Published online Jan 28, 2012. doi: 10.3748/wjg.v18.i4.331
Revised: September 2, 2011
Accepted: November 9, 2011
Published online: January 28, 2012
AIM: Тo examine the effects of nitroglycerine on portal vein haemodynamics and oxidative stress in patients with portal hypertension.
METHODS: Thirty healthy controls and 39 patients with clinically verified portal hypertension and increased vascular resistance participated in the study. Liver diameters, portal diameters and portal flow velocities were recorded using color flow imaging/pulsed Doppler detection. Cross-section area, portal flow and index of vascular resistance were calculated. In collected blood samples, superoxide anion radical (O2-), hydrogen peroxide (H2O2), index of lipid peroxidation (measured as TBARS) and nitric oxide (NO) as a marker of endothelial response (measured as nitrite-NO2-) were determined. Time-dependent analysis was performed at basal state and in 10th and 15th min after nitroglycerine (sublingual 0.5 mg) administration.
RESULTS: Oxidative stress parameters changed significantly during the study. H2O2 decreased at the end of study, probably via O2- mediated disassembling in Haber Weiss and Fenton reaction; O2- increased significantly probably due to increased diameter and tension and decreased shear rate level. Consequently O2- and H2O2 degradation products, like hydroxyl radical, initiated lipid peroxidation. Increased blood flow was to some extent lower in patients than in controls due to double paradoxes, flow velocity decreased, shear rate decreased significantly indicating non Newtonian characteristics of portal blood flow.
CONCLUSION: This pilot study could be a starting point for further investigation and possible implementation of some antioxidants in the treatment of portal hypertension.
- Citation: Vujanac A, Jakovljevic V, Djordjevic D, Zivkovic V, Stojkovic M, Celikovic D, Andjelkovic N, Skevin AJ, Djuric D. Nitroglycerine effects on portal vein mechanics and oxidative stress in portal hypertension. World J Gastroenterol 2012; 18(4): 331-339
- URL: https://www.wjgnet.com/1007-9327/full/v18/i4/331.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i4.331
Oxidative stress is a condition in which the delicate balance that exists between prooxidant (free radicals) production and their subsequent amelioration via the antioxidant defense system (ADS) becomes skewed in favor of free radical expression[1]. An increasing body of evidence suggests that oxidative stress is involved in the pathogenesis of many cardiovascular diseases, including hypertension, hypercholesterolaemia, atherosclerosis, diabetes and heart failure[2-5]. The existence of an interference of increased oxidative stress with the vasodilatating properties of veins is now a well known fact. The term endothelium dysfunction is usually used to refer to an impairment of endothelium-dependent vasorelaxation caused by a loss of nitric oxide (NO) bioactivity in the vessel wall, which is, in part, induced by accelerated NO degradation by reactive oxygen species (ROS)[2-8]. Endothelial function is most commonly assessed as a vasodilatatory response to various pharmacological agonists or mechanical stimuli that induce endothelium-dependent or endothelium-independent vasodilatation[9]. One such agonist is nitroglycerine (GTN)[9]. The aim of our study was to determine how sublingual administration of GTN might be used for the evaluation of portal endothelium-independent vasodilatation through estimating parameters of portal vascular mechanics and oxidative stress in patients suffering from portal hypertension. We hypothesized that the endothelium independent vasodilatation of the portal vein induced by sublingual GTN administration would lead to increased oxidative stress and significant changes in portal haemodynamics. The precise mechanism of increased oxidative stress is to be determined; we favor the importance of Haber-Weiss and Fenton’s mechanism. Also, increased blood flow through the portal vein and reduced shear rate may be involved in the previously mentioned process of enhanced oxidative stress. Indeed, portal blood flow is known to be affected by factors and circumstances of chronic liver diseases, and its changes evolved from basic haemodynamic laws[10-15].
This research was performed with a group of 39 patients with chronic liver disease and 30 healthy controls. Patients with chronic liver disease were recruited from the Department of Gastroenterology, Internal Clinic, Clinical Centre Kragujevac, while controls were recruited from the medical staff. The only obligatory inclusion criterion for the participants of the experimental group referred to the existence of previously clinically confirmed serious chronic liver disease (by ultrasonographical assessments, patient’s anamnesis and biochemical parameters). Thirty eight patients had previously confirmed hepatic cirrhosis and one patient was recruited with clinical diagnosis of Hepatitis B. Patients were defined as preascitic if they had never been diagnosed with ascites according to clinical and ultrasonographical examinations. None of the subjects took any medication known to affect vascular tonus or blood flow. Written informed consent was obtained from all patients and the study protocol was approved by the local Ethics Committee prior to the onset of the study. The investigation was conducted in accordance with the principles outlined in the Declaration of Helsinki (Last updated in 2005) and principles of Good Clinical Practice (GCP).
The examinations were performed in a quiet, air-conditioned, temperature controlled room (22-24 °C). The antecubital vein was cannulated by using a 19-gauge polyethylene catheter for taking blood samples. Blood samples were taken at rest and in the 10th and 15th min after endothelium-independent vasodilatation induced by sublingual GTN administration (0.3 mg). Parameters of portal vascular mechanics were recorded in the 10th and 15th min after endothelium-independent vasodilatation by using a Doppler 2D machine. All ultrasonographical measurements were performed by 3 independent physicians and mean value was used for the final calculations.
Blood samples were taken from the antecubital veins into a Vacutainer test tube containing sodium citrate anticoagulant. Blood was centrifuged to separate plasma and red blood cells (RBCs). Biochemical parameters were measured spectrophotometrically.
Nitric oxide was assessed as nitrite and quantified by a spectrophotometric method using Griess reagent. 0.5 mL of perfusate was precipitated with 200 μL of 30% sulfosalicylic acid, vortexed for 30 min and centrifuged at 3000 ×g. Equal volumes of the extracted plasma and Griess’s reagent, containing 1% sulfanilamide in 5% phosphoric acid/0.1% napthalene ethylenediamine-dihydrochloride was added and incubated for 10 min in the dark and read at 543 nmol/L. The nitrite levels were calculated by using sodium nitrite as a standard[16].
The level of superoxide anion radical (O2-) was measured using Nitro Blue Tetrazolium (NBT) reaction in TRIS-buffer with plasma and read at 530 nm. Bidistilled water was used as a blank probe[17].
The level of hydrogen peroxide (H2O2) was measured using Phenol Red reaction in TRIS-buffer with plasma and read at 230 nm. Bidistilled water was used as a blank probe[18].
The degree of lipid peroxidation in coronary venous effluent was estimated by measuring of thiobarbituric acid reactive substances (TBARS) using 1% thiobarbituric acid (TBA) in 0.05 NaOH incubated with plasma TCA extracts (using 28% Trichloracetic acid-TCA) at 100 °C for 15 min and read at 530 nm. Bidistilled water solution was used as a blank probe[19].
The following haemodynamic and biomechanical parameters were calculated: (1) Portal flow-blood flow rate (l/min) through portal vein; (2) Shear rate-the velocity gradient between the moving planes, ΔV/ΔX (s-1); (3) Portal cross sectional area-area normal to flow direction (cm2); (4) Resistance index-relative maximal velocity gradient; (5) Inlet length-point where a constant flow regime is established (cm); (6) Expected (ideal) portal vein flow ratio-ideal flow ratio with regard to Poiseuille's equation; (7) Pressure ratio-estimated pressure changes derived from Poiseuille`s equation; and (8) Portal vein flow ratio-experimentally obtained value quotient flow.
The portal flow (Q) was calculated by using equation 1.1a, where D is portal diameter and Vmean is average blood flow velocity:
(1.1a)
It is of interest to emphasize that portal blood flow calculated by equation 1.1a has to be in concordance with theoretical consideration of venous blood return and modified Poiseuille’s law (equation 1.1b and 1.1c). MCP is mean circulatory pressure, RAP is right atrial pressure, Rv and Ra are vein and arterial resistance, η is viscosity, ΔP is pressure difference, P is pressure, L is length, E is incremental elastic modulus, h is wall thickness.
(1.1b)
(1.1c)
Shear rate (Sr) was calculated according to equation 1.2, where Q is portal flow and r is portal radius:
(1.2)
Portal cross sectional area (CSA) was calculated with regard to equation 1.3, where D is portal diameter:
(1.3)
RI represent portal resistance index and it was estimated by using maximal and minimal blood flow velocity (Vmax and Vmin) according to the formula 1.4:
(1.4)
The inlet length (L) was taken as:
(1.5)
Expected (ideal) portal vein flow ratio (Fideal) with regard to Poiseuille’s equation was calculated by using equation 1.6. We made the assumption that α is 1. α is ratio of the viscosity, Dt is diameter in 10th or 15th min of test and D0 represents initial diameter.
(1.6)
Pressure ratio (ΔPratio) was calculated according to equation 1.7. Qt is portal blood flow in the 10th or 15th min of test, Q0 is basal portal flow, while Dt and D0 are portal vein diameters in the same time intervals apparently.
(1.7)
We used equation 1.8 to approximately assess portal vein pressure difference between 10th min of the test and basal pressure with regard to Bernoulli’s rule. In the following equation Vmean0 and Vmeant are the initial and 10th or 15th min mean blood flow velocity. ρ is the density of blood with assumption that it’s value is 1060 kg/m3.
(1.8)
Portal vein flow ratio (F) was calculated with regard to experimentally obtained portal vein flow values:
Q10/Q0 and Q15/Q0 (1.9)
Descriptive data were expressed as means ± SEM. The significance of difference between the two groups was assessed by Student’s t-test, while differences between parameters in different time measurements were assessed by analysis of variance test with repeated measures and paired samples t-test as post-hoc. Statistical analysis of interobserver agreement for quantitative variables (D, Vmax, Vmin) was performed using the intraclass correlation coefficient. Results were interpreted as poor (< 0.04), regular (0.41-0.75) or excellent (> 0.76). P values < 0.05 were considered significant. All statistical analysis was performed using the SPSS (version 15).
Demographic and clinical characteristics of the study population are presented in Table 1.
| Clinical parameters | Patients(n = 39) | Control(n = 30) | P value |
| Average age (yr) (mean ± SE) | 54.8 ± 1.3 | 42.5 ± 0.9 | < 0.01 |
| Gender | |||
| Male | 34 (87.1) | 17 (56.7) | < 0.01 |
| Female | 5 (12.9) | 13 (43.3) | < 0.01 |
| Body mass (kg ± SE) | 72.5 ± 2.0 | 72.8 ± 2.6 | NS |
| Height (cm ± SEM) | 170.4 ± 1.4 | 171.1 ± 2.1 | NS |
| Body mass index (mean ± SE) | 41.9 ± 1.2 | 42.3 ± 1.3 | NS |
| Diagnosis | < 0.01 | ||
| Cirrhosis hepatis | 38 (97.4) | 0 (0) | |
| Hepatitis B | 1 (2.6) | 0 (0) | |
| Diameter liver (mm ± SE) | 168.4 ± 3.1 | 147.3 ± 2.2 | < 0.01 |
| Ascites | < 0.01 | ||
| Yes | 4 (10.3) | 0 (0) | |
| No | 35 (89.7) | 30 (100) | |
| Varices esophagi | < 0.01 | ||
| Yes (Grade III) | 3 (7.7) | 0 (0) | |
| No | 36 (92.3) | 30 (100) | |
| AST (U/I ± SEM) | 90.5 ± 15.5 | 21.4 ± 1.4 | < 0.01 |
| ALT (U/I ± SEM) | 45.3 ± 8.6 | 24.1 ± 1.5 | < 0.01 |
| GGT (U/I ± SEM) | 267.5 ± 41.6 | 35.6 ± 1.6 | < 0.01 |
| AST | < 0.01 | ||
| Increased | 25 (64.1) | 0 (0) | |
| Normal | 14 (35.9) | 30 (100) | |
| ALT | < 0.01 | ||
| Increased | 12 (30.7) | 0 (0) | |
| Normal | 27 (69.3) | 30 (100) | |
| GGT | < 0.01 | ||
| Increased | 22 (56.4) | 0 (0) | |
| Normal | 17 (43.6) | 30 (100) | |
| Total bilirubin (μmol/L ± SE) | 43.3 ± 8.0 | 18.6 ± 0.8 | < 0.01 |
| Direct bilirubin (μmol/L ± SE) | 18.2 ± 3.9 | 2.9 ± 0.2 | < 0.01 |
| Total bilirubin | < 0.01 | ||
| Increased | 25 (64.1) | 0 (0) | |
| Normal | 14 (35.9) | 30 (100) | |
| Direct bilirubin | < 0.01 | ||
| Increased | 29 (74.4) | 0 (0) | |
| Normal | 10 (25.6) | 30 (100) |
Investigated haemodynamic and biomechanical parameters of patients and controls before and after GTN administration are summarized in Table 2.
| Parameters | Groups | 0 min | P value | 10 min | P value | 15 min | P value |
| D (mm) | Patients | 11.60 ± 0.32 | < 0.01 | 13.81 ± 0.31b | < 0.01 | 13.63 ± 0.33b | < 0.01 |
| Controls | 9.19 ± 0.33 | 11.02 ± 0.34b | 11.72 ± 0.37b | ||||
| CSA (cm2) | Patients | 1.08 ± 0.06 | < 0.01 | 1.52 ± 0.69b | < 0.01 | 1.49 ± 0.70b | < 0.01 |
| Controls | 0.68 ± 0.05 | 1.09 ± 0.06b | 1.11 ± 0.06b | ||||
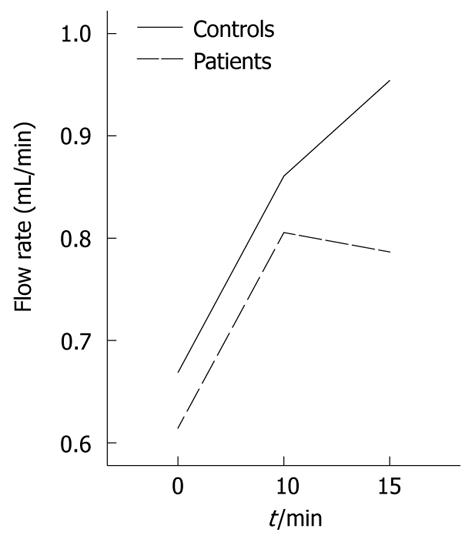
| Q (l/min) | Patients | 0.61 ± 0.05 | NS | 0.80 ± 0.07b | NS | 0.78 ± 0.71b | < 0.05 |
| Controls | 0.66 ± 0.06 | 0.86 ± 0.05b | 0.95 ± 0.08b | ||||
| Sr (1/s) | Patients | 66.50 ± 3.91 | < 0.01 | 49.85 ± 3.07b | < 0.01 | 51.71 ± 3.30b | < 0.01 |
| Controls | 139.87 ± 5.25 | 111.29 ± 5.57b | 83.83 ± 5.58b | ||||
| Vmean (cm/s) | Patients | 9.39 ± 0.50 | < 0.01 | 8.55 ± 0.55a | < 0.01 | 8.69 ± 0.57 | < 0.01 |
| Controls | 15.63 ± 0.48 | 13.13 ± 0.44b | 14.34 ± 0.54b | ||||
| Vmax (cm/s) | Patients | 10.60 ± 0.57 | < 0.01 | 9.77 ± 0.65b | < 0.01 | 9.84 ± 0.62b | < 0.01 |
| Controls | 19.31 ± 0.56 | 16.51 ± 0.51b | 18.03 ± 0.69b | ||||
| Vmin (cm/s) | Patients | 8.18 ± 0.43 | < 0.01 | 7.34 ± 0.48b | < 0.01 | 7.53 ± 0.53b | < 0.01 |
| Controls | 11.96 ± 0.48 | 9.75 ± 0.46b | 10.65 ± 0.47b | ||||
| RI | Patients | 0.22 ± 0.01 | < 0.01 | 0.24 ± 0.01 | < 0.01 | 0.24 ± 0.01 | < 0.01 |
| Controls | 0.37 ± 0.02 | 0.41 ± 0.02 | 0.40 ± 0.01 | ||||
| L (cm) | Patients | 13.69 ± 1.12 | NS | 17.95 ± 1.68b | NS | 17.52 ± 1.58b | NS |
| Controls | 15.08 ± 1.56 | 17.00 ± 1.56a | 21.26 ± 1.78b | ||||
| Fideal | Patients | / | / | 2.32 ± 0.21d | NS | 2.11 ± 0.17d | < 0.05 |
| Controls | / | 2.54 ± 0.34d | 3.38 ± 0.51d | ||||
| ΔPratio | Patients | / | / | 0.66 ± 0.03 | NS | 0.68 ± 0.03 | < 0.05 |
| Controls | / | 0.68 ± 0.04 | 0.49 ± 0.04 | ||||
| F | Patients | / | / | 1.34 ± 0.07 | NS | 1.31 ± 0.06 | NS |
| Controls | / | 1.45 ± 0.10 | 1.48 ± 0.08 | ||||
| ΔP (mmHg) | Patients | / | / | 0.004 ± 0.003 | < 0.01 | 0.005 ± 0.003 | < 0.05 |
| Controls | / | 0.029 ± 0.006 | 0.014 ± 0.003e |
Groups significantly differed in the majority of investigated parameters in all three times of measurement.
Following GTN administration, the majority of portal vein vascular mechanic and hemodynamic parameters reached a maximum after 10 min in both groups and then slightly changed in the opposite direction during the last 5 min of the performed test. Figure 1 illustrates the effects of GTN administration on mean portal flow.
Vasodilatation after GTN administration was associated with a significant difference between obtained and ideal portal flow rate ratio in patients both in 10th and 15th min of the test. However, there was no significant difference in F between the controls and patients.
In the 15th min of the test, the decrease of the pressure ratio in controls resulted in significant differences in its values between controls and patients and this phenomenon is in concordance with the decrease in hydrodynamic pressure in controls but still significantly higher hydrodynamic pressure in controls. Bernoulli’s equation was used with regard to significantly shorter portal vein (about 8 cm) when compared to the inlet length and therefore flat velocity profile. Shear rate observation could be of interest with regard to study of the boundary level and served to assess flow velocity profile. During our study velocity profile was flat, with a very thin boundary level. Table 3 shows data (diameter, maximal and minimal blood flow velocity) measured by three independent observers. Interobserver agreement evaluated by the intraclass correlation coefficient showed excellent and regular results for the quantitative variables as shown in Table 3.
| Parameters | Groups | 0 min | 10 min | 15 min | |||||||||
| I | II | III | IA | I | II | III | IA | I | II | III | IA | ||
| D (mm) | Patients | 11.47 ± 0.31 | 11.70 ± 0.33 | 11.59 ± 0.31 | 0.84 | 13.79 ± 0.31 | 13.86 ± 0.32 | 13.80 ± 0.31 | 0.89 | 13.61 ± 0.33 | 13.65 ± 0.35 | 13.64 ± 0.32 | 0.95 |
| Controls | 9.22 ± 0.32 | 9.19 ± 0.34 | 9.22 ± 0.32 | 0.96 | 11.07 ± 0.35 | 10.91 ± 0.29 | 11.00 ± 0.35 | 0.84 | 11.80 ± 0.43 | 11.77 ± 0.39 | 11.55 ± 0.34 | 0.9 | |
| Vmax | Patients | 10.55 ± 0.53 | 10.99 ± 0.64 | 10.17 ± 0.50 | 0.83 | 9.80 ± 0.63 | 10.84 ± 0.72 | 8.94 ± 0.52 | 0.73 | 9.91 ± 0.64 | 10.01 ± 0.62 | 9.52 ± 0.59 | 0.79 |
| (cm/s) | Controls | 19.74 ± 0.56 | 19.11 ± 0.52 | 19.07 ± 0.61 | 0.80 | 17.11 ± 0.53 | 16.44 ± 0.48 | 16.02 ± 0.50 | 0.79 | 18.11 ± 0.72 | 18.77 ± 0.81 | 17.43 ± 0.55 | 0.75 |
| Vmin | Patients | 8.11 ± 0.40 | 8.59 ± 0.51 | 7.73 ± 0.36 | 0.76 | 7.48 ± 0.51 | 7.22 ± 0.50 | 7.32 ± 0.44 | 0.83 | 7.68 ± 0.50 | 7.90 ± 0.59 | 7.01 ± 0.51 | 0.79 |
| (cm/s) | Controls | 12.12 ± 0.55 | 12.26 ± 0.60 | 11.46 ± 0.34 | 0.79 | 9.86 ± 0.48 | 9.89 ± 0.48 | 9.55 ± 0.42 | 0.84 | 10.89 ± 0.55 | 10.22 ± 0.43 | 10.78 ± 0.40 | 0.81 |
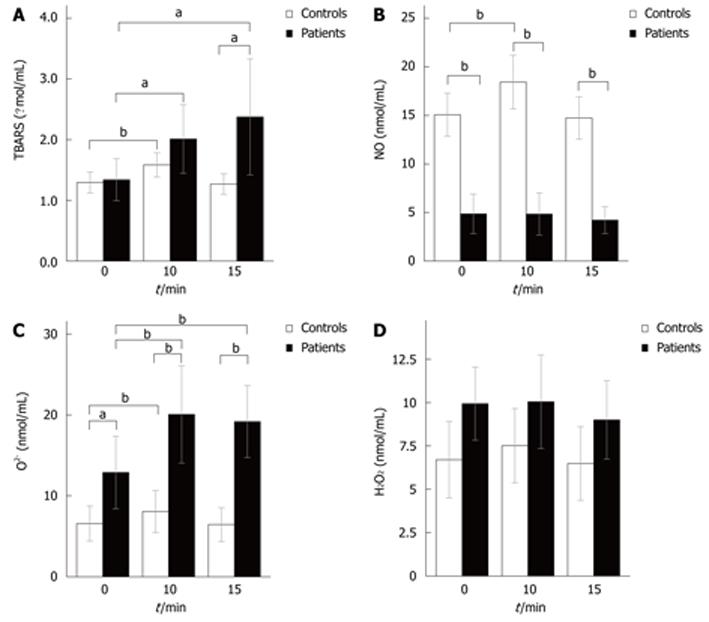
The dynamics of oxidative stress parameters in patients and controls can be seen in Figures 2A-D. At rest, significant differences between patients and controls were observed in levels of O2- (12.88 ± 2.24 nmol/mL vs 6.57 ± 1.07 nmol/mL, P < 0.05) and NO (4.85 ± 1.01 nmol/mL vs 15.06 ± 1.11 nmol/mL, P < 0.01). The same situation was observed 10 min after GTN administration, while in 15th min of the test patients experienced a significant rise in the level of the index of lipid peroxidation (2.37 ± 0.47 μmol/L vs 1.26 ± 0.08 μmol/L, P < 0.05), so groups now differed in three oxidative stress parameters.
GTN administration induced a significant rise in NO levels only in controls (NO levels were increased in the 10th min), but their NO levels returned to the basal values 15 min after GTN administration. The responses of O2- and TBARS to GTN administration were similar: 10 min after GTN administration levels of O2- and TBARS were elevated in both groups. In the 15th min of the test, controls’ O2- and TBARS levels decreased to the levels similar to that in rest, while patients experienced further increases in the level of the index of lipid peroxidation and their O2- levels remained elevated. Hydrogen peroxide did not change significantly throughout the study.
Endothelium-independent vasodilatation observed after GTN administration was caused by smooth muscle relaxation of the portal vein. Portal vein diameter rose significantly throughout the study and concomitantly statistically significant enhancement in portal blood flow (15th min) appear to be in concordance with equation 1.1b. With regard to equation 1.1b it is clear that small changes in vein resistance lead to huge blood flow changes, whereas the influence of arterial resistance is dampened due to high value of capacitance. However, there was no statistically significant difference in portal flow rate between the groups in the first ten minutes of the test, moreover flow rate was higher in controls compared to patients. In controls portal flow rate rose continuously compared to a decreasing pattern in patients after the 10th min of the test. One possible explanation for the lower flow rate in patients vs controls is increased incremental elastic modulus (with regard to equation 1.1c) and consequent leftward shift of the pressure-volume curve. However, in patients we could expect a parallel shift of the pressure-volume curve. The latter does not imply changes in compliance. It is of interest to emphasize hyperdynamic circulation in patients with liver cirrhosis. During the first 10 min, average blood flow velocity decreases simultaneously in both groups due to higher increases in portal cross-sectional area compared to flow rate. Our study disclosed significantly reduced mean blood flow velocity during the first 10 min of the test due to discrepancies between blood flow and CSA. Table 2 shows that blood flow velocity parameters were significantly higher in controls than in patients.
A similar pattern was obtained by using ideal and measured portal blood flow values. The ideal (expected) value was calculated by using equation 1.6 with regard to assumption of the equality of blood viscosity during the study. If the assumption about the equality viscosity is true, then α = 1. Nevertheless, a significantly reduced shear rate in patients after GTN administration (49.85 ± 3.07 vs 66.50 ± 3.91) led to a nonlinear correlation with shear stress, so α might be different in comparision to the ideal value. In the 10th min of the test, expected (ideal) flow ratio rose up to 2.32 in patients and 2.54 in controls, while at the same time obtained flow ratio (F) was 1.34 vs 1.45 and both these differences were not statistically significant between groups. At the same time ideal flow ratio was significantly higher in both groups compared to the experimentally obtained flow ratio. However, ideal flow ratio was significantly higher in controls vs patients in the 15th min of test. High compliance does not reflect good elastic properties of vein, as one could presume, it rather reflects a change in geometry[20]. At low pressure the vein’s cross section is ellipsoidal and every rise in pressure causes the vein to become more circular, without change in diameter, but with a great increase in cross-sectional area the vein becomes fully rounded and concomitantly the flow velocity decreases due to an inverse relationship with CSA. At higher steady state pressure, every further rise in pressure causes changes in diameter.
This concept is very similar to the observations made in our study. Table 2 shows a significant difference between the diameter in patients vs controls after GTN administration. This is, together with mentioned changes in viscoelastic properties (increased elastic modulus) and observed low shear rate in patients, a possible explanation for the proposed “double paradoxes”[21], phenomenon seen in cirrhotic patients, due to obviously increased intrahepatic resistance as a consequence of elevated vasoconstrictor levels. At the same time increased systemic vasodilatation dampened responses to endogenous vasoconstrictors, and the overall effect was markedly increased systemic blood flow. Under physiological conditions vein capacitance does not allow any apparent decline in venous system pressure[22]. However, we assumed that under physiological conditions, increased flow rate (volume overload) due to GTN administration means the portal vein wall is still working in less steep part of the tension-volume curve which further leads to a significant hydrodynamic pressure drop in the control group (15th min). The net effect is lower resistance to pressure changes than we could expect, reflecting the inherently limited distensibility of the portal vein. On the contrary, in some liver diseases increased sinusoidal resistance is responsible for the parallel and leftward shift of the pressure-volume curve. The result of this phenomenon could be a significantly lower hydrodynamic pressure difference in patients vs controls and observed significant difference between the groups in 15th min of test with regard to pressure ratio. Indeed, it is obvious that the lower initial velocity in patients led to a significant difference in hydrodynamic pressure during the whole test.
The most interesting observation is lower pressure ratio in controls vs patients in the 15th min of the test, which suggested proposed mechanism of geometry and biomechanical changes in portal vein wall. Shear stress is in good linear correlation to the shear rate (equation 1.2) only in Newtonian fluid. The very low shear rate in patients, observed in our study (66.50 ± 24.14 1/s in basal conditions), rules out linear correspondence between these parameters. At low shear rates the apparent viscosity (η) increases markedly. Shear rate measured in the 10th min of the test was significantly lower than the basal value in both groups, while in controls shear rate was significantly higher compared to patients. Below a value of 2001/s the fluid behaviour is non Newtonian[23]. The significance of this observation was stated above in discussion about blood flow discrepancy (double paradoxes). Using the inlet length value, given previously in Table 2, the parabolic velocity profile would not be expected to show complete development, already held flat profile. The reason is a much shorter portal vein (usually 8 cm) compared to inlet length. We used equation 1.8 to approximately assess portal vein pressure difference between the beginning of the test and the 10th min of the test with regard to Bernoulli’s rule and Poiseuille’s equation. Bernoulli’s equation was used, as we mentioned above, due to greater unsheared region in the flat velocity profile and it could at least be useful for explaining pressure change.
Increased oxidative stress is a well-known condition in many diseases. Oxidative stress is defined as the tissue damage resulting from an imbalance between an excessive generation of oxidant compounds and insufficient anti-oxidant defence mechanisms[1]. Different cellular enzymes, including xanthine oxidase, cyclooxygenases, lipoxygenase, have been identified as cellular source of ROS.
NO: Nitric oxide, as we expected, did not change significantly in patients in our study. Controls had significantly higher levels of NO at rest, and GTN administration induced its significant increase, observed in the 10th min of the test. NO excessive synthesis might be possible due to mechanisms of flow mediated vasodilatation via opening of stretch-activated calcium channels and further intracellular calcium accumulation, which in turn stimulate NO production[24,25]. Increased NO synthesis is also expected in liver cirrhosis environment conditions. However, several mechanisms counteracted the flow-mediated increase in NO synthesis in patients: decreased shear stress induced NO synthesis inhibition; superoxide mediated peroxynitrite formation (superoxide was dramatically higher in patients verus controls). We propose that in portal hypertension, high pressure (P) mediated an exponentially decreased reaction rate constant (K2) of the ion channel, and altered gating properties of the channel[26]. This mechanism may be explained according to equation 2.0:
(2.0)
K2 represents the reaction rate constant at pressure P, k1 is the channel activation constant in basal condition, T is temperature. However, the pressure ratio is similar in both groups, so it is more likely to presume that increased peroxynitrite formation and extensive synthesis of asymmetric dimethyl arginine (ADMA), a potent NOS inhibitor[27], are involved in maintaining the same values of NO in patients. ADMA is downregulated and very much depends on the activity of the enzyme dimethyl-diamino-hydrolase (DDHA) which transforms ADMA into citrulline. Increased oxidative stress should be able to reduce the availability of NO, so counteracting excessive NO production in liver cirrhosis. The present data demonstrate that excessive NO synthesis seen in patients with liver cirrhosis might be significantly modified by several described mechanisms.
H2O2: Hydrogen peroxide is created in the reaction of superoxide anion and hydrogen cation but this reaction is too slow (K2 < 1.0 M-1S-1, t½ = 1 min) despite the high redox potential (0.89 V). Almost certainly the Fenton and Haber-Weiss reactions are essential for H2O2 disassembling. The total redox potential of Haber Weiss and Fenton reaction is 0.78 V, very close to redox potential of synthesis reaction (0.89 V), indicating equilibrium between these opposite reactions (probability that hydrogen peroxide will change it's value in this case is zero, see later) and giving a possible explanation for unexpected lack of changes in hydrogen peroxide values throughout the performed study. Haber-Weiss and Fenton reactions can be deleterious, giving rise to the formation of the highly reactive hydroxyl radical (OH.), which induces lipid peroxidation. The concentrations of hydrogen peroxide and superoxide prior to test were in approximately equimolar equilibrium (in controls O2-: 12.88 nmol/mL and H2O2: 9.93 nmol/mL; in patients O2-: 6.57 nmol/mL and H2O2: 6.70 nmol/mL). The possibility (P) of some reaction and it's correlation to the redox potentials difference (ΔU ≈ φ1 - φ2) is theoretically determined by using equation 2.1:
(2.1)
O2-: Superoxide basal value was markedly higher in the patients compared to controls, and this observation suggests increased oxidative stress in patients with chronic liver disease. Significantly higher initial nitric oxide levels in controls compared to patients may be due to a “mirror pattern” with superoxide. Taken overall, these findings suggest that superoxide is a good indicator of oxidative stress in patients with chronic liver disease.
Superoxide is produced in accordance to Hund's rule so the probability of it's formation is higher than the probability of reduction with two electrons. The redox potential of superoxide generation is 0.16 V. Superoxide was significantly higher in patients compared to controls during the entire test. There is the possibility, albeit not undisputed, that one major contributing factor is the diameter, as the larger diameter in patients vs controls created greater circumferential wall stress according to Laplace's law. Increased oxygen consumption, promoted by increased tension, leads to increased superoxide production. However, there are doubts about whether superoxide levels are in better correlation with shear stress and Voigt's model[28] compared to Laplace's law.
TBARS: Oxidative stress could firstly be evidenced by an increase in TBARS concentration. TBARS continuously increased significantly (compared to basal value) in patients during the test. Basal values did not differ between the groups, but a significant difference was revealed after GTN administration. Indeed, we assumed that increased production of superoxide interferes with increased lipid peroxidation and TBARS concentration.
In conclusion, our study showed that endothelium-independent vasodilatation leads to a significant increase in blood flow and significant decline of mean blood flow velocity and shear rate in participants with chronic liver disease. Increased blood flow was to some extent lower than expected, probably due to increased liver sinusoidal resistance and mentioned double paradoxes. We proved, as a consequence of decreased shear rate far below the critical value, non Newtonian behaviour of portal vein blood flow. Tentative changes in O2- and H2O2 levels revealed the crucial role of ROS as trigger factors of lipid peroxidation. The preceding findings are in coherence with our assumption of O2- and H2O2 mediated lipid peroxidation via Haber Weiss and Fenton reactions.
Our study could be of great clinical importance, especially regarding the role of oxidative stress in portal vein haemodynamics. This pilot study could be a starting point for further investigation and possible implementation of some antioxidants in the treatment of portal hypertension.
Oxidative stress is a condition in which the delicate balance that exists between prooxidant (free radicals) production and their subsequent amelioration via the antioxidant defense system becomes skewed in favor of free radical expression. The existence of an interference of increased oxidative stress with the vasodilative properties of veins is now a well known fact, resulting in endothelial dysfunction i.e., a loss of nitric oxide (NO) bioactivity in the vessel wall. We hypothesized that the endothelium independent vasodilatation of the portal vein induced by sublingual nitroglycerine administration would lead to increased oxidative stress and significant changes in portal hemodynamics.
Nitroglycerine is one of the most often used drugs in the treatment of vascular diseases. In some previous studies it was shown that nitroglycerine-induced vasodilation is impaired in some vascular diseases which points to the possible use of that drug in the diagnosis of different vascular diseases.
In previous investigations, application of nitroglycerine in order to test brachial artery reactivity showed that patients suffering from coronary artery disease exhibit loss of endothelium-independent (induced by nitroglycerine) as well as endothelium-dependent vasodilation (induced by short-term artery occlusion) compared to controls. Taking into consideration hyperdynamic portal vein circulation in patients suffering from portal hypertension it seems that some basic vascular mechanisms can correlate with other vascular diseases such as coronary artery disease. In concordance with that, the aim of our study was to determine how sublingual administration of nitroglycerine might be used for the evaluation of portal endothelium-independent vasodilatation through estimating parameters of portal vascular mechanics with special interest in oxidative stress in patients suffering from portal hypertension.
The study results suggest that nitroglycerine-induced vasodilation could be used as a potential new diagnostic test for evaluation of severity in portal hypertension.
Portal hypertension: Increase in blood pressure in the veins of the portal system caused by obstruction in the liver (often associated with alcoholic cirrhosis), causing enlargement of the spleen and collateral veins associated with regional hyperdynamic circulation; Nitroglycerine: Commonly used drug for treatment of vascular diseases, basically coronary artery disease, which acts by inducing endothelium-independent vasodilation; Oxidative stress: A condition in which the delicate balance that exists between prooxidant (free radicals) production and their subsequent amelioration via the antioxidant defense system becomes skewed in favor of free radical expression.
This is an original article, important for the development of the field of study.
Peer reviewer: Eduardo Garcia Vilela, Professor, PhD, Department of Internal Medicine, Faculty of Medicine, Federal University of Minas Gerais, Avenida Professor Alfredo Balena, 190/2 andar, Belo Horizonte 30130-100, Brazil
S- Editor Tian L L- Editor O’Neill M E- Editor Zhang DN
| 1. | Halliwell B, Cross CE. Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect. 1994;102 Suppl 10:5-12. [PubMed] [DOI] [Full Text] |
| 2. | Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840-844. [PubMed] |
| 3. | Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 967] [Cited by in RCA: 985] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 4. | Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1142] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 5. | Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8502] [Cited by in RCA: 9063] [Article Influence: 453.2] [Reference Citation Analysis (0)] |
| 6. | Harrison DG. Endothelial function and oxidant stress. Clin Cardiol. 1997;20:II-11-II-17. [PubMed] |
| 7. | Cahill PA, Redmond EM, Sitzmann JV. Endothelial dysfunction in cirrhosis and portal hypertension. Pharmacol Ther. 2001;89:273-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (. Oxf). 2009;196:193-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 561] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 9. | Moyna NM, Thompson PD. The effect of physical activity on endothelial function in man. Acta Physiol Scand. 2004;180:113-123. [PubMed] |
| 10. | Mittal MK, Gupta TK, Lee FY, Sieber CC, Groszmann RJ. Nitric oxide modulates hepatic vascular tone in normal rat liver. Am J Physiol. 1994;267:G416-G422. [PubMed] |
| 11. | Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol. 1980;239:H14-H21. [PubMed] |
| 12. | Pizcueta P, Piqué JM, Fernández M, Bosch J, Rodés J, Whittle BJ, Moncada S. Modulation of the hyperdynamic circulation of cirrhotic rats by nitric oxide inhibition. Gastroenterology. 1992;103:1909-1915. [PubMed] |
| 13. | Bomzon A, Huang YT. Vascular smooth muscle cell signaling in cirrhosis and portal hypertension. Pharmacol Ther. 2001;89:255-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Quilley J, Fulton D, McGiff JC. Hyperpolarizing factors. Biochem Pharmacol. 1997;54:1059-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Yang W, Benjamin IS, Alexander B. Localisation of hepatic vascular resistance sites in the isolated dual-perfused rat liver. Eur J Pharmacol. 1999;364:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131-138. [PubMed] |
| 17. | Auclair C, Voisin E. Nitroblue tetrazolium reduction. Handbook of methods for oxygen radical research. Florida: CRC Press, Boca Raton 1985; 123-132. |
| 18. | Pick E, Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 869] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 19. | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17627] [Cited by in RCA: 19194] [Article Influence: 408.4] [Reference Citation Analysis (1)] |
| 20. | Boron WF, Boulpaep EL. Medical physiology. Philadelphia: Elsevier Saunders 2005; 456. |
| 21. | Djuric D, Knezevic S, Stojkovic M, Jakovljevic V. Acute effects of L-Arginine and nitroglycerine in patients with portal hypertension. Vaskulare inflammation und endotheliale dysfunktion. Tübingen: Deutsche Gesellschaft für Arterioskleroseforschung 2004; 104-116. |
| 22. | Berne RM, Levy MN. Physiology. 4th ed. St. Louis: Mosby 1998; 458-477. |
| 23. | Ronco C, Ghezzi PM, Brendolan A, Crepaldi C, La Greca G. The haemodialysis system: basic mechanisms of water and solute transport in extracorporeal renal replacement therapies. Nephrol Dial Transplant. 1998;13 Suppl 6:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519-560. [PubMed] |
| 25. | Hoyer J. Endothelial vasoregulation and mechanosensitive ion channels in hypertension. Nephrol Dial Transplant. 1997;12:6-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Withers PC. Comparative animal physiology. Fort Worth: Saunders College Publishing 1992; 220. |
| 27. | Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C, Zoccali C. Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transplant. 2003;18:1272-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 511] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 28. | Nichols WW, O`Rourke MF. Mc Donald`s blood flow in arteries: theoretical, experimental and clinical principles. 4th ed. New York: Oxford University Press 1998; 61. |