cochrane Library |
| 1. Diphosphonates [MH] OR diphosphonates [ALL] OR diphosphonate [ALL] | 1. Bisphosphonates/de | 1. Bisphosphonates [ALL] |
| 2. Bisphosphonates [ALL] | 2. Diphosphonate/de | 2. Diphosphonate [ALL] |
| 3. Alendronate [MH] OR alendronate [ALL] | 3. Alendronate/de | 3. Alendronate [ALL] |
| 4. Clodronic acid [MH] OR clodronic acid [ALL] OR clodronate [ALL] | 4. Clodronate/de | 4. Clodronate [ALL] |
| 5. Etidronic acid [MH] OR etidronic acid [ALL] OR etidronate [ALL] | 5. Etidronate/de | 5. Etidronate [ALL] |
| 6. Ibandronic acid [NM] OR ibandronic acid [ALL] OR ibandronate [ALL] | 6. Ibandronate/de | 6. Ibandronate [ALL] |
| 7. Minodronate [ALL] OR YM 529 [NM] OR YM 529 [ALL] | 7. Minodronate/de | 7. Minodronate [ALL] |
| 8. Neridronate [ALL] OR 6-amino-1-hydroxyhexane-1,1-diphosphonate [NM] OR 6-amino-1-hydroxyhexane-1,1-diphosphonate [ALL] | 8. Neridronate/de | 8. Neridronate [ALL] |
| 9. Olpadronic acid [NM] OR olpadronic acid [ALL] OR olpadronate [ALL] | 9. Olpadronate/de | 9. Olpadronate [ALL] |
| 10. Pamidronate [NM] OR pamidronate [ALL] | 10. Pamidronate/de | 10. Pamidronate [ALL] |
| 11. Risedronic acid [NM] OR risedronic acid [ALL] OR risedronate [ALL] | 11. Risedronate/de | 11. Risedronate [ALL] |
| 12. Tiludronic acid [NM] OR tiludronic acid [ALL] OR tiludronate [ALL] | 12. Tiludronate/de | 12. Tiludronate [ALL] |
| 13. Zoledronic acid [NM] OR zoledronic acid [ALL] OR zoledronate [ALL] | 13. Zoledronate/de | 13. Zoledronate [ALL] |
| 14. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 | 14. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 | 14. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 |
| 15. Observational studies [ALL] | 15. Observational studies/de | |
| 16. CohORt studies [ALL] | 16. CohORt studies/de | [ALL] - in all text |
| 17. Case control studies [ALL] OR case-control studies [ALL] | 17. Case control studies/de OR case-control studies/de | |
| 18. Case referent studies [ALL] OR case-referent studies [ALL] | 18. Case referent studies OR case-referent studies | |
| 19. 15 OR 16 OR 17 OR 18 | 19. 15 OR 16 OR 17 OR 18 | |
| 20. 14 OR 19 | 20. 14 OR 19 | |
| 21. Humans [MH] | 21. [Humans]/lim | |
| 22. 20 AND 21 | 22. [Embase]/lim OR [embase classic]/lim | |
| 23. 20 AND 21 AND 22 | ||
| Date of search: April 29, 2011 (1973 April 2011); Result: 1709 articles found | Date of search: April 29, 2011 (1977 April 2011);Result: 2129 articles found | Limitation: cochrane database of systematic reviews;Date of search: April 29, 2011 (1971 April 2011); Result: 47 articles found |
The inclusion criteria were: (1) observational studies (case-control or cohort studies) on bisphosphonate use; (2) with at least 2 years of follow-up; and (3) reported data on the incidence of cancer diagnosis.
To compute a pooled relative risk (RR) with 95% confidence interval (CI), we used the RRs or odds ratios and 95% CIs that were adjusted for most confounders. Because the incidence of cancer is generally low, we did not distinguish between the various measures of RR. If the outcome measures were unsuitable for meta-analysis, we calculated a crude estimate using a two-by-two contingency table.
We also assessed the heterogeneity for each meta-analysis by using the I2 value which measures the percentage of total variation across that is attributable to heterogeneity rather than chance. High value of I2 index suggests increased heterogeneity. We also calculated P value of Q-test which represents heterogeneity. If P value is less than 0.10, it represents there is heterogeneity.
Because of the known clinical and methodological heterogeneity of the studies we analyzed, we calculated the pooled RR with 95% CI based on the DerSimonian and Laird random effects model. We used Stata Version 11.0 (Stata Corp., College Station, Texas) for the statistical analysis.
Subgroup meta-analyses were carried out for the study design (cohort and case-control) and type of cancer. For the cancer subgroup analyses, esophageal, gastric and colorectal cancers were analyzed independently.
We also performed sensitivity analyses to examine the effect sizes when only studies with long-term follow-up (mean 5 years; subgroup 3 years) were included.
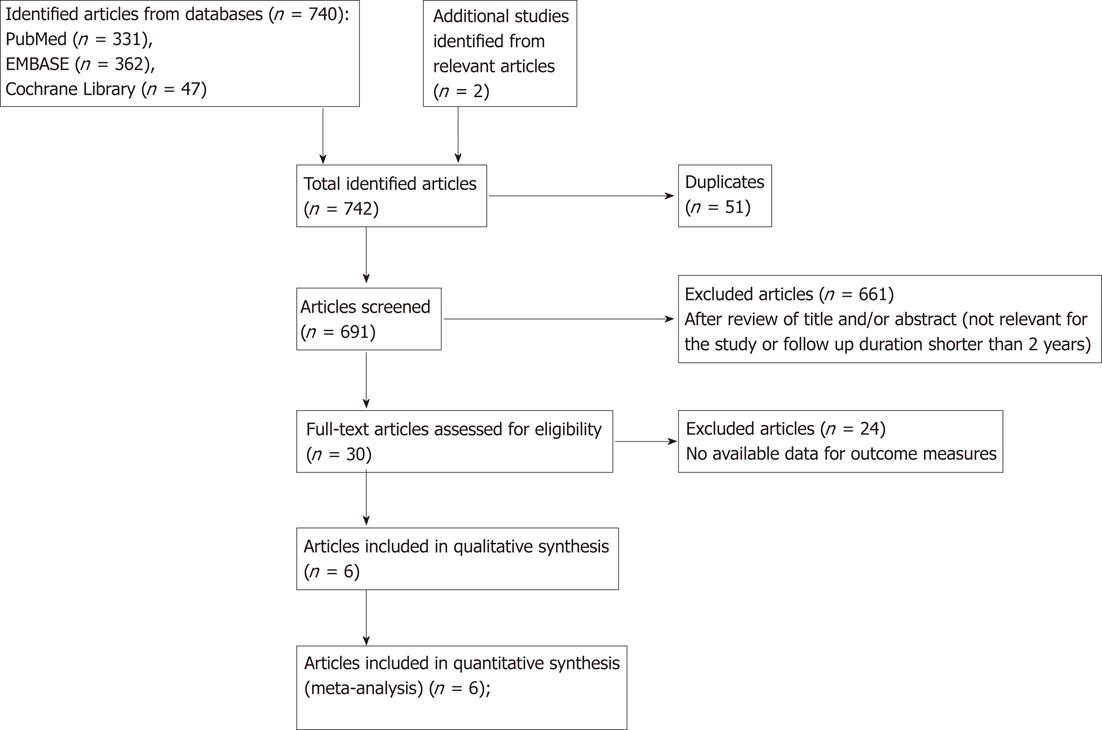
Figure 1 is a flow diagram of how we identified the relevant studies. Of the 740 articles identified, 4 cohort studies[7,9-11] and 3 case-control studies[8,12,13] were selected for the analyses. Many of initial 742 articles are not in our scope. They are mainly about effect of bisphosphonates, effects of several types of bisphosphonate regimen, other adverse effects of bisphosphonate, such as atrial fibrillation, osteonecrosis of jaw syndrome, and anti-cancerous bisphosphonate use for breast cancer and prostate cancer. We performed review of titles and abstract of the articles, then excluded irrelevant for our study. Thirty articles were remained after overall process, but 23 of 30 articles included no available data for outcome measures. One cohort study[9] was excluded because the cancer outcomes were not categorized by type of gastrointestinal cancer.
We also contacted Dr. Chris Cardwell to ask the data for the adjusted hazards ratio and 95% CI for gastric cancer only in bisphosphonate users[7].
More than 124 686 subjects participated in the 3 cohort studies[7,10,11] used in our meta-analyses. The mean follow-up time in all of the cohort studies combined was approximately 3.88 years (range: 0.5-13 years). The 3 case-control studies[8,12,13] used in our meta-analyses reported the number of cases and controls: 3070 esophageal cancer cases and 15 417 controls, 2018 gastric cancer cases and 10 007 controls, and 11 574 colorectal cancer cases and 53 955 controls. The percentage of study participants who used bisphosphonate was 2.8% among the cases and 2.9% among the controls. Tables 2 and 3 summarize the general characteristics of the studies included in our analyses.
| Ref. | Country | Cohort formation (population) | Study period | Mean F/U duration, yr (range) | Age, yr (mean±SD) | Sex ratio (reference group) | Type of cancer | Type of drug (reference group) | Risk estimate | Adjustment | No. of cancer development/ no. of study population | No. of total cohort | |
| Bisphosphonate user | Reference group | ||||||||||||
| Abrahamsen et al[10] | Denmark | Patients with fracture who had prescription for oral bisphosphonate (national registers) | E:1995-2005; F/U: -2005 | 2.8 (NA) | 74.3 ± 8.8 | M: 11; F: 89 | Esophageal cancer, gastric cancer | Any bisphosphonate use (no bisphosphonate use) | Esophageal or gastric cancer 0.78 (0.49-1.26); esophageal cancer 0.35 (0.14-0.85); gastric cancer 1.23 (0.68-2.22) | Age, sex, fracture type, Charlson index, number of concomitant medication, time to event | NA/13 678 | NA/27 356 | 41 034 |
| Solomon et al[11] | United States | Patients who had prescription for oral bisphosphonate (health care utilization records of medicare beneficiaries) | NA | NA (NA) | NA | NA | Esophageal cancer | Any bisphosphonate use (other osteoporosis medications use) | Esophageal cancer 0.55 (0.06-4.72) | NA | Incidence rate 26.7/100 000 | Incidence rate 48.4/100 000 | NA |
| Cardwell et al[7] | United Kingdom | Patients receiving prescription for oral bisphosphonate (GPRD) | E:1996-2006; F/U: -2008 | 4.5 (0.5-12.9) | 70.0 ± 11.4 | M: 19; F: 81 | Esophageal cancer, gastric cancer | Any bisphosphonate use (regardless of bisphosphonate use) | Gastric cancer 0.78 (0.50-1.23); esophageal cancer 1.07 (0.77-1.49) | Age, sex, general practice, BMI, cigarette smoking, alcohol intake, hormone therapy, NSAID use, Barrett's esophagus, GERD, H2 receptor antagonist use, proton pump inhibitor use | Esophageal or gastric cancer 116/41 826 (gastric cancer 37/41 826; esophageal cancer 79/41 826) | Esophageal or gastric cancer 115/41 826 (gastric cancer43/41 826; esophageal cancer 72/41 826) | 83 652 |
| Ref. | Country (study type) | Case selection method | Control sampling method | Medication data collection method (period) | Age, yr (mean±SD) | Sex ratio of cases (reference group) | Site of cancer | Type of drug (reference group) | Odd’s ratio (95% CI) | Adjustment | No. of cases/no. of controls | |
| Exposed | Unexposed | |||||||||||
| Green et al[8] | United Kingdom (nested case-control) | Review of computerized information (within participants of GPRD between 1995 -2005) | Matched on age (within 2 yr), sex, observation period in the database and general practice attended) | Review of computerized medical records (from 1995 until cancer diagnosis) | 72 ± 11 | M: 57; F: 43 | Gastrointestinal cancer (esophageal, gastric, colorectal) | Any bisphosphonate use (no bisphosphonate use) | Esophageal cancer 1.30 (1.02-1.66); Gastric cancer 0.87 (0.64-1.19); colorectal cancer 0.87 (0.77-1.00) | Age, sex, observation period, general practice, BMI, cigarette smoking, alcohol intake | Esophageal cancer 90/345; gastric cancer 49/270; colorectal cancer 276/1555 | Esophageal cancer 2864/14 376; gastric cancer 1969/9737; colorectal cancer 10 365/51 467 |
| Nguyen et al[12] | United States (nested case-control) | Review of computerized information (within patients with Barrett’s esophagus in the national veterans affair database between 2000-2002) | Matched on age(interval of 5 yr) and Barrett’s esophagus index date | Review of computerized medical records (from Barrett’s esophagus diagnosis until 3 mo before cancer diagnosis) | 65.0 ± 10.3 | M: 2.6; F: 97.4 | Esophageal cancer | Any bisphosphonate use, mostly alendronate (no bisphosphonate use) | 0.81 (0.18-3.72) | Age, Barrett’s esophagus index date, race, non cancer disease comorbidity index, NSAID use, PPI use | 2/13 | 114/683 |
| Rennert et al[13] | Israel (conventional case-control) | Review of a computerized information (within postmenopausal women in CHS database between 2000-2006) | Matched on age, sex, residence, and ethnic group in CHS database | Review of CHS pharmacy records | 71.1 ± NA | F: 100 | Colorectal cancer | Any bisphosphonate use more than 1 yr (no bisphosphonate use) | 0.41 (0.25-0.67) | BMI, family history of colorectal cancer, vegetable consumption, sports participation, use of low-dose aspirin, statins, vitamin D, postmenopausal hormones | 53/100 | 880/833 |
The methods of the studies we included were assessed on the basis of 5 predetermined quality assessment items (Table 4). All of the studies included in our analyses were based on the secure record linkage regarding medication use and cancer diagnosis.
| Study | Quality assessment items | ||||
| Representativeness | Ascertainment of exposure: Secure record or structured interview | Demonstration that outcome of interest | Assessment of outcome: Independent blind assessment or record linkage was not present at start of study | Study controls for age, cigarette smoking, BMI status | |
| Cohort studies | |||||
| Steinbuch et al[9] | - (female) | + | + | + | - (randomized controlled trials) |
| Abrahamsen et al[10] | + | + | - | + | + (age) |
| Solomon et al[11] | - (medicare beneficiaries) | + | - | + | - (not reported) |
| Cardwell et al[7] | + | + | + | + | +++ (age, cigarette smoking, BMI) |
| Case-control studies | |||||
| Green et al[8] | + | + | - | + | +++ (age, cigarette smoking, BMI) |
| Nguyen et al[12] | - (Barrett’s esophagus) | + | + | + | + (age) |
| Rennert et al[13] | - (female) | + | + | + | ++ (age, BMI) |
There was no negative association between bisphosphonate use and the incidence of esophageal cancer in the overall analysis (RR 0.96, 95% CI: 0.65 1.42, I2 = 52.8%, P = 0.076; Figure 2A). In 2 studies with long-term follow-up, there was a tendency for bisphosphonate users to develop esophageal cancer; however, this finding was not statistically significant (RR 1.74, 95% CI: 0.97-3.10, I2 = 58.8%, P = 0.119). No significant association was found in the studies[8,14] reporting the risk of gastric cancer (RR 0.89, 95% CI: 0.71-1.13, I2 = 0.0%, P = 0.472; Figure 2B).
Bisphosphonate users were less likely to receive a diagnosis of colorectal cancer; however, this finding was not statistically significant in the overall analysis (RR 0.62, 95% CI: 0.30-1.29, I2 = 88.0%, P = 0.004) or in the analysis limited to studies with long-term follow-up (RR 0.61, 95% CI: 0.28-1.35, I2 = 84.6%, P = 0.011; Figure 2C).
In the present study, we examined the association between oral bisphosphonate use and the incidence of gastrointestinal tract cancer by analyzing the results of previous observational studies. Our meta-analyses found no significant association between the use of oral bisphosphonate and the overall gastrointestinal cancer incidence.
In the study design subgroup meta-analysis, no statistically significant association was found for the cohort studies or case-control studies. The cancer type subgroup analysis identified no association between bisphosphonate use and esophageal cancer or gastric cancer. Colorectal cancer was less likely to be diagnosed in bisphosphonate users; however, this finding was not statistically significant. The results of the sensitivity analyses of the studies with more than 3 years of oral bisphosphonate use were similar to those of the overall analyses.
According to our analyses, oral bisphosphonate seems to have little association with the incidence of gastrointestinal cancer.
It is well known that the use of oral bisphosphonate induces gastrointestinal problems, such as erosive esophagitis[15]. The endoscopic and histological findings of mucosal injury of the esophagus in patients using oral bisphosphonates[15-20] suggest that prolonged use may increase the risk of esophageal cancer. Green et al[8] analyzed the United Kingdom General Practice Research Database and reported that bisphosphonate use increases esophageal cancer risk (RR 1.30, 95% CI: 1.02-1.66). The cancer-promoting effect was even greater in patients who used the drug for more than 3 years (RR 2.24, 95% CI: 1.47-3.43). We can infer from this result that bisphosphonate should be restricted among people with risk factors for esophageal cancer, such as Barrettv's esophagus. However, according to the study of Nguyen et al[12], the use of oral bisphosphonate does not increase the risk of esophageal adenocarcinoma in patients with Barrett's esophagus (incidence density ratio 0.92; 95% CI: 0.21-4.15). The risk of esophageal adenocarcinoma in patients with Barrett's esophagus is 30- to 125-fold greater than the risk in the general population[21]. The carcinogenic effect of oral bisphosphonate, including damage to the esophagus due to the toxicity of the drug itself and the effect of contact between the pill and the esophageal mucosa[15], may expedite the development of esophageal cancer in patients with Barrett's esophagus. However, a correlation between the risk of esophageal adenocarcinoma and use of oral bisphosphonate by patients with Barrett's esophagus is inconsistent with the concept of the so-called Barrett pathway.
Several in vivo and in vitro studies suggest that bisphosphonate has anticancerous properties[1-5]. Clinical studies also implicate the anticancerous effect of bisphosphonate in breast cancer[22]. It can be inferred from these studies that there is no significant association between the use of oral bisphosphonate and the risk of esophageal cancer because bisphosphonate has anticancerous effects. As for procancerous effects, oral bisphosphonate directly irritates the esophageal mucosa and induces erosive esophagitis. On the contrary, bisphosphonate directly and indirectly interferes with cancer cell growth. Our finding about the correlation between the risk of esophageal cancer and oral bisphosphonate use suggests that the procancerous and anticancerous effects of bisphosphonate may cancel each other out.
There is also an implication about esophageal cancer within the statistical results in our study. Two observational studies that have great significance in our meta-analyses Caldwell et al[7] and Green et al[8], reported discrepant results (Figure 2A). According to the analyses of Dixon et al[23], the time-dependent RRs of esophageal cancer and oral bisphosphonate indicate no significant increased risk for esophageal cancer within 3 years of oral bisphosphonate use. However, when these investigators restricted their analyses to bisphosphonate use for more than 3 years, their results were different[23]. The Caldwell study[7] reported a RR of 1.01 (95% CI: 0.48-2.12); Green et al[8] reported a RR of 2.24 (95% CI: 1.47-3.43). The result of our meta-analysis was a RR of 1.74 (95% CI: 0.97-3.10) for the follow-up longer than 3 years. Our results show no statistical correlation between the long-term oral bisphosphonate use and the risk of esophageal cancer. However, in the study of Caldwell et al[7], the RR increased to 1.23 (95% CI: 0.66-2.3) when the analysis was restricted to a group of patients with a mean bisphosphonate use duration of 6.8 years. These results indicate that there is somewhat an association between prolonged oral bisphosphonate use and risk of esophageal cancer. Both studies used data from the Uinted Kingdom General Practice Research Database. Inconsistency between the 2 studies may be explained by differences in study design and confounding that could not be measured.
As stated previously, oral bisphosphonates induce esophagitis[15] and esophageal cancer that can be developed from reflux esophagitis through the Barrett pathway[19,20], and this is not a one-time process; it takes time. For this reason, recurrent injuries and healing processes induced by prolonged oral bisphosphonate use could be clinically meaningful factors. The long-term effect of oral bisphosphonate could be confirmed by an observational study that focuses on much longer periods of oral bisphosphonate use.
Regarding gastric cancer, our results show no significant association with oral bisphosphonate use. There is only 1 study that estimates RR dependent on duration of oral bisphosphonate use. Although there is no statistical significance, Green et al[8] found that RR is less for those using oral bisphosphonate longer than 3 years (RR 0.54, 95% CI: 0.24-1.18) than for those using oral bisphosphonate shorter than 3 years (RR 1.03, 95% CI: 0.67-1.59 for less than 1 year; RR 0.89, 95% CI: 0.52-1.53 for 1-3 years). This implies that there is no cumulative effect of oral bisphosphonate on the risk of gastric cancer. If bisphosphonate has competing procancerous and anticancerous properties, it may not affect the risk of cancer.
We performed a random-effects model analysis of the data on colorectal cancer because of high heterogeneity (I2 = 88.0%, P = 0.004). According to our meta-analysis, oral bisphosphonate use demonstrates no effect on the risk of colorectal cancer (RR 0.62, 95% CI: 0.30-1.29). The analysis of the long-term use (more than 3 years) revealed that there is no statistically significant association between oral bisphosphonate use and colorectal cancer (RR 0.61, 95% CI: 0.28-1.35). However, the subgroup analysis shows a significant negative association between oral bisphosphonate use and the incidence of colorectal cancer (RR 0.87, 95% CI: 0.77-1.00; RR 0.41, 95% CI: 0.25-0.67). Therefore, it may be hasty to conclude on the basis of overall meta-analysis that oral bisphosphonate use does not affect colorectal cancer. In the study by Rennert et al[13], cases and controls were matched for age, ethnicity, family history of colorectal cancer, sports activity, vegetable consumption, body mass index (BMI), low-dose aspirin use, statin use, postmenopausal hormone use, calcium supplement use, and vitamin use, all of which can affect the risk of colorectal cancer. However, in Green et al[8], RRs were only adjusted for smoking status, alcohol intake and BMI. Thus, there were important differences in the study design and quality of methods.
The study by Rennert et al[13] implies that oral bisphosphonate has a protective effect against colorectal and breast cancers. The anticancerous effects of bisphosphonate, such as promoting apoptosis[2], inhibiting tumor cell adhesion and invasion[3], inhibiting angiogenesis[4], altering tumor-associated macrophage function[4], and enhancing immune surveillance, as previously mentioned, may have a key role in such a protective effect. One study reported that ibandronate reduces the incidence of colorectal dysplasia in mice with induced ulcerative colitis[24]. In colorectal cancer, different from esophageal cancer, oral bisphosphonate does not directly injure the intestinal mucosa or induce chronic mucosal inflammation and healing processes.
Considering this background, the results of our meta-analyses should be interpreted as inconclusive. A well-designed randomized controlled study or prospective cohort study is needed to confirm the preventive effect of oral bisphosphonate against colorectal cancer.
Our study has a few limitations. First, the number of studies we analyzed is small. There have been few studies about the correlation between oral bisphosphonate use and the risk for gastrointestinal cancer. For this reason, each study that reported on colorectal cancer reported a negative association, but the overall meta-analysis showed no statistical significance. Thus, oral bisphosphonate use is seemingly irrelevant to colorectal cancer. Second, the quality of our study depends on data from original publications. Our study inevitably inherits some problems from the observational studies, such as selection bias, surveillance bias, and confounding. For example, more esophageal and gastric abnormalities might be observed in bisphosphonate users simply because they receive more endoscopic exams for abdominal discomfort caused by oral bisphosphonate.
We discussed the small number of studies regarding bisphosphonate use and development of gastrointestinal cancer in our article as a limitation. However, the actual number of overall subjects in our meta-analysis is not too small. In cohort studies, 124 686 subjects with mean follow up of 3.88 years were included in the final analysis. In case of case-control studies, there were 3070 esophageal cancer cases, 2018 gastric cancer cases and 11 574 colorectal cancer cases were included as well. Moreover, the results of two large observational studies were inconsistent. Caldwell et al[7] showed no significant association between bisphosphonate use and esophageal cancer. On the other hand, Green et al[8] had revealed the significant association. The inconsistent results of observational studies suggest the need of further studies as well as a meta-analysis.
Despite the negative results, our study is meaningful since it provides not an ultimate but a reasonable interim conclusion regarding the safety of bisphosphonate use before definite accumulation of long-term observational studies.
In summary, our meta-analyses indicate that there is no significant association between oral bisphosphonate use and the risk of gastrointestinal cancer. Oral bisphosphonate use has no significant association with the risk of esophageal cancer. There is an increased, though not statistically significant, risk of esophageal cancer in long-term users of oral bisphosphonate. An observational study focused on long-term use of bisphosphonate is needed to confirm this finding. The risk of gastric cancer is not associated with oral bisphosphonate use. Each study reporting on colorectal cancer indicates a negative association between the risk of colorectal cancer and oral bisphosphonate use, but our meta-analysis showed no statistically significant association. The confidence intervals were large (95% CI: 0.30-1.29). Thus, a randomized controlled trial or prospective cohort study should be performed to confirm the preventive effect of oral bisphosphonate.
There rises concerns about bisphosphonate use after the reports of 23 cases of esophageal cancer in the United States and another 31 cases in Europe and Japan, all occurring from 1995 through 2008 among patients using oral bisphosphonates. Bisphosphonate induced esophagitis, Barrett's esophagus and gastric ulcer can be the precancerous condition. There were observational studies to evaluate the risk between esophageal cancer and bisphosphonate use. But the results were inconsistent. So the overall evaluation of the association between gastrointestinal cancer and bisphosphonate is required.
Meta-analysis was used to evaluate the risk of bisphosphonate for gastrointestinal cancer (esophageal, stomach, and colorectal cancer) in this study.
This meta-analysis systemically assessed the relation between bisphosphonate use and gastrointestinal cancer risk, and also showed site specific, long-term follow up results.
The results of meta-analysis in this study show that use of bisphosphonate has no significant association with gastrointestinal cancers. According to the results, bisphosphonate can be used without charge of carcinogen for now. But there should be a far more long-term observational studies to guarantee the long-term safety.
Meta-analysis is method focused on contrasting and combining results from different studies to show the overall conclusion. It is essential component of a systematic review procedure.
This is a good descriptive study in which authors perform a meta-analysis of observational studies to further elucidate the relationship between oral bisphosphonate use and gastrointestinal cancer risk. The results are interesting and suggest that oral bisphosphonate use had no significant effect on gastrointestinal cancer risk.
| 1. | Green J, Lipton A. Anticancer properties of zoledronic acid. Cancer Invest. 2010;28:944-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Hiraga T, Williams PJ, Ueda A, Tamura D, Yoneda T. Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clin Cancer Res. 2004;10:4559-4567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 176] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Boissier S, Ferreras M, Peyruchaud O, Magnetto S, Ebetino FH, Colombel M, Delmas P, Delaissé JM, Clézardin P. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000;60:2949-2954. [PubMed] |
| 4. | Coscia M, Quaglino E, Iezzi M, Curcio C, Pantaleoni F, Riganti C, Holen I, Mönkkönen H, Boccadoro M, Forni G. Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J Cell Mol Med. 2010;14:2803-2815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 5. | Sato K, Kimura S, Segawa H, Yokota A, Matsumoto S, Kuroda J, Nogawa M, Yuasa T, Kiyono Y, Wada H. Cytotoxic effects of gammadelta T cells expanded ex vivo by a third generation bisphosphonate for cancer immunotherapy. Int J Cancer. 2005;116:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Wysowski DK. Reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009;360:89-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Cardwell CR, Abnet CC, Cantwell MM, Murray LJ. Exposure to oral bisphosphonates and risk of esophageal cancer. JAMA. 2010;304:657-663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ. 2010;341:c4444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 9. | Steinbuch M, D'Agostino RB, Mandel JS, Gabrielson E, McClung MR, Stemhagen A, Hofman A. Assessment of mortality in patients enrolled in a risedronate clinical trial program: a retrospective cohort study. Regul Toxicol Pharmacol. 2002;35:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Abrahamsen B, Eiken P, Eastell R. More on reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009;360:1789; author reply 1791-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 11. | Solomon DH, Patrick A, Brookhart MA. More on reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009;360:1789-1790; author reply 1789-1790. [PubMed] |
| 12. | Nguyen DM, Schwartz J, Richardson P, El-Serag HB. Oral bisphosphonate prescriptions and the risk of esophageal adenocarcinoma in patients with Barrett's esophagus. Dig Dis Sci. 2010;55:3404-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Rennert G, Pinchev M, Rennert HS, Gruber SB. Use of bisphosphonates and reduced risk of colorectal cancer. J Clin Oncol. 2011;29:1146-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Robins HI, Holen KD. More on reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009;360:1790; author reply 1791-1792. [PubMed] |
| 15. | Abraham SC, Cruz-Correa M, Lee LA, Yardley JH, Wu TT. Alendronate-associated esophageal injury: pathologic and endoscopic features. Mod Pathol. 1999;12:1152-1157. [PubMed] |
| 16. | Singh SP, Odze RD. Multinucleated epithelial giant cell changes in esophagitis: a clinicopathologic study of 14 cases. Am J Surg Pathol. 1998;22:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Graham DY, Malaty HM. Alendronate and naproxen are synergistic for development of gastric ulcers. Arch Intern Med. 2001;161:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Graham DY. What the gastroenterologist should know about the gastrointestinal safety profiles of bisphosphonates. Dig Dis Sci. 2002;47:1665-1678. [PubMed] |
| 19. | Chow WH, Finkle WD, McLaughlin JK, Frankl H, Ziel HK, Fraumeni JF. The relation of gastroesophageal reflux disease and its treatment to adenocarcinomas of the esophagus and gastric cardia. JAMA. 1995;274:474-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 151] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2042] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 21. | Pera M. Trends in incidence and prevalence of specialized intestinal metaplasia, barrett's esophagus, and adenocarcinoma of the gastroesophageal junction. World J Surg. 2003;27:999-1008; discussion 1006-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Chlebowski RT, Col N. Bisphosphonates and breast cancer prevention. Anticancer Agents Med Chem. 2012;12:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Dixon WG, Solomon DH. Bisphosphonates and esophageal cancer--a pathway through the confusion. Nat Rev Rheumatol. 2011;7:369-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Sassa S, Okabe H, Nemoto N, Kikuchi H, Kudo H, Sakamoto S. Ibadronate may prevent colorectal carcinogenesis in mice with ulcerative colitis. Anticancer Res. 2009;29:4615-4619. [PubMed] |
Peer reviewer: Dr. Kok Sun Ho, Department of Colorectal Surgery, Singapore General Hospital, Outram Road, Singapore 169608, Singapore
S- Editor Gou SX L- Editor A E- Editor Xiong L