Published online Oct 21, 2012. doi: 10.3748/wjg.v18.i39.5622
Revised: April 28, 2012
Accepted: May 5, 2012
Published online: October 21, 2012
AIM: To assess the safety and efficacy of carbon dioxide (CO2) insufflation during endoscopic retrograde cholangiopancreatography (ERCP).
METHODS: The Cochrane Library, Medical Literature Analysis and Retrieval System Online, Excerpta Medica Database, Science Citation Index Expanded, Chinese Biomedical Literature Database, and references in relevant publications were searched up to December 2011 to identify randomized controlled trials (RCTs) comparing CO2 insufflation with air insufflation during ERCP. The trials were included in the review irrespective of sample size, publication status, or language. Study selection and data extraction were performed by two independent authors. The meta-analysis was performed using Review Manager 5.1.6. A random-effects model was used to analyze various outcomes. Sensitivity and subgroup analyses were performed if necessary.
RESULTS: Seven double-blind RCTs involving a total of 818 patients were identified that compared CO2 insufflation (n = 404) with air insufflation (n = 401) during ERCP. There were a total of 13 post-randomization dropouts in four RCTs. Six RCTs had a high risk of bias and one had a low risk of bias. None of the RCTs reported any severe gas-related adverse events in either group. A meta-analysis of 5 RCTs (n = 459) indicated that patients in the CO2 insufflation group had less post-ERCP abdominal pain and distension for at least 1 h compared with patients in the air insufflation group. There were no significant differences in mild cardiopulmonary complications [risk ratio (RR) = 0.43, 95% CI: 0.07-2.66, P = 0.36], cardiopulmonary (e.g., blood CO2 level) changes [standardized mean difference (SMD) = -0.97, 95% CI: -2.58-0.63, P = 0.23], cost analysis (mean difference = 3.14, 95% CI: -14.57-20.85, P = 0.73), and total procedure time (SMD = -0.05, 95% CI: -0.26-0.17, P = 0.67) between the two groups.
CONCLUSION: CO2 insufflation during ERCP appears to be safe and reduces post-ERCP abdominal pain and discomfort.
- Citation: Cheng Y, Xiong XZ, Wu SJ, Lu J, Lin YX, Cheng NS, Wu TX. Carbon dioxide insufflation for endoscopic retrograde cholangiopancreatography: A meta-analysis and systematic review. World J Gastroenterol 2012; 18(39): 5622-5631
- URL: https://www.wjgnet.com/1007-9327/full/v18/i39/5622.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i39.5622
Endoscopic retrograde cholangiopancreatography (ERCP) refers to radiographic visualization of the pancreatobiliary system by retrograde injection of contrast media through the ampulla of Vater[1]. ERCP, which was first introduced in the late 1960s[2], is now widely performed by endoscopists to diagnose various pancreatic and biliary diseases[1,3]. Although the exact number of patients undergoing ERCP worldwide each year is unknown, it has been reported that approximately 54 000 and 500 000 patients undergo ERCP annually in the United Kingdom and United States, respectively[4-6]. ERCP has become an invaluable tool in the diagnosis of numerous pancreatic and biliary diseases and is considered to be the gold standard study for the pancreatobiliary system[1,3-5].
Gas is deliberately insufflated into the bowel lumen during ERCP to provide operating space to ensure adequate visualization by the camera and manipulation of instruments in the duodenum[1-4]. Currently, air is the most commonly used gas for insufflation during ERCP worldwide[6]. However, air has some potential disadvantages. Air is not absorbed by the bowel and must be passed from the gastrointestinal tract in the form of flatus, which may lead to post-ERCP abdominal pain and discomfort (e.g., abdominal distension) because of gas retention in the gastrointestinal tract[7]. Recently, carbon dioxide (CO2) has been introduced as an alternative to air for insufflation during ERCP[8-14].
CO2 is rapidly absorbed from the bowel and is delivered directly to the lungs by the circulation[15,16]. Ultimately, it is excreted by the lungs during respiratory exchange[15,16]. Theoretically, CO2 has the potential to reduce post-ERCP abdominal pain and discomfort due to lower gas retention[15,16]. However, the absorption of CO2 may cause hypercapnia and acidosis, which must be prevented through hyperventilation[17]. The absorption of CO2 may be associated with various cardiopulmonary side effects such as tachycardia, cardiac arrhythmias, hypoxemia, and pulmonary edema[17]. Elderly patients with cardiopulmonary diseases are more likely to suffer from these adverse events[9,17].
The use of CO2 insufflation during ERCP is controversial. Some authors suggest that the application of CO2 insufflation reduces post-ERCP abdominal pain and discomfort[8,11,12,14], thus they recommend the use of CO2 in ERCP, whereas others do not think so[9,10,13]. To date, we have been unable to identify any meta-analysis that assesses the role of carbon dioxide insufflation during ERCP. We conducted a meta-analysis and systematic review to assess the safety and efficacy of CO2 insufflation during ERCP for reduction of post-ERCP abdominal pain and discomfort.
We searched the following databases up to December 2011 to identify randomized controlled trials (RCTs): The Cochrane Library, Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica Database (EMBASE), Science Citation Index Expanded, and Chinese Biomedical Literature Database (CBM). Search strategies for these databases are shown in Table 1. We also hand searched the references in relevant publications to explore additional relevant clinical trials. After completing all searches, we merged the search results using the software package Endnote X4 (reference management software) and removed duplicated records. Two independent authors (Lin YX and Xiong XZ) scanned the title and abstract of every record identified by the searches for inclusion. If compliance with inclusion criteria was not clear from the abstract, we retrieved full texts for further assessment. Only RCTs comparing CO2 insufflation with air insufflation during ERCP were considered for the review, irrespective of sample size, publication status, or language. Two independent authors (Wu SJ and Lu J) independently extracted and confirmed the data and entered them into an electronic data collection form. We resolved all differences between authors by discussion.
| Databases | Period of search | Search strategies |
| The Cochrane library | Until 1st December 2011 | 1. MeSH descriptor Cholangiopancreatography, Endoscopic Retrograde explode all trees |
| 2. (endoscopic retrograde cholangiopancreatograph*): ti, ab, kw OR (ERCP): ti, ab, kw | ||
| 3. MeSH descriptor Carbon Dioxide explode all trees | ||
| 4. (carbon dioxide): ti, ab, kw OR (CO2): ti, ab, kw | ||
| 5. 1 OR 2 | ||
| 6. 3 OR 4 | ||
| 7. 5 AND 6 | ||
| MEDLINE via PubMed | Until 1st December 2011 | 1. “Cholangiopancreatography, Endoscopic Retrograde” [MeSH] OR endoscopic retrograde cholangiopancreatograph* [tiab] OR ERCP [tiab] |
| 2. “Carbon Dioxide” [Mesh] OR carbon dioxide [tiab] OR CO2 [tiab] | ||
| 3. 1 AND 2 | ||
| EMBASE via embase.com | Until 1st December 2011 | 1. ‘endoscopic retrograde cholangiopancreatography’/exp OR ‘endoscopic retrograde cholangiopancreatography’ |
| 2. ‘ercp’/exp OR ercp | ||
| 3. ‘carbon dioxide’/exp OR ‘carbon dioxide’ | ||
| 4. ‘CO2’/exp OR CO2 | ||
| 5. 1 OR 2 | ||
| 6. 3 OR 4 | ||
| 7. 5 AND 6 | ||
| Science citation index expanded | Until 1st December 2011 | 1. TS = (‘endoscopic retrograde cholangiopancreatograph*’ OR ERCP) |
| 2. TS = (‘carbon dioxide’ OR CO2) | ||
| 3. 1 AND 2 | ||
| CBM | Until 1st December 2011 | Search strategy in Chinese. Includes search terms similar to the terms used in MEDLINE |
Data for the following outcomes were extracted: abdominal pain (pain scores via visual analogue scale and number of pain-free patients at various time points after ERCP), abdominal distention, gas-related complications (severe gas-related adverse events and mild cardiopulmonary complications), cardiopulmonary changes (heart rate, blood pressure, blood pH, etc.), cost analysis, and total procedure time.
Two authors (Cheng Y and Cheng NS) independently assessed the methodological quality of the included trials using the quality checklist recommended by the Cochrane Handbook[18]. We assessed the risk of bias of the trials based on the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias[18]. Following the evaluation of the above domains, an included trial was judged as a trial with a low risk of bias if it was evaluated as “low” in all of the above domains. If the risk of bias was judged as “unclear” or “high”, then the trial was listed under the group of trials with “high risk of bias”. We resolved all disagreements by discussion and referral to a third author (Wu TX) for adjudication.
We performed the meta-analysis using the software package Review Manager 5.1.6. Two authors (Wu SJ, Lu J) confirmed and entered all data into Review Manager independently. For dichotomous outcomes, we calculated the risk ratio (RR) with 95% CI[19]. For continuous outcomes, we calculated the mean difference (MD) with 95% CI[19]. For continuous outcomes with different measurement scales in different RCTs, we calculated the standardized mean difference (SMD) with 95% CI[19]. We described the heterogeneity with the Chi-squared test[19]. A P value less than 0.10 was considered to be significant heterogeneity[19]. We also used the I2 statistic to measure the quantity of heterogeneity[19]. For all analysis, we employed the random-effects model. We intended to perform funnel plots and assessed their visual asymmetry to determine reporting biases[20]. In case of missing data, we contacted the original investigators to request further information. If there was no reply, we performed the analysis on an “intention-to-treat” (ITT) principle, if applicable[21]. Otherwise, we adopted the available-case analysis (also known as per-protocol analysis and PP analysis). We conducted the meta-analysis and systematic review according to the Cochrane Handbook for Systematic Reviews of Interventions[22] and Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA)[23].
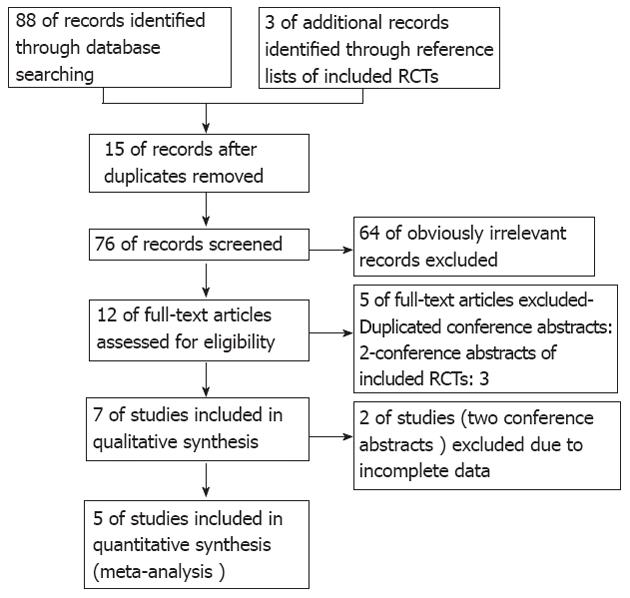
We identified a total of 88 records through electronic searches of The Cochrane Library (n = 4), MEDLINE (n = 16), EMBASE (n = 47), Science Citation Index Expanded (n = 20), Chinese Biomedical Literature Database (n = 1), and hand search of the references of the included RCTs (n = 3). We excluded 15 duplicates and 64 clearly irrelevant records by reading titles and abstracts. Twelve full-text articles were retrieved for further assessment. We excluded five articles for the reasons listed in Figure 1.
Seven RCTs (5 articles[8-12] and 2 conference abstracts[13,14]), which were published between 2007 and 2011, were identified that fulfilled the inclusion criteria. A total of 818 patients were included: 404 were assigned to the CO2 group and 401 were allocated to the air group (13 post-randomization dropouts). Details on the included studies are shown in Table 2.
| Author | Year | Country | Study design | Participants | Participants (CO2/Air) | Mean age (CO2/Air) |
| Bretthauer et al[8] | 2007 | Norway | Multi-centers | Low risk | 118 (58/58) | 57/54 |
| Maple et al[12] | 2009 | United States | Single-center | Low risk | 105 (50/50) | 57/51.7 |
| Dellon et al[9] | 2010 | United States | Single-center | High risk and low risk | 78 (36/38) | 60.1/59.7 |
| Kuwatani et al[10] | 2011 | Japan | Multi-centers | Low risk | 80 (40/40) | 66.1/68.7 |
| Luigiano et al[11] | 2011 | Italy | Single-center | Low risk | 78 (39/37) | 66.1/67.1 |
| Mei et al[13] | 2011 | Australia | Single-center | Not mentioned | 61 (34/27) | Not mentioned |
| Arjunan et al[14] | 2011 | India | Single-center | Low risk | 298 (147/151) | Not mentioned |
The risk of bias is summarized in Table 3. Six RCTs[8,10-14] had a high risk of bias and one RCT[9] had a low risk of bias. All trials were double-blind RCTs with a parallel group study design. There were a total of 13 post-randomization dropouts in four RCTs[8,9,11,12] which were not included in the analysis. Only one RCT[9] reported costs.
| Studies | Sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting |
| Bretthauer et al[8] | Low risk | Low risk | Low risk | Low risk | High risk | High risk |
| Maple et al[12] | Low risk | Low risk | Low risk | Low risk | High risk | High risk |
| Dellon et al[9] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Kuwatani et al[10] | Low risk | Unclear risk | Low risk | Low risk | Low risk | High risk |
| Luigiano et al[11] | Low risk | Low risk | Low risk | Low risk | High risk | High risk |
| Mei et al[13] | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk | Unclear risk |
| Arjunan et al[14] | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk | Unclear risk |
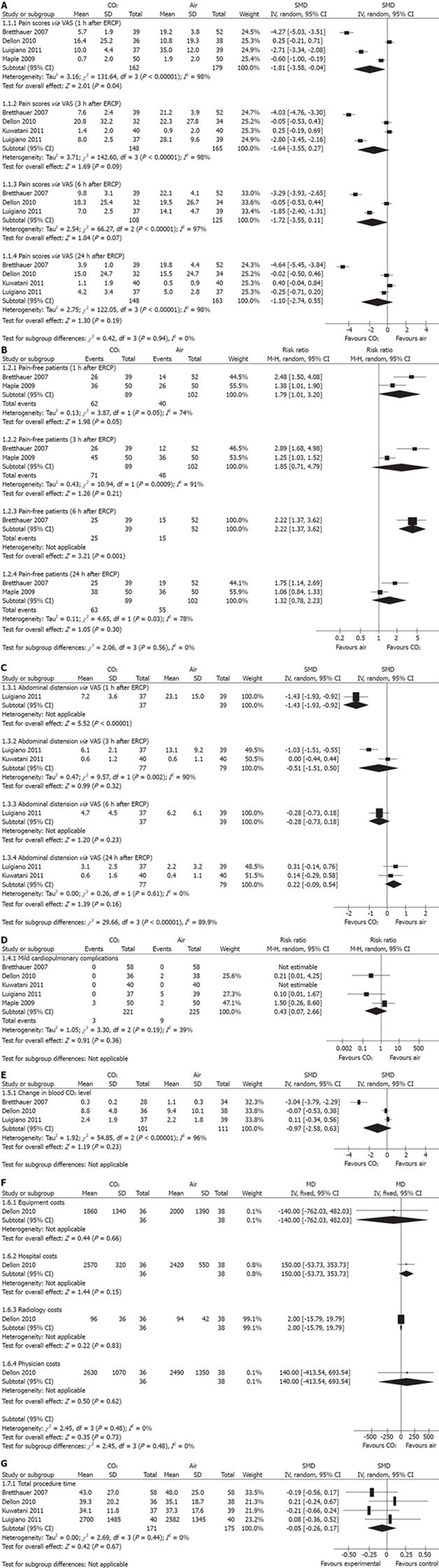
Abdominal pain scores (intensity of pain): Seven RCTs reported the abdominal pain scores. Pain was measured with a 10-point visual analogue scale (VAS)[10,12-14] or a 100-mm VAS[8-9,11] at various time points, including before the procedure, during the procedure, 30 min, 1 h, 90 min, 3 h, 6 h and 24 h after ERCP. Four RCTs[8,11,12,14] showed decreased post-ERCP abdominal pain scores in the CO2 group compared with the air group, while the other three RCTs[9-10,13] did not. The meta-analysis of 5 RCTs[8-12] showed that the abdominal pain scores 1 h after ERCP was significantly lower in the CO2 group than the air group (I2 = 98%; SMD = -1.81, 95% CI: -3.58--0.04, P = 0.04). The pain scores 3 h, 6 h and 24 h after ERCP were also lower in the CO2 group than in the air group, but the differences were not significant (Figure 2A).
Pain-free patients (incidence of pain): Only two RCTs[8,12] reported the number of pain-free patients at various time points, including 1, 3, 6 and 24 h after ERCP. The meta-analysis showed that the rate of pain-free patients 1 h and 6 h after ERCP was significantly higher in the CO2 group than in the air group: (I2 = 74%; RR = 1.79, 95% CI: 1.01-3.20, P = 0.05) and (RR = 2.22, 95% CI: 1.37-3.62, P = 0.001), respectively. The rate of pain-free patients 3 h and 24 h after ERCP were also higher in the CO2 group than in the air group, but the differences were not significant (Figure 2B).
Abdominal distention: Abdominal distension was measured with a 100-mm VAS in two RCTs[10,11]. The meta-analysis showed that abdominal distension 1 h after ERCP was significantly lower in the CO2 group than in the air group (SMD = -1.43, 95% CI: -1.93--0.92, P < 0.00001). There was no significant difference in abdominal distension between the two groups at 3 h, 6 h and 24 h after ERCP (Figure 2C). Three RCTs[9,12,14] reported the increase in abdominal girth. Maple et al[12] and Arjunan et al[14] stated that CO2 insufflation was associated with less increase in abdominal girth than air insufflation. However, Dellon et al[9] indicated that there was no significant difference between groups with regard to increase in abdominal girth. One RCT[8] reported the number of patients with bowel distention as assessed by X-ray photographs. There were fewer patients with bowel distention in the CO2 group than in the air group (RR = 0.76, 95% CI: 0.60-0.98, P = 0.03).
Complications: None of the RCTs reported any severe gas-related adverse events in either group (e.g., death, embolism, cardiac arrhythmias, and significant respiratory events). Mild cardiopulmonary complications (e.g., respiratory depression, hypotension, and bradycardia) were reported in five RCTs[8-12]. There was no significant difference in the number of patients with any mild cardiopulmonary complications between groups (I2 = 39%; RR = 0.43, 95% CI: 0.07-2.66, P = 0.36) (Figure 2D).
Cardiopulmonary changes: Changes in blood CO2 levels were reported in three RCTs[8,9,11]. There was no significant difference in the change in blood CO2 level between the two groups (I2 = 96%; SMD = -0.97, 95% CI: -2.58-0.63, P = 0.23). Only one RCT[10] reported blood oxygen saturation (SpO2); Kuwatani et al[10] stated that there was no significant difference between the two groups at any time point (Figure 2E).
Cost analysis: Only one RCT[9] reported the cost analysis. There were no significant differences in the total costs for ERCP (I2 = 0%; MD = 3.14, 95% CI: -14.57-20.85, P = 0.73), including equipment costs, hospital costs, radiology costs, and physician costs (Figure 2F).
Total procedure time: Four RCTs were included in the meta-analysis[8-11]. There was no significant difference in the total procedure time between the two groups (I2 = 0%; SMD = -0.05, 95% CI: -0.26-0.17, P = 0.67). Maple et al[12] stated that there was no difference in total procedure time between the two groups (Figure 2G).
This systematic review with meta-analysis of RCTs assessed the safety and efficacy of CO2 insufflation vs air insufflation during ERCP for reduction of abdominal pain and discomfort. The meta-analysis of 5 RCTs indicated that CO2 insufflation during ERCP was associated with less post-ERCP abdominal pain and distension for at least 1 h. There were no significant differences in mild cardiopulmonary complications, cardiopulmonary changes, cost analysis, and total procedure time between the two groups.
Post-ERCP abdominal pain and discomfort are common in clinical practice[24]. Abdominal pain related to insufflation is nonspecific and may mimic the symptoms of severe post-ERCP complications, including pancreatitis and perforation, which can be a source of stress to both patients and endoscopists[9]. Some patients need hospitalization for further evaluation and observation of post-ERCP abdominal pain and discomfort[7,15]. Post-ERCP abdominal pain and discomfort may result from air insufflation, as air is difficult to dissolve in blood and stays in the bowel for several hours after ERCP[7,16]. CO2 has unique characteristics in that it is cheap, colorless, nonflammable, non-explosive, easily excreted, and non-toxic to patients[16]. CO2 insufflation during ERCP was first introduced by Bretthauer et al[8] in 2007. Bretthauer et al[8] found that CO2 insufflation effectively reduced post-ERCP abdominal pain and discomfort and recommended its routine use in ERCP. Then the benefits of CO2 insufflation were further demonstrated by three later RCTs[11,12,14]. On the contrary, three additional RCTs[9,10,13] showed no differences in post-ERCP abdominal pain or discomfort between CO2 insufflation and air insufflation. Although this meta-analysis suggested that CO2 insufflation was associated with less abdominal pain and discomfort for at least 1 h, it included only 5 RCTs and all had small sample sizes. Consequently, the data from three ongoing RCTs performed by Mei et al[13], Arjunan et al[14] and Janssens et al[15] (Trial number: UMIN000005755) are anticipated to resolve the controversy.
The safety of CO2 insufflation during ERCP is another major concern for patients and endoscopists. Air insufflation is associated with rare but severe adverse events such as combustion when using electrocautery and gas embolisms[16,25]. CO2 is non-flammable and more soluble than air. In theory, CO2 insufflation is safer than air insufflation with regard to combustion and embolisms. CO2 is the most commonly used gas for insufflation in laparoscopy. The safety of CO2 insufflation for endoscopy has been well established in colonoscopy[16]. None of the included RCTs reported any severe CO2-related adverse events (e.g., death, embolism, cardiac arrhythmias, or significant respiratory events). With regard to other adverse effects from CO2 insufflation, the meta-analysis showed there were no differences in mild cardiopulmonary complications and cardiopulmonary changes between the two groups. However, all RCTs except one[9] excluded patients with chronic obstructive pulmonary diseases (COPD). The safety of CO2 insufflation during ERCP for high-risk patients (e.g., patients with cardiopulmonary diseases or American Society of Anesthesiologists (ASA) Physical Status classification III or IV) needs further evaluation.
There are other potential benefits of CO2 insufflation for ERCP, such as the possibility of immediate computed tomography cholangiopancreatography after ERCP, which has recently been introduced to obtain clear images, and the possibility of intraoperative ERCP during laparoscopy[16,26].
The feasibility of CO2 insufflation during ERCP is as follows. First, to date there have been three types of commercial CO2 insufflators available: Olympus KeyMed ECR, Olympus UCR, and CO2-EFFICIENT[15]. In addition, the cost of a CO2 insufflator is not very high, at approximately 7000 euros[15]. CO2 gas is inexpensive and convenient to obtain[15]. Dellon et al[9] found that the total costs for ERCP (including equipment costs, hospital costs, radiology costs, and physician costs), is similar between groups ($7170 in the CO2 group vs $7000 in the air group). Moreover, the safety of CO2 insufflation during ERCP has been well documented in the RCTs (see above). Thus, it appears that widespread implementation of CO2 insufflation during ERCP is anticipated.
This review included a total of seven RCTs. Most excluded patients with COPD, whereas only one study[9] included patients with COPD. Thus, the results of this review may only be relevant to low-risk patients without cardiopulmonary diseases.
The overall quality of the current best evidence was low. Only one RCT had a low risk of bias. There were a total of 13 post-randomization dropouts in four RCTs. All four RCTs adopted the available-case analysis (also known as per-protocol analysis and PP analysis) without performing an intension-to-treat (ITT) analysis. Only one RCT reported the cost analysis. Further RCTs are anticipated to perform both PP analysis and ITT analysis in case of post-randomization dropouts (missing data) and report the costs of ERCP.
Dellon et al[7] conducted a systematic review published in 2009 which assessed the role of CO2 insufflation for flexible sigmoidoscopy, colonoscopy, ERCP, and double-balloon enteroscopy. This review included nine RCTs, but only one used CO2 insufflation for ERCP. In addition, the authors did not perform a meta-analysis because of obvious heterogeneity. Our findings are similar to the previous systematic review in terms of the safety of CO2 insufflation. In contrast to our study, the previous systematic review showed a reduction in abdominal pain for at least 6 h in the CO2 insufflation group when compared with the air group.
The limitations of our review are as follows. The first concerns the small number of included RCTs and the small sample size of each RCT. We included only seven RCTs with 404 patients undergoing ERCP with CO2 insufflation; thus, there is a lack of available data on this issue to date. In addition, we did not perform funnel plots to assess the publication bias due to the small number of included RCTs.
This review currently provides the best available evidence for comparison of CO2 insufflation versus air insufflation during ERCP. On the basis of this evidence, CO2 insufflation during ERCP appears to be safe for the majority of patients and results in less post-ERCP abdominal pain and discomfort than air insufflation. Further RCTs with a low risk of bias and a greater number of patients are necessary to assess the role of CO2 insufflation in high-risk patients. Future RCTs need to be conducted and reported according to the Consolidated Standards of Reporting Trials (CONSORT) statement[27].
We would like to thank Jing-Qiu Cheng, Hui Ye, Jie Zhang, and Tian-Fu Wen, who assisted with the development of the review; and Rong-Xing Zhou, Yi-Lei Deng, and Yan-Wen Jin, who developed the search strategies and performed the literature searches.
Endoscopic retrograde cholangiopancreatography (ERCP) is now widely performed by endoscopists to diagnose various pancreatic and biliary diseases. Currently, air is the most commonly used gas for insufflation during ERCP worldwide. However, air has some potential disadvantages.
Recently, carbon dioxide (CO2) has been introduced as an alternative to air for insufflation during ERCP. Many studies, including randomized controlled trials (RCTs), have been conducted to assess the safety and efficacy of CO2 insufflation during ERCP for reduction of post-ERCP abdominal pain and discomfort. However, the use of CO2 insufflation during ERCP is controversial.
The authors identified all RCTs comparing CO2 insufflation with air insufflation during ERCP. They conducted a meta-analysis and systematic review according to the Cochrane Handbook for Systematic Reviews of Interventions and Preferred Reporting Items for Systematic reviews and Meta-Analysis. They found that CO2 insufflation during ERCP appears to be safe and reduces post-ERCP abdominal pain and discomfort.
Due to reduced post-procedure abdominal pain and discomfort, CO2 insufflation may be preferable to air insufflation during ERCP and should be recommended in clinical practice.
ERCP refers to radiographic visualization of the bile duct and pancreatic duct by retrograde injection of contrast media into the pancreatobiliary system; RCTs refer to trials in which people are allocated at random to receive one of several clinical interventions.
This is the first well-designed meta-analysis on the role of CO2 insufflation in ERCP. The results are interesting and suggest that CO2 insufflation may be preferable to air insufflation during ERCP. However, this article has several limitations, such as a small number of RCTs.
| 1. | Spangler CC, Gardner TB, Mukherjee S, Windle ML, Roberts KE. Endoscopic retrograde cholangiopancreatography. Available from: http: //emedicine.medscape.com/article/1829797-overview#showall. |
| 2. | McCune WS. ERCP--the first twenty years. Gastrointest Endosc. 1988;34:277-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Baron TH. Endoscopic retrograde cholangiopancreatography. Endoscopy. 2010;42:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Williams EJ, Taylor S, Fairclough P, Hamlyn A, Logan RF, Martin D, Riley SA, Veitch P, Wilkinson M, Williamson PJ. Are we meeting the standards set for endoscopy? Results of a large-scale prospective survey of endoscopic retrograde cholangio-pancreatograph practice. Gut. 2007;56:821-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Available from: http: //www.endonurse.com/articles/2008/06/developments-in-ercp.aspx. |
| 6. | Isaacs P. Endoscopic retrograde cholangiopancreatography training in the United Kingdom: A critical review. World J Gastrointest Endosc. 2011;3:30-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Dellon ES, Hawk JS, Grimm IS, Shaheen NJ. The use of carbon dioxide for insufflation during GI endoscopy: a systematic review. Gastrointest Endosc. 2009;69:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Bretthauer M, Seip B, Aasen S, Kordal M, Hoff G, Aabakken L. Carbon dioxide insufflation for more comfortable endoscopic retrograde cholangiopancreatography: a randomized, controlled, double-blind trial. Endoscopy. 2007;39:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Dellon ES, Velayudham A, Clarke BW, Isaacs KL, Gangarosa LM, Galanko JA, Grimm IS. A randomized, controlled, double-blind trial of air insufflation versus carbon dioxide insufflation during ERCP. Gastrointest Endosc. 2010;72:68-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Kuwatani M, Kawakami H, Hayashi T, Ishiwatari H, Kudo T, Yamato H, Ehira N, Haba S, Eto K, Kato M. Carbon dioxide insufflation during endoscopic retrograde cholangiopancreatography reduces bowel gas volume but does not affect visual analogue scale scores of suffering: a prospective, double-blind, randomized, controlled trial. Surg Endosc. 2011;25:3784-3790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Luigiano C, Ferrara F, Pellicano R, Fabbri C, Cennamo V, Bassi M, Ghersi S, Billi P, Polifemo A, Festa C. Carbon dioxide insufflation versus air insufflation during endoscopic retrograde cholangiopancreatography under general anesthesia. Minerva Med. 2011;102:261-269. [PubMed] |
| 12. | Maple JT, Keswani RN, Hovis RM, Saddedin EZ, Jonnalagadda S, Azar RR, Hagen C, Thompson DM, Waldbaum L, Edmundowicz SA. Carbon dioxide insufflation during ERCP for reduction of postprocedure pain: a randomized, double-blind, controlled trial. Gastrointest Endosc. 2009;70:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Mei SLCY, Ashby A, George B, Tarn W, Singh R. A prospective double blind randomised controlled trial of carbon dioxide versus air insufflation during ERCP: Is it worth the pain? Gastrointest Endosc. 2011;73:Suppl 1: AB351. |
| 14. | Arjunan S, Darishetty S, Tandan M, Lakhtakia S, Gupta R, Ramchandani M, Monga A, Wee E, Reddy DN. Randomized, double-blind, controlled trial showing carbon dioxide is superior to air insufflation during Endoscopic Retrograde Cholangio Pancreatography. J Gastroenterol Hepatol. 2011;26:Suppl 5: 2. |
| 15. | Janssens F, Deviere J, Eisendrath P, Dumonceau JM. Carbon dioxide for gut distension during digestive endoscopy: technique and practice survey. World J Gastroenterol. 2009;15:1475-1479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (5)] |
| 16. | Bretthauer M. Turning science into clinical practice - the case of carbon dioxide insufflation. Endoscopy. 2010;42:1104-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Gurusamy KS, Samraj K, Davidson BR. Low pressure versus standard pressure pneumoperitoneum in laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2009;CD006930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). : The Cochrane Collaboration 2011; Available from: http://www.cochrane-handbook.org. |
| 19. | Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). : The Cochrane Collaboration 2011; Available from: http://www.cochrane-handbook.org. |
| 20. | Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). : The Cochrane Collaboration 2011; Available from: http://www.cochrane-handbook.org. |
| 21. | Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. Int J Epidemiol. 1992;21:837-841. |
| 22. | Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. : The Cochrane Collaboration 2011; Available from: http://www.cochrane-handbook.org. |
| 23. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMAGroup. Preferred reporting items for systematic reviewsand meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 18034] [Article Influence: 1060.8] [Reference Citation Analysis (1)] |
| 24. | Borckardt JJ, Romagnuolo J, Reeves ST, Madan A, Frohman H, Beam W, George MS. Feasibility, safety, and effectiveness of transcranial direct current stimulation for decreasing post-ERCP pain: a randomized, sham-controlled, pilot study. Gastrointest Endosc. 2011;73:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Finsterer J, Stöllberger C, Bastovansky A. Cardiac and cerebral air embolism from endoscopic retrograde cholangio-pancreatography. Eur J Gastroenterol Hepatol. 2010;22:1157-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Sugimoto M, Yasuda H, Koda K, Suzuki M, Yamazaki M, Tezuka T, Kosugi C, Higuchi R, Watayo Y, Yagawa Y. Carbon dioxide-enhanced virtual MDCT cholangiopancreatography. J Hepatobiliary Pancreat Sci. 2010;17:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5694] [Cited by in RCA: 6972] [Article Influence: 435.8] [Reference Citation Analysis (108)] |
Peer reviewers: Yoshiharu Motoo, Professor, Department of Medical Oncology, Kanazawa Medical University, 1-1 Daigaku, Uchinada, Ishikawa 920-0293, Japan; Dr. Francesco Manguso, Gastroenterology Unit, AORN A Cardarelli, Via A. Cardarelli 9, 80122 Napoli, Italy
S- Editor Gou SX L- Editor Kerr C E- Editor Lu YJ