Published online Oct 28, 2012. doi: 10.3748/wjg.v18.i40.5753
Revised: June 18, 2012
Accepted: July 18, 2012
Published online: October 28, 2012
AIM: To determine the usefulness of arrival time parametric imaging (AtPI) using contrast-enhanced ultrasonography (CEUS) with Sonazoid in evaluating early response to sorafenib for hepatocellular carcinoma (HCC).
METHODS: Fourteen advanced HCC patients who received sorafenib 400/800 mg/d for at least 4 wk and were followed up by CEUS were enrolled in this study. CEUS was performed before treatment and 2 and 4 wk after treatment, and images of the target lesion in the arterial phase were recorded for each patient. The images were analyzed by AtPI. Color mapping (CM) images obtained by AtPI were compared before and after the treatment. In these CM images, the mean arrival time of the contrast agent in the region of interest from the starting point [mean time (MT)] was calculated. In each patient, differences between MT before and MT 2 and 4 wk after the treatment were compared with responses evaluated 4-8 wk after the treatment by dynamic computed tomography (CT), and statistical analysis was performed. Modified response evaluation criteria in solid tumors was used for the response evaluation.
RESULTS: In CM images both 2 and 4 wk after the treatment, delays in the arrival time of the contrast agent were noted in 8 of the 14 patients. In the other 6 patients, no color changes were observed in the tumor, or red and/or yellow increase, suggesting a decrease in blood flow velocity between images 2 and 4 wk after the treatment and those before the treatment. Dynamic CT could be performed 4-8 wk after the treatment in 13 of the 14 patients. Median differences in the MT were 1.13 s and 1.015 s, 2 and 4 wk after the treatment, respectively, in the 8 patients who showed stable disease (SD)/partial response (PR) on dynamic CT. Median differences in the MT were -0.39 s and -0.95 s, 2 and 4 wk after the treatment, respectively, in the 5 patients who showed progressive disease (PD). Differences in the median MT between SD/PR and PD groups were significant 2 and 4 wk after the treatment with P = 0.019 and P = 0.028, respectively.
CONCLUSION: AtPI by CEUS using Sonazoid is suggested to be useful for evaluating early responses to sorafenib.
- Citation: Shiozawa K, Watanabe M, Kikuchi Y, Kudo T, Maruyama K, Sumino Y. Evaluation of sorafenib for hepatocellular carcinoma by contrast-enhanced ultrasonography: A pilot study. World J Gastroenterol 2012; 18(40): 5753-5758
- URL: https://www.wjgnet.com/1007-9327/full/v18/i40/5753.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i40.5753
We previously evaluated the hemodynamics of hepatocellular carcinoma (HCC) and metastatic liver tumors based on the time intensity curve (TIC)[1] and arrival time parametric imaging (AtPI)[2,3] employing contrast-enhanced ultrasonography (CEUS) using Sonazoid (Daiichi Sankyo, Tokyo, Japan). As a result, CEUS using Sonazoid has been suggested to allow continuous observation of hemodynamics and is applicable to evaluate the hemodynamics of HCC and the effects of therapeutics on metastatic liver tumors[1,2]. Recently, molecular biological characteristics related to the progression and proliferation of HCC have been clarified, accelerating the development of various molecular-targeting agents[4-6]. Sorafenib[7-9] (Bayer, Leverkusen, Germany) is a multikinase inhibitor that targets multiple molecules, and was approved for use in the treatment of unresectable advanced HCC in Japan in May, 2009. Since it targets tumor growth (RAF-MEK-ERK) and angiogenesis (vascular endothelial growth factor receptor and platelet-derived growth factor receptor) signal transduction pathways, it is expected to be useful for the treatment of HCC[10-14].
The evaluation of chemotherapeutic agents that exert their cytotoxic effects against advanced HCC by inhibiting nucleic acid metabolism has been conducted mainly based upon the tumor volume-reducing effect, according to the response evaluation criteria in solid tumor (RECIST)[15]. The tumor volume-reducing effect of molecular-targeting agents such as sorafenib appears to be slow, although the survival period is considered to be prolonged, even with only a minor volume-reducing effect. Indeed, in the Sorafenib HCC Assessment Randomized Protocol trial in advanced HCC, while the response rate based upon RECIST was only 2%, the survival period in the sorafenib group was significantly longer than in the placebo group, demonstrating its clinical efficacy[10]. Therefore, evaluation of the antineoplastic effect of sorafenib, which exhibits an antitumor effect primarily by inhibiting angiogenesis, is difficult by conventional criteria, and the evaluation of hemodynamics is expected to become important in the assessment of its efficacy[16]. Recently, the modified RECIST (mRECIST)[17] and Choi criteria[18] have been recommended for the evaluation of therapeutic effects[19]. Also, in consideration of the serious complications of sorafenib reported to date[10], and moreover the fact that sorafenib is an expensive drug, early evaluation of its therapeutic effects is considered to be necessary to assess whether the treatment should be continued.
In this study, we performed image analysis by AtPI using CEUS with Sonazoid before and after the sorafenib administration and evaluated the usefulness of AtPI in evaluating early responses to sorafenib.
Of the 45 patients with advanced HCC in whom treatment with sorafenib was initiated at our hospital between June 2009 and October 2011, 14 who consented to the study and were orally treated with sorafenib for at least 4 wk, and who could be followed up by CEUS, were selected as subjects. All patients were males with a mean age of 70.4 years (62-82 years). Underlying liver disease was hepatitis C in 8, alcoholic hepatitis in 4, and others in 2. Child-Pugh liver function class was A in all subjects, median alpha-fetoprotein level before administration was 150.9 ng/mL (7.1-22 516 ng/mL), and median protein induced by vitamin K absence-II level was 1781 mAU/mL (12-259 000 mAU/mL). The initial dose of sorafenib was 800 mg/d for 5 patients and 400 mg/d for 9 patients.
CEUS was performed before and 2 and 4 wk after the sorafenib administration. One lesion or portal vein tumor thrombus (PVTT) that could be followed for a period was selected on employing ultrasonography in each patient to standardize evaluations, and CEUS was performed in the same cross-section and under the same conditions at all time points. The ultrasound equipment used in this examination was SSA-790A (Toshiba Medical Systems, Tokyo, Japan) with a convex probe (PVT-375BT, 3.75-MHz center frequency). The imaging mode used was wideband harmonic imaging (pulse subtraction) with transmission/reception frequencies of 1.8 and 3.5 MHz, respectively. The mechanical index for acoustic output was set to 0.2; the dynamic range was set to 60-65 dB. A single focus point was set at the deep site of the lesion, and a bolus intravenous injection of Sonazoid (0.5 mL) was administered via a left cubital venous line followed by 10 mL normal saline flush. After injection of Sonazoid, the patients were asked to hold their breaths. The arterial phase (0-40 s) was observed and video images were recorded and analyzed by an off-line procedure using AtPI.
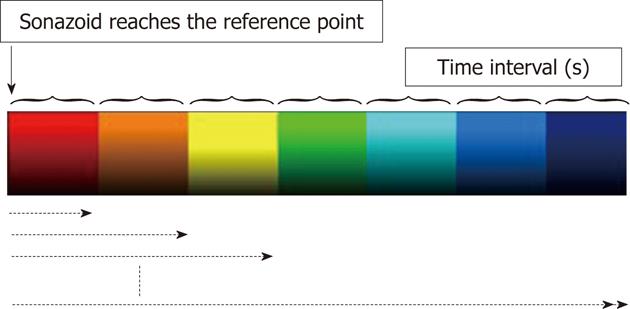
AtPI was performed using image analysis software for Aplio/Xario on the basis of the report by Watanabe et al[2]. It was performed by determining a starting point at an appropriate site such as an intrahepatic artery and a tumor vessel, regarding the time when the contrast agent reached this site as the zero point, measuring the difference in the arrival time between the target and starting points throughout the entire diagnostic image, and coloring the time differences [color mapping (CM)]. In this study, the moment of arrival of the contrast agent at a large artery near the tumor or PVTT was regarded as starting point.
Qualitative analysis: Delays in the arrival of the contrast agent at the target site compared with that at the reference point (0 s) were represented by red→orange→yellow→green→light blue→blue→dark blue at 0.5 s intervals (Figure 1). CM images obtained were grossly compared in each patient before and after the treatment.
Quantitative analysis: In prepared CM images, a maximum region of interest (ROI) was determined for each subject, and the mean arrival time of the contrast agent in the ROI from the starting point [mean time (MT)] was calculated. In each patient, differences in the MT 2 and 4 wk after the initiation of the treatment compared with the MT before treatment were determined. Blood flow velocity was judged to have been reduced when the difference was zero or greater [MT (+) group] and to have been increased when the difference was less than zero [MT (-) group]. Differences in the MT 2 and 4 wk after the treatment were compared with responses evaluated 4-8 wk after the treatment by dynamic computed tomography (CT), and statistical analysis was performed. mRECIST[7] was used for the response evaluation. The Mann-Whitney test was performed for statistical analyses at the P < 0.05 level of significance. This study was approved by the Ethical Review Board of Toho University Medical Center, Omori Hospital.
In 14 patients, the mean duration of sorafenib administration was 177 d, and the mean daily dose was 542.9 mg/d.
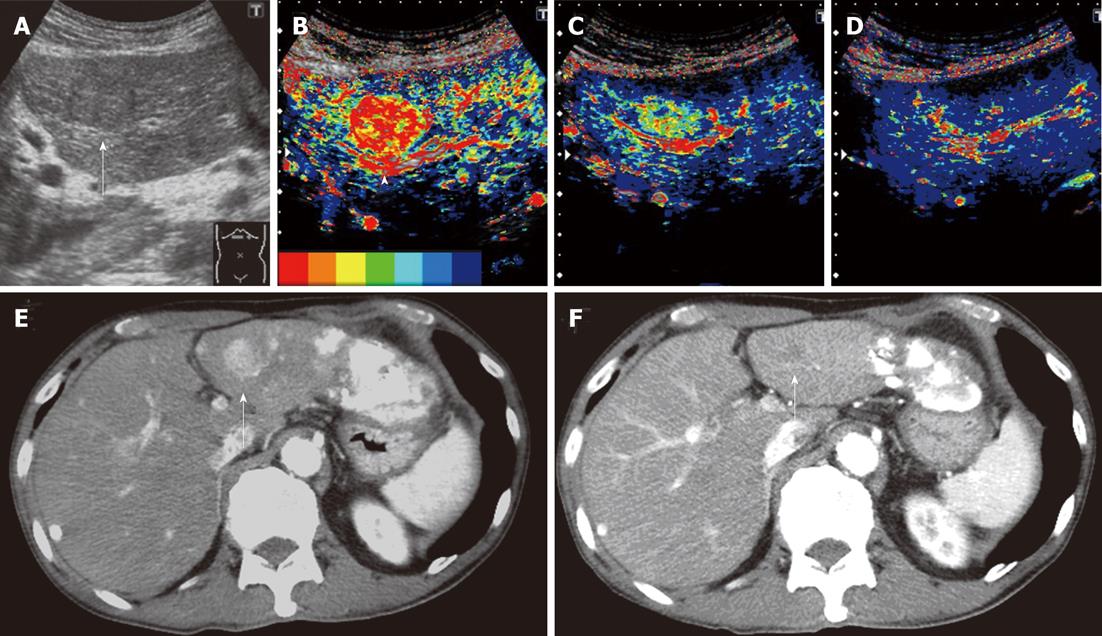
In CM images 2 wk after the treatment, color in the tumor changed to primarily blue or dark blue from primarily red or yellow before the treatment in 8 of the 14 patients, and time-dependent changes (delays in the arrival time of the contrast agent) were noted. These 8 patients also showed similar changes in CM images 4 wk after the treatment (Figure 2). In the other 6 patients, no color changes were observed in the tumor, or red and/or yellow increases, between images 2 wk after the treatment and those before the treatment. These 6 patients also showed similar CM images 4 wk after the treatment.
The MT was (+) in 8 patients but (-) in 6 two weeks after the treatment, and mean differences in MT were 1.21 ± 0.75 s and -0.85 ± 0.78 s, respectively. Four weeks after the treatment, the MT was (+) in 7 and (-) in 7, and the mean difference in MT was 1.18 ± 0.4 s and -1.11 ± 0.62 s, respectively. The MT was (+) but changed to (-) from 2 to 4 wk after the treatment in 1 of the 8 patients in whom CM images showed gross delays in the arrival time of the contrast agent in the tumor 2 and 4 wk after the treatment.
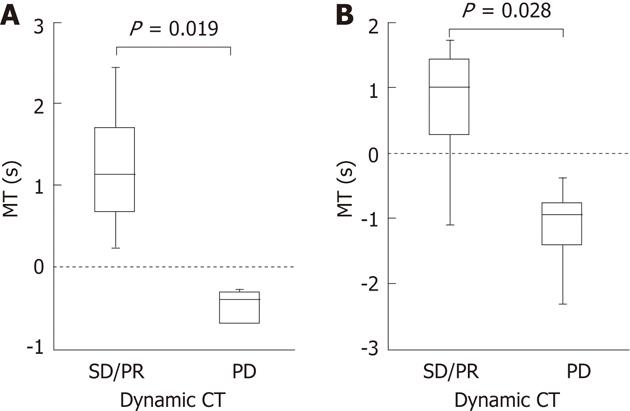
Dynamic CT could be performed 4-8 wk after the treatment in 13 of the 14 patients. Median differences in the MT were 1.13 s and 1.015 s, 2 and 4 wk after the treatment, respectively, in the 8 patients who showed stable disease (SD)/partial response (PR) on dynamic CT. Median differences in the MT were -0.39 s and -0.95 s, 2 and 4 wk after the treatment, respectively, in the 5 patients who showed progressive disease (PD). Differences in the median MT between SD/PR and PD groups were significant 2 and 4 wk after the treatment with P = 0.019 and P = 0.028, respectively (Figure 3). The finding on dynamic CT after treatment was SD in the above patient in whom the MT changed from (+) to (-) 4 wk after the treatment.
Some studies have evaluated the responses of gastrointestinal stromal tumor (GIST) and metastatic lesions of renal cell carcinoma to molecular-targeted agents by CEUS using Levovist (Schering, Berlin, Germany) and SonoVue (Bracco, Milan, Italy)[20-24]. Lassau et al[20] evaluated the responses of GIST to imatinib by CEUS using Levovist and SonoVue, and suggested that CEUS was useful for detailed evaluation early after the treatment, and that decreases in tumor staining early after the treatment were related to progression-free survival (PFS) and may serve as a response-predicting factor. Lamuraglia et al[21] also evaluated the efficacy of sorafenib against liver metastases of renal cell carcinoma by CEUS using SonoVue, and suggested that PFS or overall survival (OS) may be prolonged by the suppression of vascularization.
In this study, we evaluated the early responses of advanced HCC to sorafenib by AtPI using CEUS with Sonazoid. Differences in the arrival time of the contrast agent between a large artery near the target lesion and ROI were evaluated according to CM images or the MT. Evaluations of differences in the arrival time are considered equivalent to evaluations of blood flow velocity to the tumor or inflow volume of blood flow in the tumor.
In this study, a delay in the arrival time of the contrast agent, i.e., a decrease in blood flow velocity, could be detected visually and readily in CM images as early as 2 wk after the treatment in 8 of the 14 patients. A decrease in blood flow velocity could also be confirmed after 4 wk. However, as changes in blood flow in the tumor due to sorafenib administration were uneven, we set a ROI in the target HCC, calculated the mean arrival time of the contrast agent into the ROI, i.e., MT, and evaluated differences in the MT before and after the treatment as an objective method, not dependent on vision. In the 8 patients in whom delays in the arrival time of the contrast agent could be confirmed visually in CM images both 2 and 4 wk after the treatment, the MT was (+) in all 8 after 2 wk and in 7 after 4 wk. This suggests the possibility of quantification and objective evaluation of changes in blood flow velocity in the tumor visually detected in CM images. Comparisons between the MT and therapeutic effects according to mRECIST evaluated by dynamic CT showed significant differences in the MT between SD/PR and PD groups both 2 and 4 wk after the treatment, indicating general agreement between these parameters. The results of this study suggest that a delay in the arrival time of the contrast agent visually detected in CM images, i.e., a change of CM images, reflect the response and that changes in hemodynamics visually represented in CM images may be quantified and objectively evaluated using the MT.
Responses of HCC have been evaluated primarily using dynamic CT and magnetic resonance imaging as well as CEUS, but it has often been difficult to repeat the examination frequently during the course due to exposure, iodine allergies, and renal dysfunction. On the other hand, Sonazoid is effective at a low dose, causes few adverse reactions, and can be used safely even in patients with iodine allergies or renal dysfunction, with the exception of those with egg shell allergies. Since changes in CM images were noted in this study, and the MT showed differences from values before the treatment by 2 wk after the treatment, AtPI using CEUS with Sonazoid may be useful for the early evaluation of responses to sorafenib.
Lassau et al[22] reported that evaluation of the time to peak intensity and slope of the wash-in obtained by TIC using CEUS with SonoVue was useful for the predication of early responses of metastatic lesions of renal cell carcinoma to molecular-targeted agents. These techniques including AtPI reflect tumor hemodynamics, and their relative usefulness is impossible to discuss, but AtPI may be equally predictive of responses compared to the TIC.
This study raises the following questions: (1) Since tumors have a three-dimensional structure, can they be evaluated accurately by examination of a single cross-sectional ultrasonography image? (2) Since HCC shows multicentric carcinogenesis[25,26] and intrahepatic metastasis, the degree of differentiation may vary among lesions of multiple HCC. Therefore, is it possible to apply the findings in a single target lesion uniformly to all other lesions? (3) Visual evaluation of CM images obtained 4 wk after the treatment disagreed with the MT value in 1 patient. While CM images were found to be useful for simple comparison between conditions before and after the treatment, measurement of the MT is still a complex process, and further improvements are considered necessary in the method to determine the ROI and other aspects; and (4) Do the results obtained by this study, i.e., the MT calculated by AtPI, contribute to improvements in the OS? These problems are considered to need careful evaluation by further accumulation of cases.
In conclusion, AtPI by CEUS using Sonazoid is suggested to be useful for evaluating early responses to sorafenib. Changes in hemodynamics in advanced HCC over the course of its treatment could be visually represented in a single static CM image obtained by AtPI. By further quantification, hemodynamic changes over the course of treatment could be evaluated more objectively. Since the number of patients in this study was small, and the observation period was short, the usefulness of the procedure in evaluating the time to progression or OS cannot be discussed, but evaluation of tumor hemodynamics using AtPI is considered to contribute to the prediction of responses early after treatment.
Recently, molecular biological characteristics related to the progression and proliferation of hepatocellular carcinoma (HCC) have been clarified, accelerating the development of various molecular-targeted agents. Since sorafenib is a multikinase inhibitor and it targets tumor growth and angiogenesis signal transduction pathways, it is expected to be useful for the treatment of HCC.
Therapeutic efficacy has been assessed by Response Evaluation Criteria in Solid Tumor using dynamic computed tomography (CT) and overall survival in previous studies. Since sorafenib produces an antitumor effect primarily by inhibiting angiogenesis, evaluation of its antineoplastic effect is difficult by conventional criteria, and evaluating hemodynamics is expected to become important for its efficacy evaluation. In this study, the authors verified that arrival time parametric imaging (AtPI) by contrast-enhanced ultrasonography (CEUS) using Sonazoid is useful for evaluating early responses to sorafenib.
Dynamic CT and magnetic resonance imaging are generally used to evaluate the antineoplastic effect, but tumor hemodynamics are difficult to evaluate by these methods. AtPI by CEUS can evaluate the changes of hemodynamics in HCC over a course of treatment visually and objectively.
AtPI by CEUS using Sonazoid is suggested to be useful for evaluating early responses to sorafenib. This was a pilot study with a small series of patients. Similar studies with much larger numbers of patients are awaited.
Ultrasound contrast agents consist of microbubbles that visualize the hemodynamics. AtPI is a method using CEUS, which utilizes the arrival time of each pixel in the diagnostic image.
This is an interesting manuscript on a small series of patients followed with CEUS under treatment with sorafenib. As a pilot, it provides an interesting hypothesis to be tested in a larger series of patients. It is well conducted and also well presented.
| 1. | Shiozawa K, Watanabe M, Sumino Y. Evaluation of the hemodynamic status of focal hepatic lesions 20 mm or less in diameter by contrast-enhanced ultrasonography using Sonazoid. Intervirology. 2009;52:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Watanabe M, Shiozawa K, Takahashi M, Wakui N, Otsuka Y, Kaneko H, Tanikawa K, Shibuya K, Kamiyama N, Sumino Y. Parametric imaging using contrast-enhanced ultrasound with Sonazoid for hepatocellular carcinoma. J Med Ultrasonics. 2010;37:81-86. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Wakui N, Takayama R, Kanekawa T, Ichimori M, Otsuka T, Shinohara M, Ishii K, Kamiyama N, Sumino Y. Usefulness of arrival time parametric imaging in evaluating the degree of liver disease progression in chronic hepatitis C infection. J Ultrasound Med. 2012;31:373-382. [PubMed] |
| 4. | Yu J, Qiao L, Zimmermann L, Ebert MP, Zhang H, Lin W, Röcken C, Malfertheiner P, Farrell GC. Troglitazone inhibits tumor growth in hepatocellular carcinoma in vitro and in vivo. Hepatology. 2006;43:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Pang R, Poon RT. Angiogenesis and antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett. 2006;242:151-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Tommasi S, Pinto R, Pilato B, Paradiso A. Molecular pathways and related target therapies in liver carcinoma. Curr Pharm Des. 2007;13:3279-3287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099-7109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2966] [Cited by in RCA: 3190] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 8. | Chang YS, Adnane J, Trail PA, Levy J, Henderson A, Xue D, Bortolon E, Ichetovkin M, Chen C, McNabola A. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007;59:561-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 371] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 9. | Hotte SJ, Hirte HW. BAY 43-9006: early clinical data in patients with advanced solid malignancies. Curr Pharm Des. 2002;8:2249-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10525] [Article Influence: 584.7] [Reference Citation Analysis (9)] |
| 11. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4738] [Article Influence: 263.2] [Reference Citation Analysis (0)] |
| 12. | Kudo M, Ueshima K. Positioning of a molecular-targeted agent, sorafenib, in the treatment algorithm for hepatocellular carcinoma and implication of many complete remission cases in Japan. Oncology. 2010;78 Suppl 1:154-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Furuse J, Ishii H, Nakachi K, Suzuki E, Shimizu S, Nakajima K. Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci. 2008;99:159-165. [PubMed] |
| 14. | Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293-4300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 914] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 15. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 22783] [Article Influence: 1340.2] [Reference Citation Analysis (1)] |
| 16. | Kim MJ, Choi JI, Lee JS, Park JW. Computed tomography findings of sorafenib-treated hepatic tumors in patients with advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:1201-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3433] [Article Influence: 214.6] [Reference Citation Analysis (43)] |
| 18. | Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 1137] [Article Influence: 59.8] [Reference Citation Analysis (1)] |
| 19. | Edeline J, Boucher E, Rolland Y, Vauléon E, Pracht M, Perrin C, Le Roux C, Raoul JL. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012;118:147-156. [PubMed] |
| 20. | Lassau N, Lamuraglia M, Chami L, Leclère J, Bonvalot S, Terrier P, Roche A, Le Cesne A. Gastrointestinal stromal tumors treated with imatinib: monitoring response with contrast-enhanced sonography. AJR Am J Roentgenol. 2006;187:1267-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Lamuraglia M, Escudier B, Chami L, Schwartz B, Leclère J, Roche A, Lassau N. To predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib: pilot study using dynamic contrast-enhanced Doppler ultrasound. Eur J Cancer. 2006;42:2472-2479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Lassau N, Koscielny S, Albiges L, Chami L, Benatsou B, Chebil M, Roche A, Escudier BJ. Metastatic renal cell carcinoma treated with sunitinib: early evaluation of treatment response using dynamic contrast-enhanced ultrasonography. Clin Cancer Res. 2010;16:1216-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 23. | Williams R, Hudson JM, Lloyd BA, Sureshkumar AR, Lueck G, Milot L, Atri M, Bjarnason GA, Burns PN. Dynamic microbubble contrast-enhanced US to measure tumor response to targeted therapy: a proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapy. Radiology. 2011;260:581-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Lassau N, Chami L, Koscielny S, Chebil M, Massard C, Benatsou B, Bidault S, Cioffi A, Blay JY, Le Cesne A. Quantitative functional imaging by dynamic contrast enhanced ultrasonography (DCE-US) in GIST patients treated with masatinib. Invest New Drugs. 2012;30:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Matsui O, Kadoya M, Kameyama T, Yoshikawa J, Takashima T, Nakanuma Y, Unoura M, Kobayashi K, Izumi R, Ida M. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology. 1991;178:493-497. [PubMed] |
| 26. | Sakamoto M, Hirohashi S, Shimosato Y. Early stages of multistep hepatocarcinogenesis: adenomatous hyperplasia and early hepatocellular carcinoma. Hum Pathol. 1991;22:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 369] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
Peer reviewers: Herwig R Cerwenka, Professor, Department of Surgery, Medical University of Graz, Auenbruggerplatz 29, Graz A-8036, Austria; Bijan Eghtesad, Associate Professor, Department of General Surgery, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195, United States; Markus Peck-Radosavljevic, Professor, Department of Gastroenterologie and Hepatologie, Medizinische Universität Wien, Vienna A-1090, Austria
S- Editor Wu X L- Editor Logan S E- Editor Xiong L