Published online Oct 28, 2012. doi: 10.3748/wjg.v18.i40.5759
Revised: June 20, 2012
Accepted: June 28, 2012
Published online: October 28, 2012
AIM: To assess the nourishment status and lifestyle of non-hospitalized patients with compensated cirrhosis by using noninvasive methods.
METHODS: The subjects for this study consisted of 27 healthy volunteers, 59 patients with chronic viral hepatitis, and 74 patients with viral cirrhosis, from urban areas. We assessed the biochemical blood tests, anthropometric parameters, diet, lifestyle and physical activity of the patients. A homeostasis model assessment-insulin resistance (HOMA-IR) value of ≥ 2.5 was considered to indicate insulin resistance. We measured height, weight, waist circumference, arm circumference, triceps skin-fold thickness, and handgrip strength, and calculated body mass index, arm muscle circumference (AMC), and arm muscle area (AMA). We interviewed the subjects about their dietary habits and lifestyle using health assessment computer software. We surveyed daily physical activity using a pedometer. Univariate and multivariate logistic regression modeling were used to identify the relevant factors for insulin resistance.
RESULTS: The rate of patients with HOMA-IR ≥ 2.5 (which was considered to indicate insulin resistance) was 14 (35.9%) in the chronic hepatitis and 17 (37.8%) in the cirrhotic patients. AMC (%) (control vs chronic hepatitis, 111.9% ± 10.5% vs 104.9% ± 10.7%, P = 0.021; control vs cirrhosis, 111.9% ± 10.5% vs 102.7% ± 10.8%, P = 0.001) and AMA (%) (control vs chronic hepatitis, 128.2% ± 25.1% vs 112.2% ± 22.9%, P = 0.013; control vs cirrhosis, 128.2% ± 25.1% vs 107.5% ± 22.5%, P = 0.001) in patients with chronic hepatitis and liver cirrhosis were significantly lower than in the control subjects. Handgrip strength (%) in the cirrhosis group was significantly lower than in the controls (control vs cirrhosis, 92.1% ± 16.2% vs 66.9% ± 17.6%, P < 0.001). The results might reflect a decrease in muscle mass. The total nutrition intake and amounts of carbohydrates, protein and fat were not significantly different amongst the groups. Physical activity levels (kcal/d) (control vs cirrhosis, 210 ± 113 kcal/d vs 125 ± 74 kcal/d, P = 0.001), number of steps (step/d) (control vs cirrhosis, 8070 ± 3027 step/d vs 5789 ± 3368 step/d, P = 0.011), and exercise (Ex) (Ex/wk) (control vs cirrhosis, 12.4 ± 9.3 Ex/wk vs 7.0 ± 7.7 Ex/wk, P = 0.013) in the cirrhosis group was significantly lower than the control group. The results indicate that the physical activity level of the chronic hepatitis and cirrhosis groups were low. Univariate and multivariate logistic regression modeling suggested that Ex was associated with insulin resistance (odds ratio, 6.809; 95% CI, 1.288-36.001; P = 0.024). The results seem to point towards decreased physical activity being a relevant factor for insulin resistance.
CONCLUSION: Non-hospitalized cirrhotic patients may need to maintain an adequate dietary intake and receive lifestyle guidance to increase their physical activity levels.
- Citation: Hayashi F, Momoki C, Yuikawa M, Simotani Y, Kawamura E, Hagihara A, Fujii H, Kobayashi S, Iwai S, Morikawa H, Enomoto M, Tamori A, Kawada N, Ohfuji S, Fukusima W, Habu D. Nutritional status in relation to lifestyle in patients with compensated viral cirrhosis. World J Gastroenterol 2012; 18(40): 5759-5770
- URL: https://www.wjgnet.com/1007-9327/full/v18/i40/5759.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i40.5759
Chronic infection with hepatitis virus is one of the most common causes of liver cirrhosis worldwide. About 3.5 million people worldwide are chronically infected with hepatitis B virus (HBV). More than 70% of all newly diagnosed liver cancer patients are found in Asia, a region accounting for 75% of all patients with chronic HBV infection[1]. Hepatitis C virus (HCV) has infected approximately 1.3 million people worldwide. In the Asia-Pacific region, the prevalence of HCV infection ranges from 0.3% to 12%, with geographical variation[2]. The major cause of liver cirrhosis in Japan is the hepatitis virus. In particular, chronic HCV infection accounts for 60.9% of all liver cirrhosis cases[3].
Cirrhosis is an end-stage chronic liver disease, and the damage to the hepatocytes decreases the function of the liver, leading to a variety of nutritional metabolic disorders. Therefore, protein energy malnutrition (PEM) is frequently seen in patients with cirrhosis[4-7]. A study of subjects with cirrhosis conducted in Western countries reported that 11% had energy malnutrition, 22% had protein malnutrition, 32% had both complications of PEM, and 35% had a normal nourishment status[8]. In 2002, a study of Japanese patients with cirrhosis reported that 12% had energy malnutrition, 25% had protein malnutrition, 50% had PEM, and only 13% had a normal nourishment status[9]. Currently, the lifestyles of people in urban areas of several Asian countries are changing due to westernization and motorization, resulting in excessive food intake, decreased intensity of daily activities, and a lack of exercise (Ex). These changes may lead to obesity, metabolic syndrome, and lifestyle diseases. Is there a possibility that this lifestyle transition (over-nourishment and decline in the intensity of daily activities) has an effect on the nutrition of cirrhosis? In 2006, a study reported that body mass index (BMI) in patients with cirrhosis was almost the same as those of the same age group in the normal Japanese population[10]. Moreover, both experimental and clinical studies found that over-nutrition, fatty liver, and glucose intolerance are significant risk factors of hepatic carcinogenesis[11,12]. The combination of over-nutrition and obesity in cirrhosis patients also indicates the possibility of an impaired pathogenesis. These findings suggest that high calorie and protein intake (i.e., old dietary interventions of cirrhosis) promotes obesity, fatty liver, and hepatic carcinogenesis. Almost all subjects in Japanese reports were hospitalized having decompensated cirrhosis with hepatic failure. Patients with severe decompensated cirrhosis tend to develop a reduced food and protein intake because of abdominal ascites and hepatic encephalopathy. However, the majority of cirrhotic patients have compensated cirrhosis and so do not need to be hospitalized, and thus their daily lives may be almost the same as that of healthy people. Few researchers have studied the effect of dietary habits and lifestyle in non-hospitalized cirrhotic patients on their conditions.
If we consider cirrhosis from the point of view of a lifestyle disease, we should know the patients' actual condition in order to provide adequate lifestyle and dietary guidance. The purpose of this study is to assess the nourishment status of non-hospitalized patients with compensated cirrhosis and to create a database on nutritional and lifestyle education for these patients by analyzing their lifestyle, food intake and physical activity.
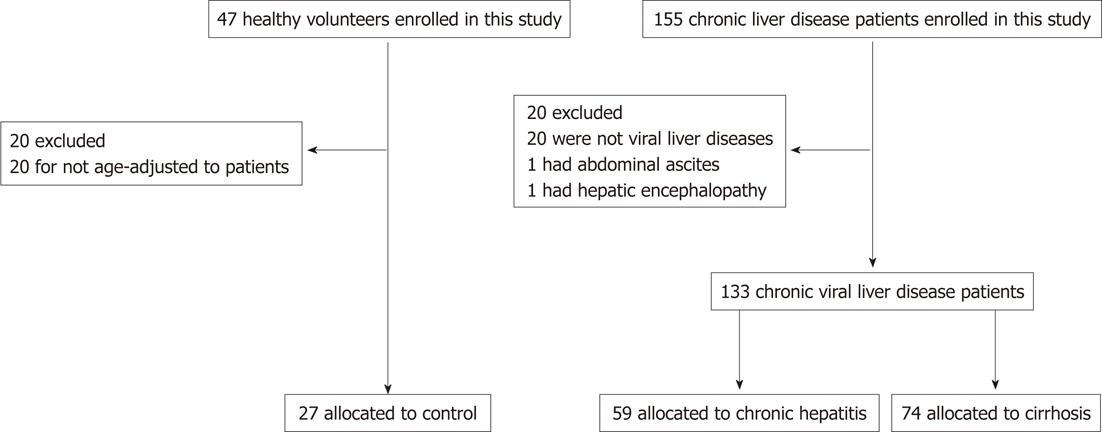
We interviewed 155 patients (age range: 45-79 years) with chronic liver disease living in urban areas. Patients with chronic viral liver disease who regularly visited the Osaka City University Hospital between March 2009 and December 2010 were enrolled. Written consent was received from all study participants. After excluding patients with non-viral liver disease, abdominal ascites and hepatic encephalopathy, 133 patients were enrolled. Next, the patients were divided into 2 groups of chronic hepatitis and cirrhosis on the basis of the clinical diagnosis made by the attending physicians. We enrolled 47 healthy volunteers from an urban area (local residents around the Osaka City University) who underwent a health check after providing their consent. The results were age-adjusted and, finally, data of 27 healthy volunteers were used for the comparison of the subjects (Figure 1). Complication of diabetes mellitus, hypertension, and hyperlipidemia were defined by taking medication for each disease. The degree of liver disease was assessed using the Child-Pugh classification. This trial was conducted in accordance with the guidelines of the World Medical Association’s Declaration of Helsinki (1996) and was approved by the ethics committee of our institution (No. 1906).
Both chronic hepatitis and cirrhosis patients were examined, and we measured the number of white blood cells (WBC; × 100/μL), number of red blood cells (RBC; × 102/μL), hemoglobin (Hb; g/dL), hematocrit level (Ht; %), platelet count (Plt; × 102/μL), prothrombin time (PT; %); total protein (TP; g/dL), albumin (Alb; g/dL), blood urea nitrogen (mg/dL), creatinine (mg/dL), total bilirubin (T-bil; mg/dL), direct bilirubin (D-bil; mg/dL), aspartate aminotransferase (AST; IU/L), alanine aminotransferase (IU/L), leucine aminopeptidase (LAP; IU/L), γ-glutamyl transpeptidase (IU/L), alkaline phosphatase (ALP; IU/L), cholinesterase (ChE; IU/L), triglycerides (TG; mg/dL), total cholesterol (Total-C; mg/dL), fasting blood sugar (FBS; mg/dL), hemoglobin A1c (HbA1c; %), immunoreactive insulin levels (IRI; μU/mL), plasma branched-chain amino acid levels (BCAA; μmol/mL), plasma tyrosine levels (Tyrosine; μmol/mL), and the molar ratio of BCAA to tyrosine (BTR). The insulin resistance index was calculated according to homeostasis model assessment-insulin resistance (HOMA-IR) from the FBS and IRI values using the following formula[13]: HOMA-IR = FBS × IRI/405.
A HOMA-IR value of ≥ 2.5 was considered to indicate insulin resistance. The data of subjects with complicated diabetes mellitus were excluded in the comparison of the HOMA-IR values.
To evaluate nourishment status in the subjects, we assessed their height (cm) and weight (kg) and calculated BMI (kg/m2). We classified patients with a BMI of < 18.5 kg/m2 as underweight, 18.5 kg/m2 to 25 kg/m2 as normal, and ≥ 25 kg/m2 as obese to estimate the rate of obesity in the subjects[14]. We measured the waist circumference (cm) by using a measuring tape to estimate the intra-abdominal fat. Arm circumference (AC; cm) and triceps skin-fold thickness (TSF; mm) were measured with Inser-Tape and Adipometer (Abbott Japan Co., Ltd., Tokyo, Japan). To minimize intra-operator variability, the averages of 3 consecutive measurements were recorded. Arm muscle circumference (AMC; cm) and arm muscle area (AMA; cm2) were calculated by using the following formula: AMC = AC - (π× TSF), AMA = (AMC)2/4π.
Age and sex difference were considered; the mean value of measured anthropometric measurements were divided by the respective age-sex-adjusted medians, which were obtained from the Japanese Anthropometric Reference Data 2001[15]. The results were expressed as a percentage (%TSF, %AC, %AMC, %AMA). In addition, right and left handgrip strength were measured once by using the Smedley Type Hand Dynamometer Grip D (Takei Kiki Kogyo Co., Ltd., Japan). The mean value of both hand grip strength measurements were expressed as a percentage by dividing the average of each age acquired from the Japanese Ministry of Education, Culture, Sports, Science and Technology, which performed a test of physical strength and Ex capacity in 2009. The obtained results were used as a qualitative evaluation index of the skeletal muscle.
Dietary habits and lifestyle of the interviewed subjects were evaluated by using the specific health guideline support software Health Check for Metabolic Syndrome (Suzuken Co., Ltd., Nagoya, Japan). Originally, this software was developed as a tool for specific health guidelines to study metabolic syndromes and reserves. The software was modified so that it could be used for chronic liver disease patients. The survey consisted of 50 questions concerning daily life and dietary habits. The results were expressed as total energy or protein intake per body (BW). To compare the results of this study with the guideline of the European Society for Parenteral and Enteral Nutrition (ESPEN) Nutritional Management[16], total energy and protein intake were divided by BW. The results were expressed as total energy or protein intake per BW.
The lifestyle recorder, Lifecorder (Suzuken Co., Ltd., Nagoya, Japan), is an activity observation machine that functions as an acceleration sensor. The physical activity pattern was objectively obtained by continuously measuring activity intensity, period and frequency. This is one means to evaluate physical activity-related energy expenditure. It is possible to estimate total energy expenditure (TEE), including involuntary activities such as slow or small erratic movements performed when sedentary and during very low-level activities, by attaching the Lifecorder for 24 h. The quantity and intensity of physical activity could then be accurately determined. This was confirmed in a paper that reported the use of the metabolic chamber[17], showing correlation with the measured values of TEE. The Lifecorder was used for 1 wk (7 d). Data of daily TEE (kcal/d), physical activity (kcal/d), the number of steps (step/d), and Ex (Ex/wk) were obtained. Ex is a unit to express the quantity of physical activity, calculated by multiplying metabolic equivalents (METs) by the duration of the activity (h) as proposed in the Physical Activity Guide for Health Promotion 2006, which was published by the Ministry of Health, Labor and Welfare of Japan. The METs is an index for the intensity of physical activity, calculated as energy expenditure (oxygen uptake, mL/kg per minute) during a specific physical activity divided by sitting/ resting energy expenditure (REE). The goal for the quantity of physical activity for health promotion was set at 23 Ex or more per week to be achieved by active physical activities (including both Ex and non-Ex activities), of which 4 Ex or more should be achieved by active Ex[18].
Univariate and multivariate logistic regression modeling were used to identify the relevant factors for insulin resistance in patients with chronic liver disease. The degree of chronic hepatitis, HCV infection, the presence or absence of hepatocellular carcinoma, sex, age, %AC, %TSF, handgrip strength, Ex, Plt, Alb, BTR, total energy intake per BW, protein intake per BW, and the percentage fat in nutrition intake were chosen as potential factors. Continuous variables were divided into 2 groups according to each standard as described below. HCV infection has been reported to cause insulin resistance[19]. Therefore, patients were categorized into 2 groups according to HBV and HCV infection. Patients were categorized into 2 groups by age, with those over 65 years considered elderly. Both %AC and %TSF were divided using 100 as a standard value. Handgrip strength was divided using 69, which was the median value. Ex was divided using a value of 4.0, which is the desired amount of active Ex. Plt was divided according to a value of 100 000, with less than 100 000 as the standard for liver cirrhosis. It has been reported that Alb of 3.5 or less is related to prognosis[9]. Therefore, Alb was divided using a value of 3.5, with less than 3.5 considered malnutrition. BTR was divided into 2 groups based on 4.41, which is the lower limit of the standard range. In this study, energy intake per BW was divided based on a value of 30, which is the median of the standard range in the ESPEN nutritional management guidelines[16]. Protein intake per BW was divided based on a value of 1.2, which is the upper limit of the standard range in the ESPEN nutritional management guidelines. The percentage of fat in daily food intake was divided using 25, which is the upper limit of the standard range.
Outcomes are presented as mean ± SD, number of patients, or a percentage. The statistical analyses were performed using the Mann-Whitney U, Kruskal-Wallis, and Scheffe's multiple comparison tests, or Spearman's rank correlation coefficient. Univariate and multivariate logistic regression modeling were used to obtain the crude and adjusted odds ratios and 95% CI for the association between each factor and insulin resistance. The statistical software Dr. SPSS II for Windows Version 11.0.1J (SPSS Inc.) was used for the analysis. P value < 0.05 was considered statistically significant.
One hundred and thirty-three patients with chronic liver disease were included in the analysis. Their characteristics are summarized in Table 1. Sex was not significantly different among the groups. Age in cirrhotic patients was significantly higher than that in patients with chronic hepatitis. Seventeen patients with chronic hepatitis and 11 patients with cirrhosis were infected with HBV, while 42 patients with chronic hepatitis and 63 patients with cirrhosis were infected with HCV. Eleven patients had diabetes mellitus, 20 had hypertension, and 4 had hyperlipidemia in the chronic hepatitis patients. The cirrhotic patients were involved with complications of diabetes mellitus in 11 patients, hypertension in 20, and hyperlipidemia in 4. When cirrhotic patients were assessed by using Child-Pugh classification of the severity of liver disease, 62 patients were classified into class A, and 12 into class B.
The results of laboratory evaluation are shown in Table 2. The values of WBC, RBC, Hb, Ht, Plt, PT, TP, Alb, Total-C, TG, ChE, HbA1c and BTR in cirrhotic patients were significantly lower than those in patients with chronic hepatitis. Furthermore, the values of AST, T-bil, D-bil, ALP and LAP were also significantly higher in the latter group. The IRI of both the groups was slightly high, and the mean value of HOMA-IR was ≥ 2.5. Twenty-two of the 106 patients who had obvious diabetes, based on their measured FBS level and insulin level, were excluded. We used the remaining 84 patients as the subjects for the analysis. The number of patients with a HOMA-IR ≥ 2.5, which was considered to indicate insulin resistance, was 14 (35.9%) in the chronic hepatitis patients and 17 (37.8%) in the cirrhotic patients.
| Chronic hepatitis | Cirrhosis | |
| WBC (× 100/μL) | 55.5 ± 19.2 | 37.9 ± 13.6a |
| RBC (× 102/μL) | 441 ± 57 | 399 ± 47a |
| Hb (g/dL) | 13.8 ± 1.5 | 12.6 ± 1.6a |
| Ht (%) | 40.7 ± 3.9 | 37.2 ± 4.0a |
| Plt (× 102/μL) | 17.8 ± 3.8 | 8.6 ± 2.5a |
| PT (%) | 96.9 ± 11.2 | 83.4 ± 13.2a |
| TP (g/dL) | 7.2 ± 0.5 | 7.0 ± 0.7b |
| Alb (g/dL) | 4.0 ± 0.4 | 3.5 ± 0.5a |
| BUN (mg/dL) | 15.6 ± 6.1 | 15.5 ± 5.6 |
| Cre (mg/dL) | 0.77 ± 0.27 | 0.73 ± 0.24 |
| T-bil (mg/dL) | 0.9 ± 0.4 | 1.1 ± 0.5c |
| D-bil (mg/dL) | 0.3 ± 0.2 | 0.4 ± 0.3a |
| AST (IU/L) | 44 ± 23 | 60 ± 39d |
| ALT (IU/L) | 41 ± 27 | 53 ± 40 |
| AST/ALT | 1.2 ± 0.4 | 1.3 ± 0.5 |
| LAP (IU/L) | 59 ± 20 | 63 ± 17e |
| γ-GTP (IU/L) | 51 ± 85 | 51 ± 57 |
| ALP (IU/L) | 299 ± 144 | 356 ± 126f |
| ChE (IU/L) | 281 ± 89 | 201 ± 81a |
| TG (mg/dL) | 102 ± 44 | 87 ± 31 |
| T-cho (mg/dL) | 182 ± 37 | 155 ± 38a |
| HbA1c (%) | 5.9 ± 0.8 | 5.2 ± 0.9a |
| FBS (mg/dL) | 99.0 ± 14.1 | 99.6 ± 14.5 |
| IRI (μU/mL) | 11.1 ± 8.2 | 12.1 ± 11.1 |
| HOMA-IR | 2.7 ± 2.2 | 2.9 ± 2.5 |
| BTR | 5.43 ± 1.54 | 3.90 ± 1.23a |
| BCAA (μmol/mL) | 425 ± 92 | 387 ± 77g |
| Tyrosine (μmol/mL) | 82 ± 23 | 107 ± 36a |
Table 3 presents the outcomes of anthropometric assessments in the subjects. The skeletal muscle index (measured as %AMC and %AMA) was significantly lower in the chronic hepatitis and cirrhotic patients than in the controls. The body fat indicator (measured as %TSF) in the chronic hepatitis patients was significantly higher than in the controls. The handgrip strength in both the patient groups was significantly lower than that in the controls. We divided the subjects into male and female subgroups when comparing height, weight, BMI and abdominal circumference. There were no significant differences among the 3 groups. Among the men, the rate of obesity was 45.5% in the control, 33.3% in those with chronic hepatitis, and 33.3% in those with liver cirrhosis. Among the women, the rate of obesity was 43.8% in the control, 40.6% in those with chronic hepatitis, and 16.0% in those with liver cirrhosis. The difference in the obesity rate was not significant in the men; however, the obesity rate in women was significantly lower as liver disease advanced.
| Control | Chronic hepatitis | Cirrhosis | |
| Height (cm)1 | |||
| Male | 168.2 ± 6.3 | 168.0 ± 7.6 | 165.8 ± 6.0 |
| Female | 151.0 ± 3.5 | 152.8 ± 4.1 | 151.6 ± 6.5 |
| Weight (kg)1 | |||
| Male | 67.0 ± 8.5 | 67.5 ± 11.0 | 64.5 ± 9.0 |
| Female | 54.5 ± 6.7 | 54.6 ± 9.4 | 50.8 ± 8.8 |
| BMI (kg/m2)1 | |||
| Male | 23.7 ± 3.1 | 23.9 ± 3.1 | 23.4 ± 2.6 |
| Female | 23.9 ± 2.6 | 23.4 ± 4.0 | 22.1 ± 3.5 |
| BMI (underweight/normal/obese)2 | |||
| Male | 1/5/5 | 1/17/9 | 0/16/8 |
| Female | 1/8/7 | 2/17/13 | 6/36/8h |
| Abdominal circumference (cm)1 | |||
| Male | 86.3 ± 7.8 | 90.0 ± 8.0 | 87.7 ± 8.6 |
| Female | 84.6 ± 10.0 | 86.0 ± 9.9 | 85.1 ± 9.2 |
| %AC1 | 108.1 ± 8.4 | 107.4 ± 11.8 | 103.9 ± 11.5 |
| %TSF1 | 96.0 ± 37.0a | 129.0 ± 50.2 | 116.5 ± 48.4 |
| %AMC1 | 111.9 ± 10.5be | 104.9 ± 10.7 | 102.7 ± 10.8 |
| %AMA1 | 128.2 ± 25.1cf | 112.2 ± 22.9 | 107.5 ± 22.5 |
| Hand grip strength (%)1 | 92.1 ± 16.2dg | 74.4 ± 20.9 | 66.9 ± 17.6 |
The results of the dietary survey are presented in Table 4. The total nutrition intake and amounts of carbohydrates, protein, and fat were not significantly different among the groups. These results showed that the average food intake of patients was not different compared to that of the controls. Moreover, the intake of important microelements such as calcium, iron, salt, vitamins A, B1, B2, and C; and dietary fibers was almost the same in these 3 groups. The food intake and its contents seemed to be sufficient in cirrhotic patients.
| Control | Chronic hepatitis | Cirrhosis | |
| Energy intake (kcal) | 1814 ± 400 | 1768 ± 470 | 1730 ± 468 |
| Energy intake (kcal/BW) | 31.0 ± 7.5 | 30.0 ± 8.6 | 32.0 ± 9.0 |
| Carbohydrate percentage (%) | 57.9 ± 7.1 | 59.1 ± 9.1 | 60.1 ± 8.9 |
| Protein percentage (%) | 15.9 ± 2.9 | 16.2 ± 2.8 | 16.2 ± 3.0 |
| Fat percentage (%) | 26.1 ± 7.1 | 24.8 ± 8.4 | 23.7 ± 8.8 |
| Carbohydrate intake (g) | 248.9 ± 57.4 | 254.9 ± 76.5 | 251.2 ± 67.9 |
| Protein intake (g) | 68.0 ± 17.1 | 68.3 ± 17.1 | 70.0 ± 19.5 |
| Protein intake (g/BW) | 1.2 ± 0.3 | 1.2 ± 0.4 | 1.3 ± 0.4 |
| Fat intake (g) | 50.5 ± 19.5 | 47.4 ± 21.4 | 46.7 ± 24.4 |
| Calcium intake (mg) | 544 ± 198 | 513 ± 170 | 536 ± 216 |
| Iron intake (mg) | 7.9 ± 2.7 | 7.4 ± 1.8 | 7.2 ± 2.5 |
| Salt intake (g) | 8.7 ± 2.3 | 8.7 ± 2.9 | 8.5 ± 2.3 |
| Vitamin A intake (μgRE) | 1015 ± 391 | 1046 ± 512 | 979 ± 478 |
| Vitamin B1 intake (mg) | 0.8 ± 0.4 | 0.8 ± 0.3 | 0.9 ± 0.4 |
| Vitamin B2 intake (mg) | 1.1 ± 0.3 | 1.2 ± 0.5 | 1.1 ± 0.4 |
| Vitamin C intake (mg) | 114 ± 66 | 148 ± 191 | 118 ± 64 |
| Dietary fiber intake (mg) | 14.3 ± 4.3 | 13.9 ± 4.1 | 14.0 ± 3.8 |
| Grains (g) | 402.5 ± 111.2 | 449.5 ± 173.4 | 416.3 ± 133.0 |
| Green and yellow vegetables (g) | 121.4 ± 76.1 | 143.6 ± 170.7 | 137.8 ± 96.8 |
| Light-colored vegetables (g) | 187.1 ± 128.3 | 165.8 ± 73.0 | 167.1 ± 78.5 |
| Tubers and roots (g) | 65.8 ± 68.1 | 38.1 ± 50.5 | 37.2 ± 44.0 |
| Meat (g) | 51.9 ± 67.5 | 67.8 ± 67.2 | 66.8 ± 73.5 |
| Seafood (g) | 97.4 ± 71.4 | 82.0 ± 56.0 | 73.3 ± 56.1 |
| Egg (g) | 37.4 ± 36.2 | 40.0 ± 38.9 | 27.0 ± 26.7 |
| Pulses (g) | 61.9 ± 64.1 | 57.0 ± 59.3 | 65.6 ± 72.3 |
| Milk and daily product (g) | 124.9 ± 119.5 | 130.1 ± 117.7 | 142.3 ± 132.1 |
| Fruit (g) | 114.5 ± 113.8 | 109.2 ± 192.1 | 129.1 ± 128.8 |
| Oil and fat (g) | 11.3 ± 10.9 | 11.9 ± 11.6 | 9.4 ± 11.6 |
The outcomes of the lifestyle survey are described in Table 5. The lifestyle-related questionnaire survey revealed that the amount of alcohol consumed by cirrhotic patients was significantly lower than that of control subjects. Chronic hepatitis patients tended to consume less alcohol compared with control subjects. With regard to drinking habits, significantly more subjects in each patient group replied that they drank less compared to the controls. Significantly more subjects in both patient groups reported that they had enough rest compared to the control group. Furthermore, the sleeping time in patients of the cirrhosis group was significantly longer than that in the other 2 groups. The perceived health status in each patient group was significantly lower than in the control group.
| Control | Chronic hepatitis | Cirrhosis | P value | |
| Drinking habit2 | < 0.001 | |||
| Nothing | 10 (8.8) | 40 (67.8) | 63 (85.1) | |
| Sometimes | 6 (22.2) | 11 (18.6) | 9 (12.2) | |
| Daily | 11 (40.7) | 8 (13.6) | 2 (2.7) | |
| Alcohol intake (g/d)1 | 13.7 ± 26.6a | 4.9 ± 14.2 | 1.6 ± 9.9 | |
| Stage model of alcohol drinking2 | 0.141 | |||
| Maintenance | 4 (23.5) | 7 (36.8) | 4 (36.4) | |
| Action | 1 (5.9) | 1 (5.3) | 0 (0) | |
| Sometimes | 3 (17.6) | 6 (31.6) | 5 (45.5) | |
| Preparation | 0 (0) | 1 (5.3) | 0 (0) | |
| Contemplation | 9 (52.9) | 2 (10.5) | 1 (9.1) | |
| Pre-contemplation | 0 (0) | 2 (10.5) | 1 (9.1) | |
| Experience of dieting2 | 0.258 | |||
| No | 21 (77.8) | 37 (62.7) | 59 (79.7) | |
| Yes | 6 (22.2) | 22 (37.3) | 15 (20.3) | |
| Stage model of dietary habit2 | ||||
| Maintenance | 6 (22.2) | 10 (16.9) | 12 (16.2) | 0.540 |
| Action | 2 (7.4) | 1 (1.7) | 2 (2.7) | |
| Preparation | 2 (7.4) | 13 (22) | 11 (14.9) | |
| Contemplation | 9 (33.3) | 7 (11.9) | 19 (25.7) | |
| Pre-contemplation | 8 (29.6) | 28 (47.5) | 30 (40.5) | |
| Eating speed (eating lunch within 10 min)2 | ||||
| Fast | 8 (29.6) | 22 (37.3) | 20 (27) | 0.451 |
| General or slow | 19 (70.4) | 37 (62.7) | 54 (73) | |
| Eating and drinking | ||||
| Prior to bedtime2 | ||||
| Yes | 6 (22.2) | 12 (20.3) | 16 (21.6) | 0.975 |
| No | 21 (77.8) | 47 (79.7) | 58 (78.4) | |
| Skipping a meal2 | ||||
| Yes | 4 (14.8) | 18 (30.5) | 14 (18.9) | 0.700 |
| No | 23 (85.2) | 41 (69.5) | 60 (81.1) | |
| Resting2 | ||||
| Enough | 9 (33.3) | 38 (64.4) | 49 (66.2) | 0.026 |
| Almost enough | 9 (33.3) | 4 (6.8) | 11 (14.9) | |
| A little shortage | 7 (25.9) | 14 (23.7) | 10 (13.5) | |
| Shortage | 2 (22.2) | 3 (5.1) | 4 (5.4) | |
| Sleeping time (h)1 | 6.7 ± 1.1b | 6.8 ± 1.5c | 7.6 ± 1.6 | |
| Perceived health status2 | < 0.001 | |||
| Very healthy | 2 (7.4) | 4 (6.8) | 3 (4.1) | |
| Almost healthy | 23 (85.2) | 32 (54.2) | 28 (37.8) | |
| A little unhealthy | 2 (7.4) | 18 (30.5) | 34 (45.9) | |
| Unhealthy | 0 (0) | 5 (8.5) | 9 (12.2) | |
| Stage model of exercise2 | 0.630 | |||
| Maintenance | 12 (44.4) | 23 (39) | 27 (36.5) | |
| Action | 0 (0) | 0 (0) | 0 (0) | |
| Preparation | 4 (14.8) | 5 (8.5) | 13 (17.6) | |
| Contemplation | 10 (37) | 23 (39) | 25 (33.8) | |
| Pre-contemplation | 1 (3.7) | 8 (13.6) | 9 (12.2) | |
| Smoking custom2 | 0.140 | |||
| Yes | 5 (18.5) | 13 (22) | 8 (10.8) | |
| No | 22 (81.5) | 46 (78) | 66 (89.2) | |
| Time to smoking cigarette first after it get out of bed2 | 0.555 | |||
| Within 5 min | 2 (40) | 1 (7.1) | 1 (14.3) | |
| From 6 to 30 min | 1 (20) | 1 (7.1) | 1 (14.3) | |
| From 31 to 60 min | 2 (40) | 4 (28.6) | 4 (57.1) | |
| More than 61 min | 0 (0) | 8 (57.1) | 1 (14.3) | |
| Number of smoking cigarettes on a day2 | 0.584 | |||
| Less than 10 cigarettes | 3 (60) | 3 (21.4) | 2 (28.6) | |
| From 10 to 20 cigarettes | 2 (40) | 7 (50) | 5 (71.4) | |
| From 21 to 30 cigarettes | 0 (0) | 3 (21.4) | 0 (0) | |
| More than 31cigarettes | 0 (0) | 1 (7.1) | 0 (0) | |
| Period of no smoking2 | 0.356 | |||
| More than 10 yr | 3 (60) | 11 (61.1) | 16 (72.7) | |
| From 3 to 10 yr | 0 (0) | 6 (33.3) | 4 (18.2) | |
| From 1 to 3 yr | 1 (20) | 0 (0) | 1 (4.5) | |
| From 6 mo to less than 1 yr | 1 (20) | 1 (5.6) | 1 (4.5) | |
| Stage model of smoking2 | 0.481 | |||
| Maintenance | 5 (50) | 19 (59.4) | 22 (71) | |
| Action | 0 (0) | 0 (0) | 0 (0) | |
| Preparation | 0 (0) | 0 (0) | 3 (9.7) | |
| Contemplation | 4 (40) | 7 (21.9) | 3 (9.7) | |
| Pre-contemplation | 1 (10) | 6 (18.8) | ||
Table 6 shows the results of the physical activity survey. The physical activity levels, number of steps, and Ex in the chronic hepatitis and cirrhosis groups were significantly lower than those of the control group. Moreover, TEE in cirrhotic patients was significantly lower than that in control subjects and chronic hepatitis patients.
Table 7 shows the results of univariate and multivariate logistic regression modeling, which were used to determine the relevant factors for insulin resistance. Unadjusted univariate logistic regression modeling suggested that 6 factors, including the presence or absence of hepatocellular carcinoma, AC, Ex, BTR, total energy intake per BW, and percentage fat, were significantly associated with insulin resistance. In the multivariate logistic regression modeling adjusted for sex, age, the presence or absence of hepatocellular carcinoma, AC, Ex, BTR, total energy intake per BW and percentage fat, Ex was a significant factor associated with insulin resistance.
| Control (n) | Case (n) | Univariate | Multivariate | |||
| Odds ratio (95% CI) | P value | Odds ratio1 (95% CI) | P value | |||
| Viral infection | ||||||
| HBV infection | 15 | 3 | 1 | 0.055 | ||
| HCV infection | 38 | 28 | 3.684 (0.972-13.960) | |||
| Groups | ||||||
| Chronic hepatitis | 25 | 14 | 1 | 0.859 | ||
| Liver cirrhosis | 28 | 17 | 1.084 (0.445-2.639) | |||
| The hepatocellular carcinoma | ||||||
| Absent | 44 | 19 | 1 | 0.030 | 1 | 0.290 |
| Present | 9 | 12 | 3.087 (1.115-8.544) | 0.311 (0.036-2.713) | ||
| Sex | ||||||
| Female | 35 | 18 | 1 | 0.466 | 1 | 0.700 |
| Male | 18 | 13 | 1.404 (0.564-3.496) | 0.712 (0.126-4.010) | ||
| Age (yr) | ||||||
| < 65 | 23 | 14 | 1 | 0.875 | 1 | 0.366 |
| ≥ 65 | 30 | 17 | 0.931 (0.382-2.271) | 0.486 (0.102-2.324) | ||
| %AC | ||||||
| ≥ 100 | 33 | 26 | 1 | 0.042 | 1 | 0.091 |
| < 100 | 20 | 5 | 0.317 (0.105-0.960) | 0.223 (0.039-1.271) | ||
| %TSF | ||||||
| ≥ 100% | 31 | 24 | 1 | 0.083 | ||
| < 100% | 22 | 7 | 0.411 (0.151-1.121) | |||
| %AMC | ||||||
| ≥ 100% | 35 | 22 | 1 | 0.641 | ||
| < 100% | 18 | 9 | 0.795 (0.304-2.081) | |||
| %AMA | ||||||
| ≥ 100% | 38 | 22 | 1 | 0.943 | ||
| < 100% | 15 | 9 | 1.036 (0.389-2.759) | |||
| Handgrip strength (%) | ||||||
| ≥ 69.0 | 22 | 17 | 1 | 0.360 | ||
| < 69.0 | 30 | 13 | 1.526 (0.617-3.771) | |||
| Ex (Ex/wk) | ||||||
| ≥ 4.0 | 29 | 7 | 1 | 0.003 | 1 | 0.024 |
| < 4.0 | 10 | 14 | 5.800 (1.823-18.455) | 6.809 (1.288-36.001) | ||
| Plt (× 102/μL) | ||||||
| ≥ 10.0 | 37 | 15 | 1 | 0.054 | ||
| < 10.0 | 16 | 16 | 2.466 (0.986-6.168) | |||
| Alb (g/dL) | ||||||
| ≥ 3.5 | 43 | 20 | 1 | 0.094 | ||
| < 3.5 | 10 | 11 | 2.365 (0.864-6.476) | |||
| BTR | ||||||
| ≥ 4.41 | 31 | 11 | 1 | 0.025 | 1 | 0.238 |
| < 4.41 | 17 | 18 | 2.984 (1.148-7.756) | 2.770 (0.509-15.061) | ||
| Energy intake per BW (kcal/BW) | ||||||
| ≤ 30 | 21 | 22 | 1 | 0.007 | 1 | 0.602 |
| > 30 | 32 | 9 | 0.268 (0.104-0.695) | 0.689 (0.171-2.785) | ||
| Protein intake per BW (g/BW) | ||||||
| ≤ 1.2 | 24 | 17 | 1 | 0.339 | ||
| > 1.2 | 29 | 14 | 0.682 (0.280-1.660) | |||
| Fat percentage (%) | ||||||
| ≤ 25 | 26 | 23 | 1 | 0.027 | 1 | 0.096 |
| > 25 | 27 | 8 | 0.335 (0.127-0.882) | 0.222 (0.038-1.307) | ||
In this study, we carried out laboratory evaluations, anthropometric assessments, a diet, lifestyle, and physical activity survey in chronic liver disease patients. The results were compared with the data of healthy subjects. As shown in Table 2, a considerable number of the chronic hepatitis patients presented insulin resistance without obvious elevation of FBS. Thirty-three point nine percent in the chronic hepatitis and 37.8% in cirrhotic patients indicated insulin resistance. Insulin resistance has been reported to be correlated with liver fibrosis[20,21], carcinogenesis[22] and mortality[23] of liver cancer in chronic liver disease patients. FBS as well as IRI should be measured and HOMA-IR should be calculated for an early diagnosis and for improving insulin resistance by diet and Ex therapy.
The rate of obesity in men and women was 30.5% and 20.8%, respectively, according to the national health and nutrition examination survey conducted by the Japanese Ministry of Health, Labour and Welfare in 2009. In this study, the incidence of obesity in men with liver cirrhosis was almost the same as that in the general adult male population. However, the rate of obesity in women was significantly lower as liver disease advanced. However, BMI was not significantly different among the 3 groups, and 72.0% of the women with liver cirrhosis had a normal BMI. These results suggested that almost all women with liver cirrhosis might have normal weight. Complications such as metabolic syndrome, obesity, and fatty liver in patients with chronic liver disease were risk factors of liver fibrillization[24] and carcinogenesis[25]. These complications are related to increasing mortality of carcinoma[11] and decreased response to antiviral therapy[26]. If the condition of patients with obesity or fatty liver could be improved by correcting their diet and lifestyle, these approaches might be effective to curb the progression of the disease, carcinogenesis, and to improve the efficacy of interferon therapy. However, malnutrition in cirrhotic patients is caused by the decreased protein synthesis and disturbed energy metabolism that result from liver failure. Protein malnutrition can cause a decrease in skeletal muscle mass in cirrhotic patients. In fact, AMC and AMA in patients with chronic hepatitis and liver cirrhosis were significantly lower than those in the control subjects, while AC was not significantly different among all groups. Moreover, TSF in the chronic hepatitis group was significantly higher than that in the control group. These results suggest the possibility of a decrease in skeletal muscle mass and a relative increase in fat mass in patients with chronic liver disease. However, anthropometry might underestimate the prevalence and severity of malnutrition in patients with cirrhosis. In particular, the underestimation was more pronounced in patients with Child-Pugh A and B cirrhosis, although anthropometry is a simple and non-invasive method[27]. More attention needs to be paid to the method of nutrition assessment. In the future, the body composition of cirrhotic patients might have to be evaluated again by using more accurate methods such as dual-energy X-ray absorptiometry.
In this study, the handgrip strength in the patient groups was significantly lower than that in the control group. Handgrip strength has been reported to be strongly correlated with muscle mass[28]. Decreased handgrip strength corresponded with lower %AMC and %AMA values. These results might reflect a decrease in muscle mass. Furthermore, the handgrip strength can be used to predict morbidity of complications in cirrhotic patients[29]. Therefore, it may be a valuable predictive factor of malnutrition and its accompanying complications. Although not specific to patients with liver disease, a Japanese study published in 2007 reported that the multivariate-adjusted reactive risk of all causes of death, excluding external causes, for each 5-kg increment of grip strength was significantly lower in middle-aged and elderly individuals[30]. Maintaining grip strength might benefit cirrhotic patients.
In the dietary survey, there were no significant differences in nutrition intake between the groups. Furthermore, patients did not show a deficiency in total energy and protein intake per BW, as per the guidelines of ESPEN Nutritional Management. This finding suggests that dietary intake was almost sufficient to fulfill the nutritional requirements. The results of the biochemical blood tests and anthropometric assessments showed that cirrhotic patients were malnourished, leading to reduced values of serum albumin, AMC, AMA and handgrip strength. Although protein intake was adequate in the cirrhosis group, the BTR was low. This result suggests that improving the amino acid imbalance by dietetic treatment is difficult. Therefore, oral BCAA supplementation in cirrhotic patients has been shown to be effective in improving BTR and maintaining serum albumin and their quality of life (QoL)[31]. In addition, oral BCAA supplementation could decrease the risk of specific hepatic carcinogenesis in cirrhotic patients[10].
In the lifestyle survey, chronic liver disease patients consumed significantly less alcohol and had a lower drinking frequency. These results suggested that the patients were aware of the adverse effect of alcohol drinking. Cirrhotic patients had a significantly longer sleeping time, and more patients reported that their resting time was sufficient than the other 2 groups. This shows that patients with cirrhosis are careful in maintaining rest. Perceived health status in the patient groups was significantly lower than that in the control group; progression of the liver disease might influence the QoL of the patients.
The result of the physical activity survey suggested that cirrhotic patients in urban areas had significantly lower TEE than the control and chronic hepatitis groups. In a study that determined REE by using an indirect calorimeter, the REE of cirrhotic patients was found to vary[32]. However, the REE in cirrhotic patients was significantly higher than that in the control and chronic hepatitis groups when measured using a direct calorimeter[33]. The Lifecorder of TEE was conjectured by body motion, but this measured value does not consider the increased REE in cirrhotic patients. This suggested that the TEE of cirrhotic patients in this study might be underestimated. However, a study that validated the Lifecorder reported that there was a strong correlation between physical activity levels and measured METs while walking[17]. At the very least, this result suggested that the level of physical activity of chronic hepatitis and cirrhosis group was low. As mentioned above, the perceived health status in cirrhotic patients was also low. We think that all physical activity might be suppressed by stressing about the need for rest in the usual life guidelines for cirrhosis. Some reports stated that maintenance of weight loss and Ex in overweight patients with liver disease resulted in a sustained improvement in liver function, insulin resistance and QoL[34,35]. Obese cirrhotic patients would rather try to increase the intensity of daily activities involving Ex than continue to maintain excessive resting. However, since patients with decompensated cirrhosis with ascites, hepatic encephalopathy, and jaundice need to lie quietly and consume sufficient amounts of nutrients, we should probably exclude them from the patients with chronic hepatitis and compensated cirrhosis enrolled in this study.
In this study, univariate and multivariate logistic regression modeling were used to identify the relevant factors for insulin resistance. The results show that decreased Ex was a significant factor for insulin resistance. For patients with chronic liver disease, increasing the amount of physical activity might improve insulin resistance. Insulin resistance tended to decrease at low values of %AC, which is an index of muscle and fat mass. In this study, the muscle mass in patients with chronic liver disease was lower than in the control group. While the amount of fat mass in the liver cirrhosis group was not significantly different from that of the control group, the amount of fat mass in the chronic hepatitis group was higher than that of the control group. In patients with chronic liver disease, obesity or muscle wasting might be related to insulin resistance. Insulin resistance tended to decrease at higher fat percentages. Insulin secretion might not be as easily stimulated when fat is ingested compared with when carbohydrates are ingested. This might be associated with the insulin resistance observed in chronic liver disease. However, this study has limitations. We have not completely verified a causal relationship between Ex and insulin resistance as this is a cross sectional study. It will thus be necessary to perform a future cohort study to verify whether insulin resistance in patients with cirrhosis is directly induced by decreased physical activity.
In summary, the results of this study suggested that chronic liver disease patients in urban areas have lower skeletal muscle mass and higher relative body fat percentage. We think that the lower skeletal muscle mass may result from the decline in BTR, which indicates an amino acid imbalance, and that the intensity of daily activities was lower because of excessive resting instead of a decrease in dietary intake. Moreover, this study showed that insulin resistance might exist in early-stage chronic liver disease patients. Ex was a relevant factor for insulin resistance, and decreased physical activity was associated with insulin resistance.
Therefore, non-hospitalized cirrhotic patients in urban areas need to maintain an adequate dietary intake and receive lifestyle guidance to increase their physical activity levels.
Cirrhosis is an end-stage chronic liver disease, and one of the typical diseases that is associated with malnutrition. However, the combination of over-nutrition and obesity in cirrhosis patients indicates the possibility of an impaired pathogenesis. These findings suggest that excessive food intake, decreased intensity of daily activity, and a lack of exercise (Ex) in cirrhotic patients promotes obesity, fatty liver, and hepatic carcinogenesis. Few researchers have studied the effect of dietary habits and lifestyle in non-hospitalized cirrhotic patients on their conditions.
Recently, both experimental and clinical studies found that over-nutrition, fatty liver, and glucose intolerance are significant risk factors of hepatic carcinogenesis. Moreover, insulin resistance has been reported to be correlated with liver fibrosis and mortality of liver cancer in chronic liver disease patients. Some reports have said that maintenance of weight loss and Ex in overweight patients with liver disease resulted in a sustained improvement in liver function, insulin resistance, and quality of life. To improve insulin resistance or the loss of excess weight, correction of malnutrition and the addition of moderate Ex are effective methods of therapy. However, it is not clear if Ex that is moderate in intensity or quantity will be effective therapy for cirrhotic patients. The purpose of this study is to assess the nourishment status of non-hospitalized patients with compensated cirrhosis.
The results of this study suggested that chronic liver disease patients in urban area have lower skeletal muscle mass and higher relative body fat percentage. Moreover, this study showed that insulin resistance might exist in early-stage chronic liver disease patients. Ex was a relevant factor for insulin resistance, and decreased physical activity was associated with insulin resistance.
The results of this study suggest that non-hospitalized cirrhotic patients in urban areas need to maintain an adequate dietary intake and receive lifestyle guidance to increase their physical activity levels. Decreased physical activity was associated with insulin resistance. Therefore, increasing physical activity may improve insulin resistance and suppress hepatocellular carcinogenesis.
Ex is a unit to express the quantity of physical activity, calculated by multiplying metabolic equivalents (METs) by the duration of the activity (h) as proposed in the Physical activity guide for Health Promotion 2006, which was published by the Ministry of Health, Labor and Welfare of Japan. METs is an index for the intensity of physical activity, calculated as energy expenditure (oxygen uptake, mL/kg per minute) during a specific physical activity divided by sitting/resting energy expenditure.
In this paper, the authors investigated the nourishment status and lifestyle of non-hospitalized patients with compensated cirrhosis by using non-invasive methods in Japan. They found that insulin resistance was seen in more than 35% of patients with chronic liver disease. In addition, the handgrip strength, physical activity levels, number of steps and Ex were significantly lower in the cirrhotic group than in the control group. The authors concluded that non-hospitalized cirrhotic patients may need to maintain an adequate dietary intake and receive lifestyle guidance in order to increase their physical activity levels. Overall, theseindings are interesting.
| 1. | Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362:2089-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 590] [Article Influence: 25.7] [Reference Citation Analysis (1)] |
| 2. | McCaughan GW, Omata M, Amarapurkar D, Bowden S, Chow WC, Chutaputti A, Dore G, Gane E, Guan R, Hamid SS. Asian Pacific Association for the Study of the Liver consensus statements on the diagnosis, management and treatment of hepatitis C virus infection. J Gastroenterol Hepatol. 2007;22:615-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Michitaka K, Nishiguchi S, Aoyagi Y, Hiasa Y, Tokumoto Y, Onji M. Etiology of liver cirrhosis in Japan: a nationwide survey. J Gastroenterol. 2010;45:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Kondrup J, Müller MJ. Energy and protein requirements of patients with chronic liver disease. J Hepatol. 1997;27:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 77] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Mendenhall CL, Anderson S, Weesner RE, Goldberg SJ, Crolic KA. Protein-calorie malnutrition associated with alcoholic hepatitis. Veterans Administration Cooperative Study Group on Alcoholic Hepatitis. Am J Med. 1984;76:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 262] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Guglielmi FW, Panella C, Buda A, Budillon G, Caregaro L, Clerici C, Conte D, Federico A, Gasbarrini G, Guglielmi A. Nutritional state and energy balance in cirrhotic patients with or without hypermetabolism. Multicentre prospective study by the 'Nutritional Problems in Gastroenterology' Section of the Italian Society of Gastroenterology (SIGE). Dig Liver Dis. 2005;37:681-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Lautz HU, Selberg O, Körber J, Bürger M, Müller MJ. Protein-calorie malnutrition in liver cirrhosis. Clin Investig. 1992;70:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 172] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Tajika M, Kato M, Mohri H, Miwa Y, Kato T, Ohnishi H, Moriwaki H. Prognostic value of energy metabolism in patients with viral liver cirrhosis. Nutrition. 2002;18:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 161] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol Res. 2006;35:204-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5430] [Cited by in RCA: 5355] [Article Influence: 232.8] [Reference Citation Analysis (2)] |
| 12. | Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med. 2006;166:1871-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 410] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 13. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 25011] [Article Influence: 610.0] [Reference Citation Analysis (2)] |
| 14. | WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7065] [Cited by in RCA: 8607] [Article Influence: 391.2] [Reference Citation Analysis (0)] |
| 15. | Japanese Society of Nutritional Assessment. Japanese Anthropometric Reference Data (JARD2001). Journal of Nutritional Assessment. 2002;19 (suppl): 1-81. |
| 16. | Plauth M, Merli M, Kondrup J, Weimann A, Ferenci P, Müller MJ. ESPEN guidelines for nutrition in liver disease and transplantation. Clin Nutr. 1997;16:43-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 235] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Kumahara H, Schutz Y, Ayabe M, Yoshioka M, Yoshitake Y, Shindo M, Ishii K, Tanaka H. The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: a validation study against whole-body indirect calorimetry. Br J Nutr. 2004;91:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 359] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 18. | Exercise and Physical Activity Guide for Health Promotion 2006 (Exercise Guide 2006). Available From: URL: http://www0.nih.go.jp/eiken/programs/pdf/exercise_guide.pdf. . |
| 19. | Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 432] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 20. | Fartoux L, Poujol-Robert A, Guéchot J, Wendum D, Poupon R, Serfaty L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut. 2005;54:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 278] [Article Influence: 13.2] [Reference Citation Analysis (3)] |
| 21. | Muzzi A, Leandro G, Rubbia-Brandt L, James R, Keiser O, Malinverni R, Dufour JF, Helbling B, Hadengue A, Gonvers JJ. Insulin resistance is associated with liver fibrosis in non-diabetic chronic hepatitis C patients. J Hepatol. 2005;42:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 22. | Nkontchou G, Bastard JP, Ziol M, Aout M, Cosson E, Ganne-Carrie N, Grando-Lemaire V, Roulot D, Capeau J, Trinchet JC. Insulin resistance, serum leptin, and adiponectin levels and outcomes of viral hepatitis C cirrhosis. J Hepatol. 2010;53:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Balkau B, Kahn HS, Courbon D, Eschwège E, Ducimetière P. Hyperinsulinemia predicts fatal liver cancer but is inversely associated with fatal cancer at some other sites: the Paris Prospective Study. Diabetes Care. 2001;24:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Younossi ZM, McCullough AJ, Ong JP, Barnes DS, Post A, Tavill A, Bringman D, Martin LM, Assmann J, Gramlich T. Obesity and non-alcoholic fatty liver disease in chronic hepatitis C. J Clin Gastroenterol. 2004;38:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Kurosaki M, Hosokawa T, Matsunaga K, Hirayama I, Tanaka T, Sato M, Yasui Y, Tamaki N, Ueda K, Tsuchiya K. Hepatic steatosis in chronic hepatitis C is a significant risk factor for developing hepatocellular carcinoma independent of age, sex, obesity, fibrosis stage and response to interferon therapy. Hepatol Res. 2010;40:870-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Hanouneh IA, Feldstein AE, Lopez R, Yerian L, Pillai A, Zein CO, Zein NN. Clinical significance of metabolic syndrome in the setting of chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2008;6:584-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Figueiredo FA, Perez RM, Freitas MM, Kondo M. Comparison of three methods of nutritional assessment in liver cirrhosis: subjective global assessment, traditional nutritional parameters, and body composition analysis. J Gastroenterol. 2006;41:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol. 1990;45:M82-M88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 377] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 29. | Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 324] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 30. | Sasaki H, Kasagi F, Yamada M, Fujita S. Grip strength predicts cause-specific mortality in middle-aged and elderly persons. Am J Med. 2007;120:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 362] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 31. | Nishiguchi S, Habu D. Effect of oral supplementation with branched-chain amino acid granules in the early stage of cirrhosis. Hepatol Res. 2004;30S:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Müller MJ, Lautz HU, Plogmann B, Bürger M, Körber J, Schmidt FW. Energy expenditure and substrate oxidation in patients with cirrhosis: the impact of cause, clinical staging and nutritional state. Hepatology. 1992;15:782-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 184] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Greco AV, Mingrone G, Benedetti G, Capristo E, Tataranni PA, Gasbarrini G. Daily energy and substrate metabolism in patients with cirrhosis. Hepatology. 1998;27:346-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, Powell EE. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 289] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 35. | Konishi I, Hiasa Y, Tokumoto Y, Abe M, Furukawa S, Toshimitsu K, Matsuura B, Onji M. Aerobic exercise improves insulin resistance and decreases body fat and serum levels of leptin in patients with hepatitis C virus. Hepatol Res. 2011;41:928-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Peer reviewers: Dr. Laura E Matarese, Department of Gastroenterology, Hepatology and Nutrition, East Carolina University, 600 Moye Blvd., Greenville, NC 27834, United States; Takumi Kawaguchi, MD, PhD, School of Medicine, Kurume University, 67 Asahi-machi, Kurume 830-0011, Japan
S- Editor Gou SX L- Editor Rutherford A E- Editor Xiong L