Published online Jan 28, 2012. doi: 10.3748/wjg.v18.i4.375
Revised: August 22, 2011
Accepted: October 28, 2011
Published online: January 28, 2012
AIM: To explore the DNA image cytometry (DNA-ICM) technique as a primary screening method for esophageal squamous precancerous lesions.
METHODS: This study was designed as a population-based screening study. A total of 582 local residents aged 40 years-69 years were recruited from Linzhou in Henan and Feicheng in Shandong. However, only 452 subjects had results of liquid-based cytology, DNA-ICM and pathology. The sensitivity and specificity of DNA-ICM were calculated and compared with liquid-based cytology in moderate dysplasia or worse.
RESULTS: Sensitivities of DNA-ICM ranging from at least 1 to 4 aneuploid cells were 90.91%, 86.36%, 79.55% and 77.27%, respectively, which were better than that of liquid-based cytology (75%). Specificities of DNA-ICM were 70.83%, 84.07%, 92.65% and 96.81%, but the specificity of liquid-based cytology was 91.91%. The sensitivity and specificity of a combination of liquid-based cytology and DNA-ICM were 84.09% and 85.78%, respectively.
CONCLUSION: It is possible to use DNA-ICM technique as a primary screening method for esophageal squamous precancerous lesions.
- Citation: Zhao L, Wei WQ, Zhao DL, Hao CQ, Lin DM, Pan QJ, Li XQ, Lei FH, Wang JW, Wang GQ, Shang Q, Qiao YL. Population-based study of DNA image cytometry as screening method for esophageal cancer. World J Gastroenterol 2012; 18(4): 375-382
- URL: https://www.wjgnet.com/1007-9327/full/v18/i4/375.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i4.375
Esophageal cancer, the world’s eighth most common cancer, is curable if detected early. In China, the survival rates are over 85% when found at an early stage of esophageal squamous cell carcinoma (ESCC), but less than 10% when diagnosed at the advanced stage[1]. Patients in early stages are asymptomatic, and therefore, screening is important for detecting patients before they progress to advanced stage disease.
Presently, several screening methods have been explored in high-risk areas of China including conventional cytology[2-5], liquid-based cytology[6], occult blood detection[7] and endoscopic biopsy examination with Lugol’s iodine staining[8]. Conventional cytology had been used in high-risk areas of China in the 1970s, but it has been replaced by liquid-based cytology. Occult blood detection has been explored in several areas in China, but because of low sensitivity and specificity, this method has not been used widely yet. Endoscopic examination with Lugol’s iodine staining is mostly used in high-risk areas in China now. Although endoscopic examination with Lugol iodine staining has high sensitivity and specificity of more than 90%[8], because of the high cost, this screening method is difficult to be accepted by residents in rural high-risk areas. Meanwhile, endoscopic and pathological doctors require a lengthy qualification process. Liquid-based cytology may improve the quality of the cytological procedure and provide satisfactory diagnostic slides compared with conventional cytology, but it has limits for improving the sensitivity and specificity of screening[6,9]. Furthermore, there is a skill shortage in rural high-risk areas due to the lack of experienced cytologists.
DNA image cytometry (DNA-ICM) has been proposed as a simple, easy, sensitive and high specificity method for screening of ESCC. Changes in DNA ploidy occurring in human tumors have been shown to be a global reflection of the chromosomal and subchromosomal genetic changes which are important in tumor development and progression[10]. DNA ploidy is analyzed by DNA-ICM as a screening marker to reveal the development of ESCC. Until now, few studies have investigated the relationship between DNA ploidy and ESCC diagnosis application[11-13]. In these previous studies, researchers obtained specimens from surgical tissue, paraffin-embedded tissue and endoscopic biopsy. They have analyzed the prognostic value of DNA ploidy in the progression of ESCC, and predicted the prognosis of ESCC. Furthermore, these studies have not been performed by a population-based method, but were small sample clinical studies. DNA-ICM has been used as a screening method in cervical cancer[14], and was shown to have a higher sensitivity than that of cytology. However, no population-based screening study has focused on DNA-ICM for assessment of esophageal squamous carcinoma and precancerous lesions.
In order to evaluate sensitivity and specificity of ESCC screening by DNA-ICM, we conducted a population-based study in two high-risk areas of China.
This was a population-based screening study. In two areas at high risk of ESCC, namely Linzhou in Henan province and Feicheng in Shandong province, local trained village doctors visited every family in June 2010, gathered local residents to the village hospital, and then explained this study to them. Finally, 70% of the local residents consented to participate in our study. Inclusion criteria were: (1) local resident; (2) age 40 to 69 years; (3) no contraindications for endoscopic examinations (e.g., history of reaction to iodine or lidocaine, serious cardiovascular disease, poor health status); and (4) voluntarily consenting to participate in screening and signing the informed consent document. Exclusion criteria were: participants who had a history of liver cirrhosis, esophageal varices, hematemesis, a bleeding disorder, uncontrolled congestive heart failure, unstable angina or a reaction to topical anesthetics or iodine.
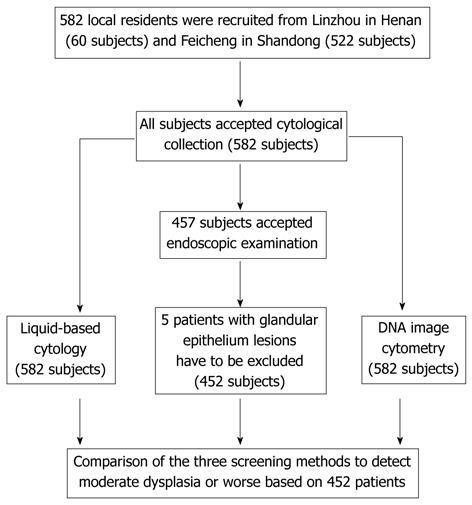
The details of subject recruitment and exclusion, those with cytology and endoscopy examinations, and the study procedure are described in Figure 1.
This study was approved by the Institutional Review Boards of the Cancer Institute/Hospital of the Chinese Academy of Medical Sciences (CICAMS) and Peking Union Medical College.
Preparation for balloon examination: All cytological examinations were performed in the villages. After completing informed consent and a short questionnaire, patients fasted overnight prior to examination. Before balloon examination, an inflatable balloon was soaked in 75% alcohol for at least 12 h.
Collection of cytological samples[6]: Balloon examination was performed by an experienced local doctor. After cleansing the oral cavity, the balloon was inserted into the back of the throat and swallowed by the patient. Once in the stomach, the balloon and covering mesh were expanded and then gradually pulled up the esophagus. When the balloon reached the upper esophageal sphincter, it was deflated and withdrawn completely. After removal, the balloon was placed in a 50 mL centrifuge tube including Thinprep digestive fluid, the catheter was cut off, and the centrifuge tube was sealed and transferred to a CICAMS laboratory.
Reparation of cytological slides: Each sample was vortexed for 10 min to remove adherent cells from the balloon, which was then taken out of the tube. The remaining cell suspension was centrifuged at 2500 r/min for 5 min. Excess supernatant was discarded and the cell deposits remained in the tube. All cell deposits were then transferred to Eppendorf tubes, which included 20 mL of Thinprep preserving cyto-solution. Every sample in the eppendorf tube was prepared for two cytological slides. Every subject had two slides, which were divided randomly for a Pap smear or Feulgen staining. We adopted a crossover design to eliminate the impact of numbers of cells on the diagnosis.
The slides were reviewed by two cytopathologists independently, and discrepancies were adjudicated by joint review. The slides were read blindly without the pathological results. Diagnostic criteria were adapted from criteria of the original Bethesda System[15].
The specimens were stained by the Feulgen-Thionin method as detailed in one previous study[16]. The hardware of the system included a MOTIC BA600 microscope, DELL370 workstation, automated microscope control-box, Moticam 1501 camera and assistant accessories. The software for the DNA-ICM system came from Canada[17].
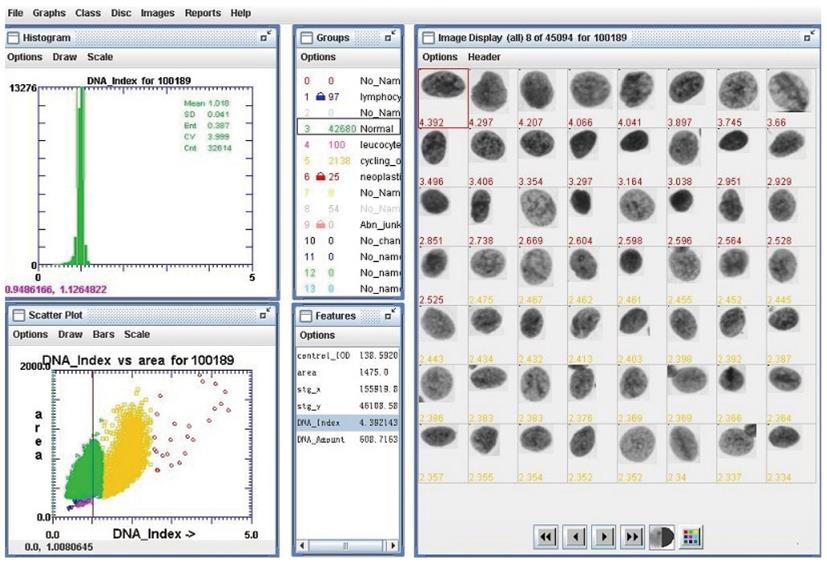
This system automatically loaded each slide, scanned the areas of Thinprep deposition, stored an image of every object detected, calculated a set of 132 features for each cell nucleus, ordered the DNA content of the nucleus, and used multilevel decision-making to classify each object as either nucleus or ‘junk’. Finally, two cytopathologists checked blindly the image of every nucleus repeatedly, then excluded an unsatisfactory nucleus. Figure 2 shows the interface of DNA-ICM which is used to assess the image of the cell nucleus with its corresponding features.
At least 400 normal epithelial cells were taken as the internal reference diploid population in each specimen. The coefficient of variation (CV) of the DNA quantity of these reference cells never exceeded 3%. This value was lower than the CV of 5% recommended by the European Society for Analytical Cellular Pathology (ESAPC)[18,19]. The DNA content of every nucleus was measured by integrated optical density (IOD).
The resulting DNA ploidy value is expressed as a “c”value for the chromosome. A DNA ploidy value of 2c indicates a normal diploid cell, 4c is a tetraploid cell, 5c is a cutoff used for aneuploidy by most authors[18].
The quality control process was implemented and depended on the reports of ESAPC[18,19].
DNA index = DNA IOD value of detected cell/Average DNA (G0/G1) IOD among reference cells nuclei
Endoscopic screening was completed by local doctors after training by and under the supervision of experienced doctors from the CICAMS. The technical processes of endoscopic screening were as follows:
Pre-endoscopy: Firstly, subjects signed the informed consent, then were anesthetized with 5 mL 1% lidocaine via the mouth.
Endoscopy: Subjects were placed in the left lateral position. The entire esophagus and stomach were visually examined including careful examination of the cardiac mucosa spinal roots.
Iodine staining: During the endoscopic procedure, Lugol’s iodine (1.2%) solution was used to stain the normal glycogen-containing tissue, which left the suspicious lesions unstained. Unstained foci were targeted and multiple biopsies were taken. Cleaning and disinfection of endoscopes was carried out using 2% alkaline glutaraldehyde solution.
Pathological diagnoses: Biopsy specimens were fixed in 10% buffered formalin, embedded in paraffin, cut in 5 μm sections, and stained with hematoxylin and eosin. The biopsy slides were read blindly by two experienced pathologists (WJW, LFH) without knowledge of the visual endoscopic results. Subjects with mild dysplasia needed to be followed up, but with moderate dysplasia or worse would be offered argon plasma coagulation and/or endoscopic mucosal resection, or surgery, depending on the grade of the lesion. Therefore, diagnosis of moderate dysplasia or worse has clinical implications.
The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of DNA-ICM were calculated by SAS 9.2 software. The results of DNA-ICM ranging from 1 to 15 aneuploid cells were based on different esophageal precancerous lesions (mild dysplasia, moderate dysplasia, severe dysplasia and squamous cell carcinoma).
The study consisted of 452 subjects, including 171 men and 281 women. The mean age of the participants was 55 years (range, 40-69 years). Overall, 60 subjects (13.3%) came from Linzhou in Henan province and 392 subjects (86.7%) from Feicheng in Shandong province. The analytic database was limited to 452 subjects who had results of liquid-based cytology, histopathology and DNA-ICM.
There were 336 of 452 (74.3%) subjects with “negative” histology, 10 (2.2%) had severe dysplasia, 29 (6.4%) had moderate dysplasia, 72 (15.9%) had mild dysplasia and 5 (1.1%) were diagnosed with ESCC. For diagnoses with liquid-based cytology, 386 subjects were normal (85.4%) and 66 (14.6%) had cytological abnormalities, of which 39 had atypical squamous cells of undetermined significance (ASCUS), 19 had low-grade squamous intraepithelial lesions (LSIL), 3 had high grade squamous intraepithelial lesion (HSIL) and 5 cases had ESCC. For DNA-ICM, 293 of 452 (64.8%) subjects had no aneuploid cells, and 159 (35.2%) subjects had aneuploid cells.
Table 1 shows diploid cells and aneuploidy classified by pathological diagnoses. The results showed that 27.4% subjects with normal esophageal epithelia presented with aneuploid cells, but the proportions of aneuploid cells for subjects with mild dysplasia, moderate dysplasia, severe dysplasia, and ESCC were 37.5%, 86.2%, 100% and 100%, respectively. In particular, among subjects with severe dysplasia or ESCC, the proportion of diploid cells was zero. Furthermore, the larger the proportion of aneuploid cells, the worse the lesions.
| DNA ploidy | Pathological diagnoses | ||||
| Normal | mD | MD | SD | ESCC | |
| Diploid | 244 (72.6) | 45 (62.5) | 4 (13.8) | 0 (0) | 0 (0) |
| Aneuploid | 92 (27.4) | 27 (37.5) | 25 (86.2) | 10 (100) | 5 (100) |
| No. of aneuploid cells ≤ 5 | 90 (26.8) | 25 (34.7) | 7 (24.1) | 1 (10) | 0 (0) |
| No. of aneuploid cells ≤ 10 | 2 (0.6) | 2 (2.8) | 2 (6.9) | 2 (20) | 0 (0) |
| No. of aneuploid cells ≤ 20 | 0 (0) | 0 (0) | 8 (27.6) | 5 (50) | 2 (40) |
| No. of aneuploid cells > 20 | 0 (0) | 0 (0) | 8 (27.6) | 2 (20) | 3 (60) |
| Total | 336 | 72 | 29 | 10 | 5 |
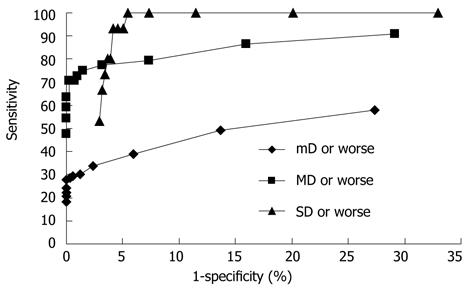
Figure 3 shows receiver operating characteristics curves of the DNA-ICM method based on mild dysplasia or worse, moderate dysplasia or worse and severe dysplasia or worse. It also shows the sensitivity and specificity of DNA ploidy by category, which is the minimum number of cells required to define a subject as aneuploid. It is difficult to determine the exact threshold value, so optimal limits of the threshold for DNA-ICM method were determined. According to Figure 3, the optimal values for sensitivity and specificity fell around a minimum number of aneuploid cells ranging from at least 1 to 5.
Table 2 shows the sensitivity, specificity, PPV and NPV of liquid-based cytology and DNA-ICM in our laboratory using these samples as determined by two blinded reviewers. ASCUS, LSIL and HSIL thresholds for cytology, and 1-5 aneuploid cells present at a DNA index of 2.5 for ploidy. For the threshold of moderate dysplasia or worse, sensitivities of the DNA-ICM method ranging from at least 1 aneuploid cell to 4 aneuploid cells were 90.91%, 86.36%, 79.55% and 77.27%, respectively. The specificities were 70.83%, 84.07%, 92.65% and 96.81%, respectively, but the sensitivity and specificity of liquid-based cytology for ASCUS or worse was 75% and 91.91%, respectively.
| Threshold for positivity | Total | Se(%) | Sp(%) | PPV (%) | NPV (%) | |
| Cytology | ASCUS+ | 452 | 75.00 | 91.91 | 50.00 | 97.15 |
| LSIL+ | 452 | 52.27 | 99.02 | 85.19 | 95.06 | |
| HSIL+ | 452 | 18.18 | 100.00 | 100.00 | 91.89 | |
| DNA ploidy | At least 1 5cER cell | 452 | 90.91 | 70.83 | 25.16 | 98.63 |
| At least 2 5cER cell | 452 | 86.36 | 84.07 | 36.89 | 98.28 | |
| At least 3 5cER cell | 452 | 79.55 | 92.65 | 53.85 | 97.67 | |
| At least 4 5cER cell | 452 | 77.27 | 96.81 | 72.34 | 97.53 | |
| At least 5 5cER cell | 452 | 75.00 | 98.53 | 84.62 | 97.34 |
Table 3 shows the sensitivity and specificity of a combination of liquid-based cytology and DNA-ICM. Liquid-based cytology took ASCUS as the threshold value. The threshold value of DNA-ICM ranged from at least 1 aneuploid cell to at least 5 aneuploid cells. The combination improved sensitivity and the ability to screen for positive cases. The group “≥ ASCUS or ≥ 3 aneuploid cells” delivered optimal sensitivity and specificity, 84.09% and 85.78%, respectively.
| Cytology or DNA ploidy | Se (%) | Sp (%) |
| ≥ ASCUS or ≥ 1 aneuploid cells | 93.18 | 67.65 |
| ≥ ASCUS or ≥ 2 aneuploid cells | 88.64 | 79.41 |
| ≥ ASCUS or ≥ 3 aneuploid cells | 84.09 | 85.78 |
| ≥ ASCUS or ≥ 4 aneuploid cells | 81.82 | 88.97 |
| ≥ ASCUS or ≥ 5 aneuploid cells | 81.82 | 90.69 |
This is the first population-based study to explore DNA image analysis technology as a screening method in ESCC. Our study indicated that the higher the grade of esophageal precancerous lesions the higher the proportion of aneuploid cells. This result is similar to the conclusion that early malignant changes in the esophagus are associated with alterations in DNA content in the clinical study by Blant et al[13] in Switzerland. Therefore, DNA image analysis may have potential clinical application as a screening method for ESCC.
Our study also showed that the sensitivities of the DNA-ICM method ranging from at least 1 aneuploid cell to 4 aneuploid cells were 90.91%, 86.36%, 79.55% and 77.27%, respectively, in identifying moderate dysplasia or worse disease. These sensitivities were much higher than that of liquid-based cytological testing (75%) using moderate dysplasia or worse as the threshold.
One study on cervical cancer showed that the sensitivity of DNA cytometry testing for cervical cancer was 91.7%, whereas the sensitivity of cytological testing was 44.5%, and the specificity was 54.1% for DNA cytometry testing and 70.6% for cytological testing[14]. Singh et al[20] proposed the idea of combination screening using the DNA-ICM method, and in cervical cancer, human papillomavirus screening with cytology would be an optimal method to detect progressive lesions with the greatest possible sensitivity and specificity. In our study, the aim of combining liquid-based cytology and DNA-ICM was to explore whether the combination could improve the sensitivity in screening for ESCC and precancerous lesions. The results indicated that the group “≥ ASCUS or ≥ 3 aneuploid cells” had the optimal sensitivity (84.09%) and specificity (85.78%). This improved the sensitivity of liquid-based cytological testing (75%) based on moderate dysplasia or worse. However, the specificities of combined cytology and DNA-ICM were lower than for the individual methodologies. If both the results of cytology and DNA-ICM are negative, the specificities of the combination were negative. This led to the low specificities. All in all, a combination of the two methods was only an indication for screening, and was not suitable for screening ESCC and precancerous lesions.
It is important to note one parameter of DNA-ICM in this study: the DNA index. In this study, we took the 5c value as the threshold value of the aneuploid cell. The ESAPC suggest that 5c is the cut-off value for aneuploidy[18]. A few studies suggested the threshold value for aneuploidy in other cancers was 5c, based on clinical samples[21-23]. DNA-ICM, used in cervical cancer screening, also set 5c as the cut-off value since results had showed that optimal values for sensitivity and specificity fell around a DNA ploidy of 5c[17]. However, this value has not been verified in esophageal cancer screening. It may be a limitation of our study, and future studies should confirm the cut-off value of DNA-ICM in esophageal cancer.
Our study is also the first study using cytological samples for DNA-ICM with Feulgen staining by inflatable balloon. Previous studies[24,25] on esophageal cancer and DNA-ICM collected biopsy specimens and then analyzed the DNA content of the nucleus. The sample collection procedure in our study was simple, and easy to perform as a screening method. Most ESCC cases occur in rural areas in China where residents have little knowledge of the capacity of medical diagnosis, and there is little heath education and inadequate treatment. These areas also lack experienced cytologists, endoscopists, and pathologists, as medical training is arduous and long. However, for DNA-ICM, a technician could work independently after 3 wk training, and 90% of slides could be diagnosed in 1 min, so this method would be easier to perform. Cytological diagnosis depends largely on the numbers of cytologists, but DNA-ICM is more objective as it is read by computer[26]. Our aim was to explore DNA-ICM as a primary screening method, which is certain to miss some positive cases compared with the “golden standard” of endoscopic examination. Nevertheless, we should not ignore the advantages of DNA-ICM: it is simple, objective, and likely to be accepted by participants. According to the data analysis, of 116 subjects with mild dysplasia or worse, 49 results of DNA-ICM were negative (false negative rate 42.2%). However, all of the 49 cases (100%) had mild or moderate dysplasia. This means that no advanced stage esophageal lesions had been missed. We also have relevant data about the efficiency of cell collection by inflatable balloon. is the so-called adequacy codes are classified into three grades: adequate for evaluation; satisfactory for evaluation but limited; and unsatisfactory. The number of subjects with adequate for evaluation cytological samples was 288, which is 63.7% of all subjects; with satisfactory for evaluation but limited samples was 163 (36.1%), and the sample of one subject was unsatisfactory (0.2%). Thus, we could say that the efficiency of cell collection by inflatable balloon was good for diagnosis by DNA-ICM.
DNA-ICM could also be used to predict the progress of lesions, as ESCC patients with diploid cells lived longer than ones with polyploidy[12]. One study in 2001 showed that the progression rate from oral dysplasia to invasive or carcinoma, based on the detection of DNA aneuploidy was only 10% after 1 year but increased significantly, to 90%, after 5 years[27]. DNA-ICM has been used as a diagnostic tool and aneuploidy tended to correlate with progression to invasive SCC[13]. Esophageal squamous cell carcinogenesis is thought to be a multi-step process, influenced by multiple factors. Changes in DNA ploidy occurring in human tumors are shown to be a global reflection of the chromosomal and subchromosomal genetic changes which play a key role in tumor development and progression[10]. It had been indicated that the aneuploid cell is important in the early stage of carcinogenesis[28,29]. Therefore, we believe that aneuploid cells as a biomarker can be detected by DNA-ICM, which not only may be used for screening precancerous lesions of ESCC, but also for clinical diagnoses, prognostic evaluation and, in particular, patient follow-up and management after screening.
In summary, this is the first population-based study to focus on the relationship between the alteration in DNA content and ESCC and its precancerous lesions. We found that higher grade esophageal precancerous lesions had a higher proportion of aneuploid cells. Confirmed by pathological diagnoses from endoscopic biopsy, DNA-ICM had higher sensitivity, based on moderate dysplasia or worse as threshold, in ESCC screening than liquid-based cytology. Thus, it is possible to use DNA-ICM as the primary screening method in ESCC in high-risk areas of China. Further larger sample studies are under consideration.
We thank the staff of Feicheng People’s Hospital, Feicheng, Shandong Province and Linzhou Cancer Hospital, Linzhou, Henan Province, China, for their cooperation with esophageal specimen and data collection.
Several screening methods have limitation in screening esophageal squamous cell carcinoma (ESCC) and precancerous lesions. Thus, it is necessary to explore a simple, objective, sensitive method as a primary screening method.
In several previous studies, researchers obtained specimens from surgical tissue and endoscopic biopsy. They analyzed the prognostic value of DNA ploidy in the progression of ESCC and predicted the prognosis of ESCC. These studies were not population-based studies, but small sample clinical studies. DNA image cytometry (DNA-ICM) has been used as a screening method in cervical cancer, which showed that the sensitivity of DNA-ICM was higher than that of cytology.
This is the first population-based study to explore DNA image analysis technology as a screening method in ESCC. Our study is also the first study using cytological sampling for DNA-ICM with Feulgen staining by inflatable balloon. According to our results, the specificities of DNA-ICM were higher than those of liquid-based cytology based for moderate dysplasia or worse.
It is possible to use DNA-ICM as a primary method in ESCC and precancerous lesions according to our results. Further larger sample studies are under consideration to verify our results.
The diploid cell is normal according to diagnosis by DNA imaging, which means it has a normal DNA content. The aneuploid cell has abnormal DNA content.
The authors address an important issue considering the shortage of experienced cytologists, pathologists and endoscopic doctors in rural high-risk areas.
Peer reviewers: Hiroyuki Uehara, MD, PhD, Chief, Division of Pancreatology, Department of Gastroenterology, Osaka Medical Center for Cancer and Cardiovascular Diseases, 1-3-3 Nakamichi, Higashinari, Osaka 537-8511, Japan; Joong-Won Park, MD, PhD, Center for Liver Cancer, National Cancer Center, 111 Jungbalsan-ro, Ilsan Dong-gu, Goyang, Gyeonggi 410-769, South Korea; Kazuaki Takabe, MD, PhD, Assistant Professor of Surgery and Assistant Professor of Biochemistry and Molecular Biology, Surgical Oncology, VCU Massey Cancer Center, Virginia Commonwealth University/Medical College of Virginia, PO Box 980011, Richmond VA 23298-0011, United States
S- Editor Tian L L- Editor Cant MR E- Editor Zhang DN
| 1. | Wang GQ, Jiao GG, Chang FB, Fang WH, Song JX, Lu N, Lin DM, Xie YQ, Yang L. Long-term results of operation for 420 patients with early squamous cell esophageal carcinoma discovered by screening. Ann Thorac Surg. 2004;77:1740-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 149] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 2. | Shu YJ. Cytopathology of the esophagus. An overview of esophageal cytopathology in China. Acta Cytol. 1983;27:7-16. [PubMed] |
| 3. | Shen O, Liu SF, Dawsey SM, Cao J, Zhou B, Wang DY, Cao SG, Zhao HZ, Li GY, Taylor PR. Cytologic screening for esophageal cancer: results from 12,877 subjects from a high-risk population in China. Int J Cancer. 1993;54:185-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Dawsey SM, Shen Q, Nieberg RK, Liu SF, English SA, Cao J, Zhou B, Wang GQ, Lewin KJ, Liu FS. Studies of esophageal balloon cytology in Linxian, China. Cancer Epidemiol Biomarkers Prev. 1997;6:121-130. [PubMed] |
| 5. | Roth MJ, Liu SF, Dawsey SM, Zhou B, Copeland C, Wang GQ, Solomon D, Baker SG, Giffen CA, Taylor PR. Cytologic detection of esophageal squamous cell carcinoma and precursor lesions using balloon and sponge samplers in asymptomatic adults in Linxian, China. Cancer. 1997;80:2047-2059. [PubMed] |
| 6. | Pan QJ, Roth MJ, Guo HQ, Kochman ML, Wang GQ, Henry M, Wei WQ, Giffen CA, Lu N, Abnet CC. Cytologic detection of esophageal squamous cell carcinoma and its precursor lesions using balloon samplers and liquid-based cytology in asymptomatic adults in Llinxian, China. Acta Cytol. 2008;52:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Qin DX, Wang GQ, Zuo JH, Zhang XH, Yuan FL, Li MS, Wu CR, Ju CL. Screening of esophageal and gastric cancer by occult blood bead detector. Cancer. 1993;71:216-218. [PubMed] |
| 8. | Dawsey SM, Fleischer DE, Wang GQ, Zhou B, Kidwell JA, Lu N, Lewin KJ, Roth MJ, Tio TL, Taylor PR. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83:220-231. [PubMed] |
| 9. | Guo HQ, Wei WQ, Lu N, Cao J, Li ZL, Wang NP, Wang GQ, Pan QJ, Qiao YL. [Liquid-based cytology for esophageal carcinoma screening]. Ai Zheng. 2009;28:1243-1247. [PubMed] |
| 10. | Veltman JA, Bot FJ, Huynen FC, Ramaekers FC, Manni JJ, Hopman AH. Chromosome instability as an indicator of malignant progression in laryngeal mucosa. J Clin Oncol. 2000;18:1644-1651. [PubMed] |
| 11. | Doki Y, Shiozaki H, Tahara H, Kobayashi K, Miyata M, Oka H, Iihara K, Mori T. Prognostic value of DNA ploidy in squamous cell carcinoma of esophagus. Analyzed with improved flow cytometric measurement. Cancer. 1993;72:1813-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Böttger T, Störkel S, Stöckle M, Wahl W, Jugenheimer M, Effenberger-Kim O, Vinh T, Junginger T. DNA image cytometry. A prognostic tool in squamous cell carcinoma of the esophagus? Cancer. 1991;67:2290-2294. [PubMed] |
| 13. | Blant SA, Ballini JP, Caron CT, Fontolliet C, Monnier P, Laurini NR. Evolution of DNA ploidy during squamous cell carcinogenesis in the esophagus. Dis Esophagus. 2001;14:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Tong H, Shen R, Wang Z, Kan Y, Wang Y, Li F, Wang F, Yang J, Guo X. DNA ploidy cytometry testing for cervical cancer screening in China (DNACIC Trial): a prospective randomized, controlled trial. Clin Cancer Res. 2009;15:6438-6445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Solomon D. The Bethesda System for reporting cervical/vaginal cytologic diagnosis: an overview. Int J Gynecol Pathol. 1991;10:323-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Tezcan A, Garner DM, Lam P. Analysis of thionin, gallocyanin and hematoxylin for automated quantitative image cytometry of cervical samples. 1993;. |
| 17. | Guillaud M, Benedet JL, Cantor SB, Staerkel G, Follen M, MacAulay C. DNA ploidy compared with human papilloma virus testing (Hybrid Capture II) and conventional cervical cytology as a primary screening test for cervical high-grade lesions and cancer in 1555 patients with biopsy confirmation. Cancer. 2006;107:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Haroske G, Giroud F, Reith A, Böcking A. 1997 ESACP consensus report on diagnostic DNA image cytometry. Part I: basic considerations and recommendations for preparation, measurement and interpretation. European Society for Analytical Cellular Pathology. Anal Cell Pathol. 1998;17:189-200. [PubMed] |
| 19. | Giroud F, Haroske G, Reith A, Böcking A. 1997 ESACP consensus report on diagnostic DNA image cytometry. Part II: Specific recommendations for quality assurance. European Society for Analytical Cellular Pathology. Anal Cell Pathol. 1998;17:201-208. [PubMed] |
| 20. | Singh M, Mehrotra S, Kalra N, Singh U, Shukla Y. Correlation of DNA ploidy with progression of cervical cancer. J Cancer Epidemiol. 2008;2008:298495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Diwakar N, Sperandio M, Sherriff M, Brown A, Odell EW. Heterogeneity, histological features and DNA ploidy in oral carcinoma by image-based analysis. Oral Oncol. 2005;41:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Krause FS, Feil G, Bichler KH, Schrott KM, Akcetin ZY, Engehausen DG. Heterogeneity in prostate cancer: prostate specific antigen (PSA) and DNA cytophotometry. Anticancer Res. 2005;25:1783-1785. [PubMed] |
| 23. | Razak IA, Usman A, Fun HK, Yamin BM, Keat GW. Dichlorobis(trimethylenethiourea-kappaS)antimony(III) chloride. Acta Crystallogr C. 2002;58:m122-m123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Klimstra DS. Pathologic prognostic factors in esophageal carcinoma. Semin Oncol. 1994;21:425-430. [PubMed] |
| 25. | Matsuura H, Sugimachi K, Ueo H, Kuwano H, Koga Y, Okamura T. Malignant potentiality of squamous cell carcinoma of the esophagus predictable by DNA analysis. Cancer. 1986;57:1810-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Böcking A, Nguyen VQ. Diagnostic and prognostic use of DNA image cytometry in cervical squamous intraepithelial lesions and invasive carcinoma. Cancer. 2004;102:41-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Kashyap V, Das BC. DNA aneuploidy and infection of human papillomavirus type 16 in preneoplastic lesions of the uterine cervix: correlation with progression to malignancy. Cancer Lett. 1998;123:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 2829] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 29. | Shackney SE, Shankey TV. Common patterns of genetic evolution in human solid tumors. Cytometry. 1997;29:1-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |