Published online Jan 28, 2012. doi: 10.3748/wjg.v18.i4.340
Revised: August 25, 2011
Accepted: August 31, 2011
Published online: January 28, 2012
AIM: To investigate whether α-fetoprotein (AFP) and vascular endothelial growth factor receptor (VEGFR)-1 correlate with early recurrence of hepatoma/hepatocellular carcinoma (HCC).
METHODS: From 2000 to 2005, 114 consecutive patients with HCC underwent primary curative hepatectomy. The mean age was 60.7 (8.7) years and 94 patients were male. The median follow-up period was 71.2 mo (range: 43-100 mo). Immediately prior to commencing laparotomy, 5 mL bone marrow was aspirated from the sternum and collected in citrate-coated test tubes. The initial 2 mL of bone marrow aspirate was discarded in each case. AFP mRNA and VEGFR-1 mRNA in the bone marrow and peripheral blood (BM- and PH-AFP mRNA and BM- and PH-VEGFR-1 mRNA, respectively) were measured by real-time quantitative reverse transcription polymerase chain reaction. As normal controls, VEGFR-1 mRNA in the bone marrow and peripheral blood was also measured in 11 living liver donors. These data were evaluated for any correlation with early recurrence, comparing clinical and pathological outcomes.
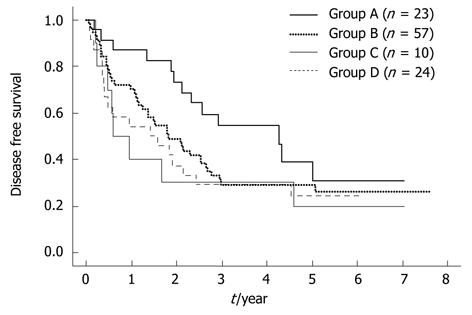
RESULTS: The cut-off value of the BM-AFP mRNA and PH-AFP mRNA level in patients with HCC was set at 1.92 × 10-7 and zero, respectively, based on data from the controls. A total of 34 (29.8%) and six (5.4%) patients were positive for BM-AFP mRNA and PH-AFP mRNA, respectively. The BM-VEGFR-1 mRNA levels in all HCC patients were higher than those in the normal controls, and this was the case also for PH-VEGFR-1mRNA. The 25-percentile values for the BM- and PH-VEGFR-1 mRNA in HCC patients were used as the cut-off values for assigning the patients into two groups based on these transcript levels. The High group for BM- VEGFR-1 mRNA contained 81 (71.1%) HCC cases and the Low group was assigned 33 (28.9%) patients. These numbers for PH-VEGFR-1mRNA were 78 (75.0%) and 26 (25.0%), respectively. HCC recurred in 80 patients; in the remnant liver in 48 cases, in the remnant liver and remote tissue in 20, and in the remote tissue alone in 12. BM-AFP mRNA-positive cases showed a significantly higher rate of early recurrence (within 1 year of surgical treatment) compared with BM-AFP mRNA-negative patients (P = 0.0091). Patients were classified into four groups according to the level/status of their BM-VEGFR-1 and BM-AFP mRNA as follows: group A (n = 23), BM-VEGFR-1/BM-AFP mRNA = low/negative; group B (n = 57) high/negative; group C (n = 10) low/positive; group D (n = 24), high/positive. This classification was found to correlate with a recurrence of this disease within 1 year (P = 0.0228). The disease-free survival curve of group A was significantly better than that of groups B, C or D (P = 0.0437, P = 0.0325, P = 0.0225). No other classification (i.e., PH-VEGF-R1/BM-AFP, BM-VEGF-R1/PH-AFP, and PH-VEGF-R1/PH-AFP mRNA) showed such a correlation.
CONCLUSION: The evaluation of BM-AFP and BM-VEGFR-1 mRNA in patients with HCC may be a valuable predictor of disease recurrence following curative resection.
- Citation: Kamiyama T, Takahashi M, Nakanishi K, Yokoo H, Kamachi H, Kobayashi N, Ozaki M, Todo S. α-fetoprotein, vascular endothelial growth factor receptor-1 and early recurrence of hepatoma. World J Gastroenterol 2012; 18(4): 340-348
- URL: https://www.wjgnet.com/1007-9327/full/v18/i4/340.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i4.340
Various factors are thought to contribute to hepatocellular carcinoma (HCC) recurrence, which commonly results in death, including multicentric carcinogenesis in the remnant liver due to an underlying hepatitis-B-virus- or hepatitis-C-virus-induced liver cirrhosis[1], hematogenic spread, or micrometastasis of HCC cells prior to surgery or during hepatectomy by manipulation of the liver[2]. Recently, using various molecular biological markers, the detection of malignant cells in the systemic circulation and bone marrow has become possible and the presence of these cells has been found to correlate with the clinical outcome[3-8]. We have also reported from our laboratory that the detection of HCC cells in the bone marrow by real-time reverse-transcriptase polymerase chain reaction (RT-PCR) analysis of α-fetoprotein (AFP) mRNA before curative hepatectomy correlates with HCC recurrence and patient survival outcomes. Although early recurrence within 1 year of curative resection for HCC is one of the most important factors affecting the prognosis and clinical outcomes[9,10], the relationship between early recurrence and disseminated cancer cells has not yet been evaluated.
It has been recently hypothesized that metastasis is dependent on both isolated cancer cells and the host response. Kaplan et al[11] have reported that bone-marrow-derived hematopoietic progenitor cells that express vascular endothelial growth factor receptor (VEGFR)-1 migrate to tumor-specific pre-metastatic sites and form cellular clusters before the arrival of tumor cells both in vitro and in vivo. Moreover, it has been reported that the simultaneous presence of isolated tumor cells and VEGFR-1 expression at pre-metastatic sites is clinically significant for disease progression in gastric cancer[12]. With regard to HCC however, there has been no study to date of the association between isolated cancer cells and the expression of VEGFR-1.
In our present study, we examined whether the expression of AFP mRNA and VEGFR-1 in the bone marrow and peripheral blood, detected by sensitive real-time quantitative RT-PCR, could predict early recurrence in consecutive HCC patients who had undergone a curative hepatic resection.
This study was approved by the Institutional Review Board of the Hokkaido University, School of Advanced Medicine. Informed consent was obtained from each patient in accordance with the Ethics Committee Guidelines at our institution.
From July 2000 to June 2005, 114 consecutive patients underwent primary curative hepatectomy at the First Department of Surgery, Hokkaido University Hospital. The mean age was 60.7 (8.7) years and 94 patients were male. The Child-Pugh staging was A in 110 patients and B in four. Patients were discharged from the hospital at an average of 17.5 (7.1) d after surgery. They were followed up at 3-mo intervals by computed tomography (CT), magnetic resonance imaging (MRI), ultrasonography (US) and laboratory tests for AFP, lens culinaris agglutinin-reactive fraction of AFP (AFP-L3), and protein induced by vitamin K absence or antagonist-II (PIVKA-II). The median follow-up period was 71.2 mo (range: 43 mo-100 mo).
As normal controls, VEGFR-1 mRNA in the bone marrow and peripheral blood was also measured in 11 living liver donors. The cut-off value for AFP mRNA/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the bone marrow and peripheral blood was set as described in our previous study[13].
Immediately prior to commencing the laparotomy, 5 mL bone marrow was aspirated from the sternum and collected in citrate-coated test tubes. The initial 2 mL of the bone marrow aspirate was discarded in each case.
Bone marrow samples were prepared for the measurement of total RNA using a Blood RNA extraction kit (QIAGEN, Hilden, Germany) according to the manufacturer’s protocol with minor modifications. Briefly, 5 mL bone marrow cells were mixed with 25 mL Reagent buffer erythrocyte lysis (EL). They were then cooled on ice for 15 min, centrifuged, and the cell pellets were collected. The pellets were suspended in 1.35 mL buffer and applied to the reagent columns, and then washed twice with reagent buffer containing ethanol. Total RNA was eluted with RNase-free water. These bone marrow RNA samples were stored at -80 °C until use. cDNA was generated from 1 μg total RNA using Moloney murine leukemia virus reverse transcriptase (SuperScript II, Life Technologies, Carlsbad, CA, United States), plus 20 pmol/L each dNTP and 10 pmol/L oligo dT primers in a 20-μL final reaction volume at 42 °C for 1 h. This was followed by heating at 99 °C for 5 min.
A LightCycler PCR and detection system (Roche Diagnostics, Mannheim, Germany) was used for amplification. Online quantification real-time RT-PCR was then performed in glass capillaries according to the manufacturer’s protocol. The cDNA was amplified in a 20-μL PCR reaction mixture containing each dNTP (with dUTP instead of dTTP), 1 × PCR buffer, specific primers, and magnesium chloride.
For the detection of AFP, two adjacent oligonucleotide probes were used: the LightCycler Red 640 fluorophore, hAFP-LCR; (5′-CTTGCACACAAAAGCCCACTCCA-3′) and a fluorophore labeled at the 3′-end with fluorescein, hAFP-FITC; (5′-TCGATCCCACTTTTCCAAGTT-3′) (Nihon Gene Research Laboratories, Sendai, Japan). The sense and antisense primers (kindly supplied by Dr. Hiroaki Nagano at Osaka University) used for the amplification of AFP were as follows: 5′-TGCAGCCAAAGTGAAGAGGGAAGA-3′) (hAFP-s) and 5′-CATAGCGAGCAGCCCAAAGAAGAA-3′ (hAFP-As). The RT-PCR amplification was carried out for one cycle of 95 °C for 10 min, followed by 35 cycles of 95 °C for 10 s, 62 °C for 15 s, and 72 °C for 15 s. The final cycle was followed by a 10-min extension step at 40 °C.
For the detection of VEGFR-1, two adjacent oligonucleotide probes were used: hVEGFR-1-LCR; 5′-TTCCGTGTCCCCACTGCCAA-3′ and hVEGFR-1-FITC; 5′-GGGAAGCTCACTGGCATGGC-3′. The sense and antisense primers for the amplification of VEGFR-1 were as follows: 5′-TCATGAATGTTTCCCTGCAA-3′ (h VEGFR-1-S) and 5′-GGAGGTATGGTGCTTCCTGA-3′ (h VEGFR-1-As). These primers were designed using sequences described in a previous report[14]. RT-PCR amplification was carried out for one cycle of 95 °C for 10 min, followed by 35 cycles of 95 °C for 10 s, 60 °C for 10 s, and 72 °C for 16 s. The final cycle was followed by a 10-min extension step at 40 °C.
For the detection of GAPDH as an internal control, two adjacent oligonucleotide probes were used: hGAPDH-LCR; 5′-TTCCGTGTCCCCACTGCCAA-3′ and hGAPDH-FITC; 5′-GGGAAGCTCACTGGCATGGC-3′. The sense and antisense primers for the amplification of GAPDH were as follows: 5′-GCCTCCTGCACCACCAACTG-3′ (hGAPDH-S) and 5′-CGACGCCTGCTTCACCACCTTCT-3′ (hGAPDH-As). The RT-PCR amplification was carried out for one cycle of 95 °C for 10 min, followed by 35 cycles of 95 °C for 10 s, 60 °C for 10 s and 72 °C for 16 s. The final cycle was followed by a 10-min extension step at 40 °C.
Quantification data were analyzed using the LightCycler analysis software (Roche Diagnostics, Mannheim, Germany) in accordance with the manufacturer’s instructions. In this analysis, the background fluorescence was removed by setting a noise band. The crossing point for the calculation of amplified PCR products was set by the intersection of the best-fit line through the log-linear lesion and the noise band. The standard curve was a plot of the “crossing point” versus the copy number of DNA fragments inserted into the cloning vector.
Cumulative survival and disease-free survival (DFS) rates were computed according to the Kaplan-Meier method and compared between groups using the Breslow-Gehan-Wilcoxon test. The Cox proportional hazards model was used for multivariate analysis. Statistical analyses using standard tests (χ2, t test) were performed where appropriate. Significance was defined as P < 0.05. Statistical analyses were performed using StatView 5.0 Windows (SAS Institute Inc., Cary, NY, United States).
The mean AFP mRNA/GAPDH ratio in the bone marrow (BM-AFP mRNA) of HCC patients, as determined by real-time quantitative RT-PCR, was 3469.27 × 10-7 (range: 0-348 526.19 × 10-7). The cut-off value of the BM-AFP mRNA level was set at 1.92 × 10-7 (with reference to a previous report)[13]. The HCC patients were then divided into two groups according to this cut-off value. Accordingly, 80 patients (70.2%) were found to be negative for BM-AFP mRNA and 34 patients (29.8%), assigned to the “High” group, were positive for this transcript.
No AFP mRNA was detectable in the peripheral blood of the control patients, therefore, the cut-off value for AFP mRNA/GAPDH in the peripheral blood (PH-AFP mRNA) was set at zero. Accordingly, six patients (5.4%) were found to be positive and 105 (94.6%) were negative for AFP mRNA. Due to some sampling loss, peripheral blood samples were unavailable for three patients.
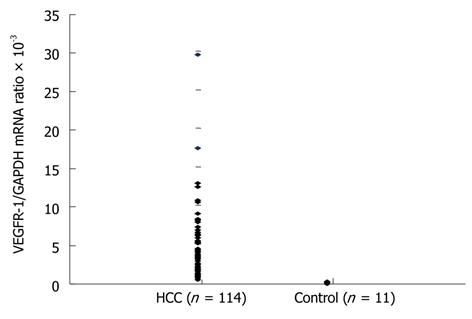
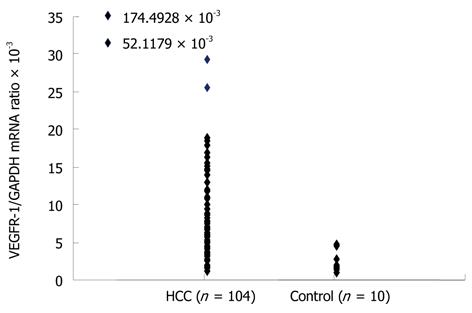
The mean VEGFR-1 mRNA/GAPDH in the bone marrow (BM-VEGFR-1 mRNA) of normal controls, again determined by real-time quantitative RT-PCR measurements, was 0.1497 × 10-3 (range: 0.0212 × 10-3 to 0.3213 × 10-3). The mean BM-VEGFR-1 mRNA level in the HCC patients was 3.8474 × 10-3 (range: 0.3481 × 10-3 to 29.5885 × 10-3). The mean BM-VEGFR-1 mRNA level of all HCC patients was higher than that of the normal controls (Figure 1). The HCC patients were then divided into two groups (“High” and “Low”) according to their BM-VEGFR-1 mRNA level; the cut-off value was 1.5664 × 10-3, which was the 25th percentile value of the BM-VEGFR-1 mRNA levels in the HCC cohort. The number of patients in the High group was 81 (71.1%) and 33 (28.9%) were assigned to the Low group.
The mean VEGFR-1 mRNA/GAPDH ratio in the peripheral blood (PH-VEGFR-1 mRNA) of the normal controls was 2.4944 × 10-3 (range: 1.0730 × 10-3 to 4.6958 × 10-3). The mean PH-VEGFR-1 mRNA level in the HCC patients was 9.1285 × 10-3 (range: 1.2774 × 10-3 to 174.4928 × 10-3). The PH-VEGFR-1 mRNA level of almost all HCC patients was higher than that of the normal controls (Figure 2). The HCC patients were divided into high and low groups according to their PH-VEGFR-1 mRNA level. The cut-off value was 4.0238 × 10-3, which was in the 25th percentile of the PH-VEGFR-1 mRNA level of HCC patients. The number of patients in the high group was 78 (75.0%) with 26 (25.0%) placed in the Low group. Peripheral blood samples were available for 104 patients only.
The status of the BM-AFP mRNA levels was correlated with microscopically detectable portal invasion, whereas that of PH-AFP mRNA was found to correlate with the serum AFP and AFP-L3 levels, the number of tumors, microscopic portal invasion, and microscopic intrahepatic metastasis (Table 1). The number of tumors, serum albumin level, and a noncancerous liver were significantly correlated with the BM-VEGFR-1 mRNA level (Table 2).
| BM-AFP mRNA | P value | PH-AFP mRNA | P value | ||||
| Positive (n = 34) | Negative (n = 80) | Positive (n = 6) | Negative (n = 105) | ||||
| Sex | Male | 27 | 67 | 0.5774 | 5 | 86 | 0.9294 |
| Female | 7 | 13 | 1 | 19 | |||
| Age (yr) | ≤ 60 | 19 | 34 | 0.1900 | 5 | 47 | 0.0655 |
| > 60 | 15 | 46 | 1 | 58 | |||
| HBsAg | + | 19 | 30 | 0.0697 | 3 | 45 | 0.7312 |
| - | 15 | 50 | 3 | 60 | |||
| HCV | + | 12 | 34 | 0.4731 | 2 | 43 | 0.7116 |
| - | 22 | 46 | 4 | 62 | |||
| Albumin | ≤ 4.0 mg/dL | 13 | 33 | 0.7641 | 3 | 41 | 0.5937 |
| > 4.0 mg/dL | 21 | 47 | 3 | 64 | |||
| Total bilirubin | ≤ 0.7 mg/dL | 21 | 45 | 0.5853 | 4 | 60 | 0.6461 |
| ≥ 0.8 mg/dL | 13 | 35 | 2 | 45 | |||
| ICGR15 | ≤ 15% | 22 | 39 | 0.1181 | 3 | 56 | 0.8736 |
| > 15% | 12 | 41 | 3 | 49 | |||
| Anatomical resection | Yes | 25 | 54 | 0.5231 | 4 | 73 | 0.8826 |
| No | 9 | 26 | 2 | 32 | |||
| AFP | ≤ 200 ng/mL | 21 | 57 | 0.3189 | 1 | 76 | 0.0040 |
| > 200 ng/nL | 13 | 23 | 5 | 29 | |||
| AFPL3 | ≤ 15% | 21 | 58 | 0.2556 | 2 | 76 | 0.0418 |
| > 15% | 13 | 22 | 4 | 29 | |||
| PIVKA-II | ≤ 40 mAU/mL | 8 | 30 | 0.1477 | 1 | 36 | 0.3732 |
| > 40 mAU/mL | 26 | 50 | 5 | 69 | |||
| Tumor number | Solitary | 25 | 57 | 0.8804 | 2 | 78 | 0.0259 |
| Multiple | 9 | 22 | 4 | 26 | |||
| Tumor size | ≤ 2 cm | 2 | 10 | 0.2922 | 0 | 12 | 0.3806 |
| > 2 cm | 32 | 70 | 6 | 93 | |||
| Differentiation | Well | 0 | 7 | 0.0737 | 0 | 7 | 0.5859 |
| Moderately | 26 | 49 | 3 | 70 | |||
| Poorly | 7 | 24 | 3 | 27 | |||
| vp | Positive | 14 | 18 | 0.0423 | 5 | 25 | 0.0014 |
| Negative | 20 | 62 | 1 | 80 | |||
| vv | Positive | 3 | 4 | 0.4366 | 1 | 5 | 0.2098 |
| Negative | 31 | 76 | 5 | 100 | |||
| im | Positive | 12 | 18 | 0.1558 | 4 | 25 | 0.0201 |
| Negative | 22 | 62 | 2 | 80 | |||
| Noncancerous liver | 11 | 28 | 0.6833 | 1 | 36 | 0.3501 | |
| Liver cirrhosis | |||||||
| Non liver cirrhosis | 23 | 49 | 5 | 66 | |||
| BM-VEGFR1 | P value | PH-VEGFR1 | P value | ||||
| High (n = 81) | Low (n = 33) | High (n = 78) | Low (n = 26) | ||||
| Sex | Male | 67 | 27 | 0.9090 | 64 | 22 | 0.7647 |
| Female | 14 | 6 | 14 | 4 | |||
| Age (yr) | ≤ 60 | 40 | 13 | 0.3322 | 37 | 12 | 0.9097 |
| > 60 | 41 | 20 | 41 | 14 | |||
| HBsAg | + | 35 | 14 | 0.9387 | 34 | 12 | 0.8197 |
| - | 46 | 19 | 44 | 14 | |||
| HCV | + | 36 | 10 | 0.1628 | 32 | 9 | 0.5624 |
| - | 45 | 23 | 46 | 17 | |||
| Albumin | ≤ 4.0 mg/dL | 38 | 8 | 0.0252 | 27 | 12 | 0.2926 |
| > 4.0 mg/dL | 43 | 25 | 51 | 14 | |||
| Total bilirubin | ≤ 0.7 mg/dL | 48 | 18 | 0.6439 | 51 | 11 | 0.0378 |
| ≥ 0.8 mg/dL | 33 | 15 | 27 | 15 | |||
| ICGR15 | ≤ 15% | 41 | 20 | 0.3322 | 44 | 13 | 0.5695 |
| > 15% | 40 | 13 | 34 | 13 | |||
| Anatomical resection | Yes | 55 | 24 | 0.6124 | 56 | 18 | 0.8026 |
| No | 26 | 9 | 22 | 8 | |||
| AFP | ≤ 200 ng/mL | 54 | 24 | 0.5278 | 57 | 16 | 0.2653 |
| > 200 ng/nL | 27 | 9 | 21 | 10 | |||
| AFPL3 | ≤ 15% | 54 | 25 | 0.3399 | 59 | 15 | 0.0802 |
| > 15% | 27 | 8 | 19 | 11 | |||
| PIVKA-II | ≤ 40 mAU/mL | 24 | 14 | 0.1888 | 25 | 10 | 0.5491 |
| > 40 mAU/mL | 57 | 19 | 53 | 16 | |||
| Tumor number | Solitary | 53 | 29 | 0.0068 | 55 | 18 | 0.8867 |
| Multiple | 28 | 3 | 23 | 7 | |||
| Tumor size | ≤ 2 cm | 9 | 3 | 0.7499 | 10 | 2 | 0.4784 |
| > 2 cm | 72 | 30 | 68 | 24 | |||
| Differentiation | Well | 4 | 3 | 0.1151 | 6 | 1 | 0.1614 |
| Moderately | 51 | 25 | 56 | 11 | |||
| Poorly | 26 | 5 | 16 | 14 | |||
| vp | Positive | 26 | 6 | 0.1337 | 21 | 7 | ... |
| Negative | 55 | 27 | 57 | 19 | |||
| vv | Positive | 6 | 1 | 0.3773 | 4 | 1 | 0.7913 |
| Negative | 75 | 32 | 74 | 25 | |||
| im | Positive | 23 | 7 | 0.4296 | 24 | 5 | 0.2559 |
| Negative | 58 | 26 | 54 | 21 | |||
| Noncancerous liver cirrhosis | 34 | 5 | 0.0061 | 26 | 9 | 0.9962 | |
| Non liver cirrhosis | 45 | 27 | 49 | 17 | |||
Mortality: By the end of our study, 42 of the HCC patients under analysis had died; 35 from HCC, three from liver failure and four from another malignant disease. The 1-, 2- and 3-year patient survival rates for this cohort were determined to be 92.1%, 85.9% and 78.7%, respectively.
HCC recurrence: HCC recurred in 80 patients (70.2%); in the remnant liver in 48 cases (60%), in the remnant liver and remote tissue in 20 (25%), and in the remote tissue alone in 12 (15%). The 1-, 2- and 3-year DFS rates were 67.5%, 49.8% and 34.4%, respectively. We found a significant tendency for patients who were positive for BM-AFP mRNA to experience recurrence within 1 year of their surgery compared with patients who were negative for this transcript (Table 3).
| Recurrence over 1 year (77) | Recurrence within 1 year (37) | P value | ||
| Sex | Male | 64 | 30 | 0.7980 |
| Female | 13 | 7 | ||
| Age (yr) | ≤ 60 | 30 | 23 | 0.0200 |
| > 60 | 47 | 14 | ||
| HBsAg | + | 24 | 25 | 0.0002 |
| - | 53 | 12 | ||
| HCV | + | 34 | 12 | 0.2322 |
| - | 43 | 25 | ||
| Albumin | ≤ 4.0 mg/dL | 24 | 22 | 0.0039 |
| > 4.0 mg/dL | 53 | 15 | ||
| Total bilirubin | ≤ 0.7 mg/dL | 47 | 19 | 0.3266 |
| ≥ 0.8 mg/dL | 30 | 18 | ||
| ICGR15 | ≤ 15% | 43 | 18 | 0.4708 |
| > 15% | 34 | 19 | ||
| Anatomical resection | Yes | 55 | 24 | 0.4769 |
| No | 22 | 13 | ||
| AFP | ≤ 200 ng/mL | 59 | 19 | 0.0066 |
| > 200 ng/nL | 18 | 18 | ||
| AFPL3 | ≤ 15% | 58 | 21 | 0.0442 |
| > 15% | 19 | 16 | ||
| PIVKA-II | ≤ 40 mAU/mL | 32 | 6 | 0.0072 |
| > 40 mAU/mL | 45 | 31 | ||
| Tumor number | Solitary | 62 | 20 | 0.0021 |
| Multiple | 14 | 17 | ||
| Tumor size | ≤ 2 cm | 36 | 6 | 0.0016 |
| > 2 cm | 41 | 31 | ||
| Differentiation | Well | 7 | 0 | 0.1631 |
| Moderately | 52 | 13 | ||
| Poorly | 18 | 24 | ||
| vp | Positive | 11 | 16 | < 0.0001 |
| Negative | 66 | 21 | ||
| vv | Positive | 2 | 5 | 0.0230 |
| Negative | 75 | 32 | ||
| im | Positive | 13 | 17 | 0.0010 |
| Negative | 64 | 20 | ||
| BM VEGFR1 | Low | 24 | 9 | 0.4506 |
| High | 53 | 28 | ||
| PH VEGFR1 | Low | 18 | 8 | |
| High | 54 | 24 | ||
| BM AFP mRNA | Positive | 17 | 17 | 0.0091 |
| Negative | 60 | 20 | ||
| PH AFP mRNA | Positive | 2 | 4 | 0.0569 |
| Negative | 74 | 31 | ||
| BM-AFP mRNA/BM-VEGFR1 | Negative/low | 20 | 3 | 0.0228 |
| Negative/high | 40 | 17 | ||
| Positive/low | 4 | 6 | ||
| Positive/high | 13 | 11 |
Patients were classified into four groups according to the level/status of their BM-VEGFR-1 and BM-AFP mRNA as follows: group A (n = 23), BM-VEGFR-1/BM-AFP mRNA = low/negative; group B (n = 57) high/negative; group C (n = 10) low/positive; group D (n = 24), high/positive. This classification was correlated with disease recurrence within or more than 1 year after surgery. Significantly, in the groups in which patients were negative for BM-AFP mRNA, only three patients (13.0%) experienced recurrence in group A, whereas 17 (29.0%) in group B experienced recurrence within 1 year of surgery (Table 3). Classification of the HCC cases in the current study cohort by their PH-VEGFR-1 and BM-AFP (P = 0.1024), BM-VEGFR-1 and PH-AFP (P = 0.2100), and PH-VEGFR-1 and PH-AFP (P = 0.2138) mRNA status showed no such correlation. The DFS curve of group A was significantly better than that of group B, C or D (P = 0.0437, P = 0.0325, P = 0.0225, respectively; Figure 3).
Univariate analysis further revealed that age, hepatitis B surface antigen (HBsAg), albumin, AFP, AFPL3, PIVKA-II, the number of tumors, tumor size, portal vein invasion, hepatic vein invasion, intrahepatic metastasis, BM-AFP mRNA and classification by BM-VEGFR-1/BM-AFP mRNA are important risk factors for HCC early recurrence (Table 3). Multivariate analysis revealed that albumin ≤ 4.0 mg/dL and positive portal vein invasion were independent risk factors for recurrence within 1 year of surgery. Although BM-AFP mRNA positivity was not a significant factor by multivariate analysis, it was still found to be an important factor in predicting an early recurrence in HCC cases (P = 0.0761, Table 4).
| P value | Risk ratio | 95% CI | |
| Age ≤ 60 yr | 0.0899 | 3.147 | 0.836-11.838 |
| HBsAg + | 0.3601 | 1.821 | 0.504-6.571 |
| Albumin ≤ 4.0 mg/dL | 0.0038 | 6.536 | 1.832-23.256 |
| AFP > 200 ng/nL | 0.2571 | 2.330 | 0.539-10.067 |
| AFPL3 ≤ 15% | 0.4379 | 1.869 | 0.385-9.090 |
| PIVKA-II > 40 mAU/mL | 0.1494 | 2.959 | 0.677-12.987 |
| Tumor number solitary | 0.9127 | 1.088 | 0.240-4.938 |
| Tumor size > 3 cm | 0.1177 | 3.026 | 0.756-12.114 |
| vp positive | 0.0069 | 6.639 | 1.681-26.219 |
| vv positive | 0.2221 | 0.234 | 0.023-2.408 |
| im positive | 0.2307 | 2.508 | 0.557-11.289 |
| BM AFP mRNA: positive | 0.0761 | 2.704 | 0.901-8.113 |
In our current study, we found a significant tendency for HCC patients who were positive for BM-AFP mRNA to experience disease recurrence within 1 year of surgery. Patients with low BM-VEGFR-1 mRNA and who were negative for BM-AFP mRNA experienced early recurrence in 3/23 cases, whereas in 57 cases with high BM-VEGFR-1 and BM-AFP mRNA, 17 recurrences were observed. Hence, BM-AFP mRNA positivity is an important predictor of early HCC recurrence after curative hepatectomy due to hematogenic spread. BM-VEGFR-1 mRNA was also found to be associated with early HCC recurrence.
The time between hepatectomy and recurrence of metachronous de novo tumors is longer than that of intrahepatic metastases[15], therefore, early recurrence of these lesions (within 1 year) is thought to be dependent on hematogenic spread. By real-time quantitative RT-PCR, we found in our current analyses that, although the AFP/GAPDH mRNA ratios in the liver tissues were generally constant among normal control subjects, they were markedly different among HCC patients. This indicated highly variable AFP synthesis activity among individual HCC cells. It has been shown that high AFP mRNA levels reflect the presence of HCC cells[13]. In our present study, the 1-year survival and DFS rates of HCC patients who were positive for AFP mRNA were 86.5% and 54.5%, respectively. Hence, we analyzed the relationship between early recurrence and the preoperative status of the BM- and PH-AFP, and the BM- and PH-VEGFR-1 mRNA.
Although we found in our present experiments that the BM-AFP mRNA status significantly correlates with early HCC recurrence, the BM-VEGFR-1, PH-VEGFR-1 and PH-AFP mRNA levels did not correlate with this outcome. However, classifying the HCC cases in our cohort using the BM-VEGFR-1/BM-AFP mRNA levels showed a correlation with early recurrence (P = 0.0228). Based on these findings, we speculate that the preoperative presence of cancer cells in the bone marrow is an important and essential driver of early HCC recurrence due to hematogenic spread, although we did not detect any changes in the AFP or VEGFR1 mRNA levels in the bone marrow and peripheral blood after surgical intervention in recurrent cases. The importance of the coexistence of disseminated cancer cells and VEGFR-1-positive hematopoietic bone marrow progenitor cells was further supported by the improved DFS curve of HCC patients that were negative for BM-AFP mRNA, and that showed low BM-VEGFR-1 transcript levels as compared with the other three patient groups. On the other hand, we surmised that the relationship between BM-VEGFR-1 mRNA and hematogenic spread in HCC during hematogenic recurrence was not stronger than that in gastric cancer, because it has been reported in a clinically relevant and widely used preclinical study model that blockade of VEGFR-1 activity does not affect the formation of spontaneous metastases[16].
In our present study, the BM-VEGFR-1 mRNA level of all HCC patients was higher than that in the normal controls, and the PH-VEGFR-1 mRNA levels of almost all of these patients were also higher than in the normal controls. Direct evidence for the role of the chemokine stromal-cell derived factor-1 [SDF-1, also known as chemokine CXC ligand (CXCL)12] in regulating the mobilization of proangiogenic bone marrow cells in vivo has been demonstrated by plasma elevation of SDF-1, which stimulates the mobilization of chemokine CXC receptor (CXCR) 4+ bone marrow cells, including hematopoietic stem cells and endothelial progenitor cells[17,18]. SDF-1 not only promotes revascularization by engaging with CXCR4 expressed on vascular cells but also supports the mobilization of proangiogenic CXCR4+ VEGFR1+ hematopoietic cells[19]. In contrast, Li et al[20] have reported a much higher expression level of the CXCL12-CXCR4 axis in HCC specimens than in adjacent, cirrhotic, adenocarcinoma or normal liver tissues. Hence, we speculate that VEGFR-1-positive hematopoietic bone marrow progenitor cells might be regulated and recruited by a mechanism similar to the SDF-1-CXCR4 pathway in most HCC patients. On the basis of our current data and the results of these earlier reports, we further predict that, in almost all patients with HCC, a pre-metastatic niche might have already been initiated by VEGFR-1-positive hematopoietic bone marrow progenitor cells. The levels of BM- and PH-VEGFR-1 mRNA were not found to correlate with early recurrence in each of the HCC patients, although BM-AFP mRNA positivity was significantly associated with early recurrence. These findings thus indicate that the initiation of a pre-metastatic niche be recognized as a first but essential step in the development of metastasis that requires the presence of disseminated cancer cells. This hypothesis is supported by our finding that patients negative for BM-AFP mRNA and with low levels of BM-VEGFR-1 mRNA show the lowest rate of recurrence among all of the groups analyzed.
It has been shown in several previous studies that the detection of micrometastases from solid tumors in bone marrow samples can be an important prognostic indicator with high specificity[3-7,11]. The release of carcinoma cells from the bone marrow into the peripheral blood can be induced by cytokine treatment[21]. Hence, the bone marrow might function as an important reservoir and a source of disseminated cancer cells that can subsequently spread into other organs. Moreover, the bone marrow itself may become altered in response to chemokines produced by the primary tumor and thereby enhance the metastatic capabilities of tumor cells that reside within it[22]. It has been reported that VEGFR-1-positive cells promote tumor adherence and growth[11]. VEGFR signaling is a crucial inducer of angiogenesis, enables primary tumor growth, and probably releases micrometastases from dormancy[23]. In our current study, only the classification by BM-VEGFR-1 and BM-AFP mRNA was correlated with early recurrence. Hence, the coexistence of bone-marrow-derived hematopoietic progenitor cells that express VEGFR-1 in the bone marrow, and not in the peripheral blood, might be advantageous for various cancer cells in the bone marrow in terms of metastasis.
In conclusion, the evaluation of BM-AFP mRNA and BM-VEGFR-1 mRNA in patients with HCC shows great promise as a predictor of recurrence in curatively resected HCC.
The authors wish to thank the staff of the Department of General Surgery, Graduate School of Medicine, Hokkaido University, for their kind cooperation.
α-fetoprotein (AFP) mRNA, which represents disseminate cancer cells, is related to recurrence of hepatocellular carcinoma (HCC). Bone-marrow-derived hematopoietic progenitor cells that express vascular endothelial growth factor receptor (VEGFR)-1 home to tumor-specific pre-metastatic sites and form cellular clusters before the arrival of tumor cells.
It has been reported that simultaneous presence of isolated tumor cells and VEGFR-1 expression at pre-metastatic sites is clinically significant for disease progression in gastric cancer. With regard to HCC, there has been no study about the association between the presence of isolated cancer cells and the expression of VEGFR-1. In the present study, we tried to determine whether expression of AFP mRNA and VEGFR-1 in bone marrow and peripheral blood detected by real-time quantitative reverse transcriptase polymerase chain reaction (RT-PCR) could predict early recurrence in consecutive patients after curative hepatic resection.
There was a significant tendency for patients who were positive for AFP mRNA in bone marrow to experience recurrence within 1 year after surgery compared to those negative for AFP mRNA in bone marrow. The VEGFR-1 mRNA level in bone marrow in all HCC patients was higher than that of normal controls. It was supposed that this initiation of pre-metastatic niche represented by the high level of VEGFR-1mRNA might be recognized as only the first step and as a necessary condition for development of metastasis, and required the subsequent presence of disseminated cancer cells represented by AFP mRNA.
The evaluation of AFP mRNA and VEGFR-1 mRNA in bone marrow in patients with HCC could be very important for the prediction of recurrence of curatively resected HCC.
This study found that the expression of AFP and VEGFR-1 mRNA in bone marrow detected by real-time quantitative RT-PCR predicted early recurrence in consecutive patients after curative hepatic resection. This finding is very important to elucidate the mechanism of metastasis and recurrence by hematogenic spread of HCC cells.
Peer reviewers: Thomas Kietzmann, Professor, Dr., Department of Biochemistry, University of Oulu, FI-90014 Oulu, Finland; Arezoo Aghakhani, MD, PhD, Assistant Professor, Clinical Research Department, Pasteur Institute of Iran, No 69, Pasteur Ave., Tehran 13164, Iran
S- Editor Lv S L- Editor Kerr C E- Editor Zhang DN
| 1. | Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, Sone Y, Toyoda H, Shimada S, Takahashi M. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology. 1997;25:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 271] [Article Influence: 9.3] [Reference Citation Analysis (3)] |
| 2. | Yamanaka N, Okamoto E, Fujihara S, Kato T, Fujimoto J, Oriyama T, Mitsunobu M, Toyosaka A, Uematsu K, Yamamoto K. Do the tumor cells of hepatocellular carcinomas dislodge into the portal venous stream during hepatic resection? Cancer. 1992;70:2263-2267. [PubMed] |
| 3. | Diel IJ, Kaufmann M, Goerner R, Costa SD, Kaul S, Bastert G. Detection of tumor cells in bone marrow of patients with primary breast cancer: a prognostic factor for distant metastasis. J Clin Oncol. 1992;10:1534-1539. [PubMed] |
| 4. | Lindemann F, Schlimok G, Dirschedl P, Witte J, Riethmüller G. Prognostic significance of micrometastatic tumour cells in bone marrow of colorectal cancer patients. Lancet. 1992;340:685-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 358] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Pantel K, Izbicki J, Passlick B, Angstwurm M, Häussinger K, Thetter O, Riethmüller G. Frequency and prognostic significance of isolated tumour cells in bone marrow of patients with non-small-cell lung cancer without overt metastases. Lancet. 1996;347:649-653. [PubMed] |
| 6. | Soeth E, Vogel I, Röder C, Juhl H, Marxsen J, Krüger U, Henne-Bruns D, Kremer B, Kalthoff H. Comparative analysis of bone marrow and venous blood isolates from gastrointestinal cancer patients for the detection of disseminated tumor cells using reverse transcription PCR. Cancer Res. 1997;57:3106-3110. [PubMed] |
| 7. | Wiedswang G, Borgen E, Kåresen R, Kvalheim G, Nesland JM, Qvist H, Schlichting E, Sauer T, Janbu J, Harbitz T. Detection of isolated tumor cells in bone marrow is an independent prognostic factor in breast cancer. J Clin Oncol. 2003;21:3469-3478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Zhang YL, Feng JG, Gou JM, Zhou LX, Wang P. Detection of CK20mRNA in peripheral blood of pancreatic cancer and its clinical significance. World J Gastroenterol. 2005;11:1023-1027. [PubMed] |
| 9. | Shah SA, Greig PD, Gallinger S, Cattral MS, Dixon E, Kim RD, Taylor BR, Grant DR, Vollmer CM. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg. 2006;202:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 219] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 10. | Regimbeau JM, Abdalla EK, Vauthey JN, Lauwers GY, Durand F, Nagorney DM, Ikai I, Yamaoka Y, Belghiti J. Risk factors for early death due to recurrence after liver resection for hepatocellular carcinoma: results of a multicenter study. J Surg Oncol. 2004;85:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2613] [Cited by in RCA: 2438] [Article Influence: 116.1] [Reference Citation Analysis (0)] |
| 12. | Mimori K, Fukagawa T, Kosaka Y, Kita Y, Ishikawa K, Etoh T, Iinuma H, Sasako M, Mori M. Hematogenous metastasis in gastric cancer requires isolated tumor cells and expression of vascular endothelial growth factor receptor-1. Clin Cancer Res. 2008;14:2609-2616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Kamiyama T, Takahashi M, Nakagawa T, Nakanishi K, Kamachi H, Suzuki T, Shimamura T, Taniguchi M, Ozaki M, Matsushita M. AFP mRNA detected in bone marrow by real-time quantitative RT-PCR analysis predicts survival and recurrence after curative hepatectomy for hepatocellular carcinoma. Ann Surg. 2006;244:451-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Kosaka Y, Mimori K, Fukagawa T, Ishikawa K, Etoh T, Katai H, Sano T, Watanabe M, Sasako M, Mori M. Identification of the high-risk group for metastasis of gastric cancer cases by vascular endothelial growth factor receptor-1 overexpression in peripheral blood. Br J Cancer. 2007;96:1723-1728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1261] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 16. | Dawson MR, Duda DG, Fukumura D, Jain RK. VEGFR1-activity-independent metastasis formation. Nature. 2009;461:E4; discussion E5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Hattori K, Heissig B, Tashiro K, Honjo T, Tateno M, Shieh JH, Hackett NR, Quitoriano MS, Crystal RG, Rafii S. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354-3360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 395] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 18. | Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625-637. [PubMed] |
| 19. | Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28:299-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 465] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 20. | Li W, Gomez E, Zhang Z. Immunohistochemical expression of stromal cell-derived factor-1 (SDF-1) and CXCR4 ligand receptor system in hepatocellular carcinoma. J Exp Clin Cancer Res. 2007;26:527-533. [PubMed] |
| 22. | Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the 'pre-metastatic niche': within bone and beyond. Cancer Metastasis Rev. 2006;25:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 245] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 23. | Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle. 2006;5:1779-1787. [PubMed] |