Published online Oct 14, 2012. doi: 10.3748/wjg.v18.i38.5470
Revised: May 7, 2012
Accepted: May 13, 2012
Published online: October 14, 2012
AIM: To explore the impact of tumor size on outcomes in patients with advanced gastric cancer in the lower third of the stomach.
METHODS: We retrospectively analyzed the clinical records of 430 patients with advanced gastric cancer in the lower third of the stomach who underwent distal subtotal gastrectomy and D2 lymphadenectomy in our hospital from January 1998 to June 2004. Receiver-operating characteristic (ROC) curve analysis was used to determine the appropriate cutoff value for tumor size, which was measured as maximum tumor diameter. Based on this cutoff value, patients were divided into two groups: those with large-sized tumors (LSTs) and those with small-sized tumors (SSTs). The correlations between other clinicopathologic factors and tumor size were investigated, and the 5-year overall survival (OS) rate was compared between the two groups. Potential prognostic factors were evaluated by univariate Kaplan-Meier survival analysis and multivariate Cox’s proportional hazard model analysis. The 5-year OS rates in the two groups were compared according to pT stage and pN stage.
RESULTS: The 5-year OS rate in the 430 patients with advanced gastric cancer in the lower third of the stomach was 53.7%. The mean ± SD tumor size was 4.9 ± 1.9 cm, and the median tumor size was 5.0 cm. ROC analysis indicated that the sensitivity and specificity results for the appropriate tumor size cutoff value of 4.8 cm were 80.0% and 68.2%, respectively (AUC = 0.795, 95%CI: 0.751-0.839, P = 0.000). Using this cutoff value, 222 patients (51.6%) had LSTs (tumor size ≥ 4.8 cm) and 208 (48.4%) had SSTs (tumor size < 4.8 cm). Tumor size was significantly correlated with histological type (P = 0.039), Borrmann type (P = 0.000), depth of tumor invasion (P = 0.000), lymph node metastasis (P = 0.000), tumor-nodes metastasis stage (P = 0.000), mean number of metastatic lymph nodes (P = 0.000) and metastatic lymph node ratio (P = 0.000). Patients with LSTs had a significantly lower 5-year OS rate than those with SSTs (37.1% vs 63.3%, P = 0.000). Univariate analysis showed that depth of tumor invasion (χ2 = 69.581, P = 0.000), lymph node metastasis (χ2 = 138.815, P = 0.000), tumor size (χ2 = 78.184, P = 0.000) and metastatic lymph node ratio (χ2 = 139.034, P = 0.000) were significantly associated with 5-year OS rate. Multivariate analysis revealed that depth of tumor invasion (P = 0.000), lymph node metastasis (P = 0.019) and tumor size (P = 0.000) were independent prognostic factors. Gastric cancers were divided into 12 subgroups: pT2N0; pT2N1; pT2N2; pT2N3; pT3N0; pT3N1; pT3N2; pT3N3; pT4aN0; pT4aN1; pT4aN2; and pT4aN3. In patients with pT2-3N3 stage tumors and patients with pT4a stage tumors, 5-year OS rates were significantly lower for LSTs than for SSTs (P < 0.05 each), but there were no significant differences in the 5-year OS rates in LST and SST patients with pT2-3N0-2 stage tumors (P > 0.05).
CONCLUSION: Using a tumor size cutoff value of 4.8 cm, tumor size is a prognostic factor in patients with pN3 stage or pT4a stage advanced gastric cancer located in the lower third of the stomach.
- Citation: Wang HM, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Lu J. Tumor size as a prognostic factor in patients with advanced gastric cancer in the lower third of the stomach. World J Gastroenterol 2012; 18(38): 5470-5475
- URL: https://www.wjgnet.com/1007-9327/full/v18/i38/5470.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i38.5470
Gastric cancer is a common gastrointestinal malignancy in China and is the second most common cause of cancer-related deaths worldwide[1,2]. The identification of prognostic factors may be helpful in predicting and improving outcomes in patients with gastric cancer. Lymph node metastasis[3-5] and depth of tumor invasion[6-8] are the most important prognostic factors and are included in the Japanese Classification of Gastric Carcinoma (JCGC) and the American Joint Committee on Cancer tumor-nodes metastasis classification (AJCC TNM). Tumor size is another valuable clinicopathological feature because it can be measured easily before or during surgery and may be prognostic for survival in patients with gastric cancer[9-11]. Although tumor size is included in staging systems for lung and breast cancer, it has not been considered prognostic in gastric cancer. We therefore, retrospectively, analyzed the impact of tumor size on the prognosis of patients with advanced gastric cancer located in the lower third of the stomach.
Patients undergoing curative resection (distal subtotal gastrectomy and D2 lymphadenectomy) for advanced gastric carcinoma (pT2-T4a stage) in the lower third of the stomach at the Department of Gastric Surgery, Affiliated Union Hospital of Fujian Medical University, Fuzhou, China, between January 1998 and June 2004 were included. Patients with gastric stump cancer, infiltration of surrounding organs (T4b) or distant metastases (hepatic, lung, peritoneal dissemination, or extraregional lymph nodes such as retropancreatic, mesenteric, and para-aortic lymph nodes) were excluded. After applying these criteria, 430 patients were included.
A surgical procedure was defined as curative if no grossly visible tumor tissue remained after the resection and the resection margins were histologically normal. Dissected lymph nodes were classified according to JCGC[12] criteria by specialist surgeons who reviewed the excised specimens after surgery. A total of 10 400 lymph nodes were dissected. The median number of dissected lymph nodes per patient was 24 (range, 6-61; mean 24.3 ± 8.8). Depth of tumor invasion, lymph node metastasis and tumor-nodes metastasis (TNM) stage were classified with respect to the seventh edition of AJCC TNM classification[13]. The metastatic lymph node ratio (MLR) was defined as the ratio of metastatic lymph nodes to the total number of dissected lymph nodes and categorized as MLR 0 (0%), MLR 1 (1%-9%), MLR 2 (10%-25%), MLR 3 (> 25%).
Routine follow-up consisted of physical examination, laboratory tests (including measurements of CEA, CA19-9 and CA125 concentrations), chest radiography, abdominopelvic ultrasonography and computed tomography (CT). Patients were followed-up every 3 mo during the first year, and every 6 mo or 12 mo thereafter, for a total of 5 years. Endoscopy was performed every 6 mo or 12 mo. All surviving patients were followed for more than five years. Survival was calculated from the date of diagnosis to last contact, date of death, or date when the survival information was collected. Of the 430 patients, 394 (93.0%) were followed-up.
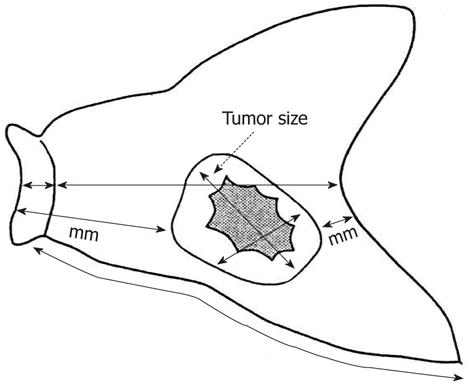
In accordance with JCGC criteria[12], the resected stomach was opened along the greater curvature so that the whole mucosa could be observed. If the tumor was located on the greater curvature, the stomach was opened along the lesser curvature. The opened stomach was placed on a flat board with the mucosal side up and examined macroscopically. The lengths of the greater and lesser curvatures, as well as the attached portion of the esophagus and/or the duodenum and the size and thickness of the tumor, were recorded. Tumor size was measured as maximum tumor diameter (Figure 1). If tumor margins were unclear, the resected stomach was fixed in formalin for 1 h to make the margins clearer.
All statistical analyses were performed using the Statistical Package for Social Science (SPSS) version 16.0 for Windows. The appropriate cutoff value for tumor size predicting 5-year survival was determined using the receiver-operating characteristic (ROC) curve, from which the area under the curve (AUC) was determined and the Youden index corresponding to each size was calculated. χ2 tests were used to evaluate differences in proportions, and Student’s t-tests were used to evaluate continuous variables. Five-year overall survival (OS) rates were calculated by the Kaplan-Meier method, with groups compared by log-rank tests. Multivariate analysis was performed using a Cox’s proportional hazard model. P values < 0.05 were considered statistically significant.
We retrospectively analyzed a total of 430 patients with pT2-T4a gastric cancer located in the lower third of the stomach. Of these, 132 patients (30.7%) were women and 298 (69.3%) were men, with 187 patients (43.5%) ≥ 60 years old. Postoperatively, 45 patients (10.5%) were classified as stage IB, 49 (11.4%) as stage IIA, 78 (18.1%) as stage IIB, 65 (15.1%) as stage IIIA, 82 (19.1%) as stage IIIB and 111 (25.8%) as stage IIIC. In addition, 117 patients (27.2%) were classified as stage pT2, 40 (9.3%) as pT3 and 273 (63.5%) as pT4a, while 105 patients (24.4%) were stage pN0, 92 (21.4%) were pN1, 94 (21.9%) were pN2, and 139 (32.3%) were pN3. Of the 430 patients, 325 (75.6%) had undifferentiated tumors. Based on the MLR classification, 70 patients (16.3%) were classified as MLR 0, 118 (27.4%) as MLR 1, 93 (21.6%) as MLR 2, and 149 (34.7%) as MLR 3.
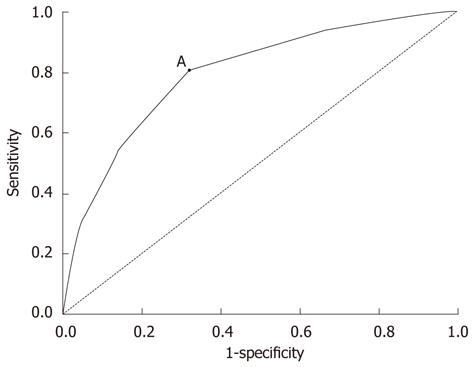
The mean ± SD tumor size was 4.9 ± 1.9 cm, and the median tumor size was 5.0 cm (range, 1.0-12.0 cm). ROC analysis indicated that a cutoff value of 4.8 cm yielded a sensitivity of 80.0% and a specificity of 68.2% in predicting survival after gastric surgery (AUC = 0.795, 95%CI: 0.751-0.839, P = 0.000) (Figure 2). Based on this cutoff value, the patients were divided into 2 groups, with 222 (51.6%) having large-sized tumors [large-sized tumors (LSTs), ≥ 4.8 cm] and 208 (48.4%) having small-sized tumors [small-sized tumors (SSTs), < 4.8 cm].
When we analyzed the correlation between other clinicopathologic factors and tumor size (Table 1), we found that tumor size was significantly correlated with histological type (P = 0.039), Borrmann type (P = 0.000), depth of tumor invasion (P = 0.000), lymph node metastasis (P = 0.000), TNM stage (P = 0.000), mean number of metastatic lymph nodes (P = 0.000) and metastatic lymph node ratio (P = 0.000). SSTs were associated with differentiated and Borrmann I/II types (P < 0.05 each), whereas LSTs were deeper and were associated with more extensive lymph node metastasis (P < 0.05). The mean number of metastatic lymph nodes was greater in patients with LSTs than with SSTs (P < 0.05).
| Factors | LST | SST | P |
| Gender | 0.221 | ||
| Male | 148 (66.7) | 150 (72.1) | |
| Female | 74 (33.3) | 58 (27.9) | |
| Age (yr) | 0.209 | ||
| < 60 | 119 (53.6) | 124 (59.6) | |
| ≥ 60 | 103 (46.4) | 84 (40.4) | |
| Histological type | 0.039 | ||
| Differentiated | 45 (20.3) | 60 (28.8) | |
| Undifferentiated | 177 (79.7) | 148 (71.2) | |
| Borrmann type | 0.000 | ||
| I/II | 50 (22.5) | 111 (53.4) | |
| III/IV | 172 (77.5) | 97 (46.6) | |
| Lymph node metastasis | 0.000 | ||
| pN0 | 35 (15.8) | 70 (33.7) | |
| pN1 | 41 (18.5) | 51 (24.5) | |
| pN2 | 52 (23.4) | 42 (20.2) | |
| pN3 | 94 (42.3) | 45 (21.6) | |
| Depth of invasion | 0.000 | ||
| pT2 | 28 (12.6) | 89 (42.8) | |
| pT3 | 19 (8.6) | 21 (10.1) | |
| pT4a | 175 (78.8) | 98 (47.1) | |
| TNM stage | 0.000 | ||
| IB | 9 (4.1) | 36 (17.3) | |
| IIA | 14 (6.3) | 35 (16.8) | |
| IIB | 33 (14.9) | 45 (21.6) | |
| IIIA | 30 (13.5) | 35 (16.8) | |
| IIIB | 54 (24.3) | 28 (13.5) | |
| IIIC | 82 (36.9) | 29 (14.0) | |
| Number of lymph nodes | 8.02 ± 8.66 | 3.98 ± 5.65 | 0.000 |
| MLR | 0.000 | ||
| 0 | 35 (15.8) | 70 (33.6) | |
| 1 | 37 (16.7) | 46 (22.1) | |
| 2 | 41 (18.5) | 43 (20.7) | |
| 3 | 109 (49.0) | 49 (23.6) |
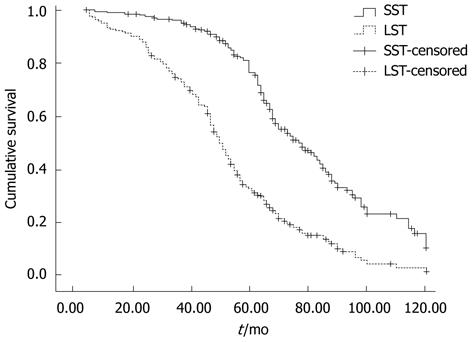
The 5-year OS rate of all patients was 53.7%, being significantly lower in patients with LSTs than with SSTs (32.9% vs 76.7%; χ2 = 78.184, P = 0.000; Figure 3).
Univariate analysis showed that depth of tumor invasion (χ2 = 69.581, P = 0.000), lymph node metastasis (χ2 = 138.815, P = 0.000), tumor size (χ2 = 78.184, P = 0.000) and metastatic lymph node ratio (χ2 = 139.034, P = 0.000) were significantly associated with 5-year OS rate, whereas patient age (P = 0.613), gender (P = 0.240) and histological type (P = 0.361) were not (Table 2). Multivariate analysis using a Cox’s proportional hazards model revealed that depth of tumor invasion (P = 0.000), lymph node metastasis (P = 0.019) and tumor size (P = 0.000) independently predicted poor prognosis (Table 3).
| Factors | n | 5-year OS (%) | χ2 | P value |
| Gender | 1.655 | 0.198 | ||
| Male | 298 | 54.8 | ||
| Female | 132 | 51.3 | ||
| Age (yr) | 0.012 | 0.911 | ||
| < 60 | 242 | 52.6 | ||
| ≥ 60 | 188 | 55.1 | ||
| Tumor size (cm) | 78.184 | 0.000 | ||
| < 4.8 | 208 | 76.7 | ||
| ≥ 4.8 | 222 | 32.9 | ||
| Depth of invasion | 69.581 | 0.000 | ||
| pT2 | 117 | 82.5 | ||
| pT3 | 40 | 56.5 | ||
| pT4a | 273 | 41.2 | ||
| Histological type | 0.835 | 0.361 | ||
| Differentiated | 105 | 66.5 | ||
| Undifferentiated | 325 | 49.8 | ||
| Lymph node metastasis | 138.815 | 0.000 | ||
| pN0 | 105 | 85.9 | ||
| pN1 | 92 | 73.0 | ||
| pN2 | 94 | 40.4 | ||
| pN3 | 139 | 21.7 | ||
| MLR | 139.034 | 0.000 | ||
| 0 | 70 | 85.9 | ||
| 1 | 118 | 70.1 | ||
| 2 | 93 | 54.1 | ||
| 3 | 149 | 23.4 |
| Characteristics | B | SE | Wald | P value | RR | 95%CI | |
| Depth of invasion | 23.143 | 0.000 | |||||
| pT3/pT2 | 0.604 | 0.228 | 7.032 | 0.008 | 1.829 | 1.171 | 2.857 |
| pT4a/pT2 | 0.775 | 0.161 | 23.135 | 0.000 | 2.171 | 1.583 | 2.977 |
| Lymph node metastasis | 9.923 | 0.019 | |||||
| pN1/pN0 | 0.578 | 0.280 | 4.268 | 0.039 | 1.783 | 1.030 | 3.085 |
| pN2/pN0 | 1.130 | 0.420 | 7.224 | 0.007 | 3.095 | 1.358 | 7.055 |
| pN3/pN0 | 1.601 | 0.528 | 9.204 | 0.002 | 4.959 | 1.763 | 13.954 |
| MLR | 0.061 | 0.170 | 0.130 | 0.719 | 1.063 | 0.762 | 1.483 |
| Tumor size (cm) | 0.762 | 0.123 | 38.524 | 0.000 | 2.143 | 1.684 | 2.726 |
Gastric cancers were divided into 12 subgroups: pT2N0; pT2N1; pT2N2; pT2N3; pT3N0; pT3N1; pT3N2; pT3N3; pT4aN0; pT4aN1; pT4aN2; and pT4aN3. The 5-year OS rates in these subgroups were compared in patients with LSTs and SSTs. We found that the 5-year survival rates of patients with pT2-3N3 stage and pT4a stage tumors were significantly lower in the LST than in the SST group (P < 0.05 each), but did not differ significantly in patients with pT2-3N0-2 stage tumors in the LST and SST groups (P > 0.05) (Table 4).
| n (5-yr OS, %) | χ2 | P value | ||
| LST | SST | |||
| pT2 | ||||
| pN0 | 9 (88.9) | 36 (96.7) | 0.260 | 0.610 |
| pN1 | 11 (72.7) | 26 (91.1) | 0.000 | 0.986 |
| pN2 | 4 (50.0) | 16 (87.1) | 0.066 | 0.797 |
| pN3 | 4 (0.00) | 11 (63.6) | 7.661 | 0.006 |
| pT3 | ||||
| pN0 | 3 (66.7) | 9 (100.0) | 1.634 | 0.201 |
| pN1 | 6 (66.7) | 4 (75.0) | 0.348 | 0.555 |
| pN2 | 2 (0.0) | 3 (66.7) | 0.825 | 0.364 |
| pN3 | 8 (12.5) | 5 (40.0) | 3.940 | 0.047 |
| pT4a | ||||
| pN0 | 23 (68.7) | 25 (83.1) | 5.108 | 0.024 |
| pN1 | 24 (54.2) | 21 (75.4) | 4.743 | 0.029 |
| pN2 | 46 (27.2) | 23 (61.9) | 7.682 | 0.006 |
| pN3 | 83 (6.9) | 28 (48.6) | 23.138 | 0.000 |
The prognostic value of tumor size in gastric cancer patients has recently received greater attention because tumor size can be measured easily before or during surgery; however, there is as yet no consensus formula to calculate the appropriate cutoff value for gastric tumor size. For example, tumor size of gastric cancer patients has been stratified into four subgroups (≤ 2 cm, ≤ 3 cm, ≤ 5 cm, and > 5 cm) by minimizing the estimated average expected distance (AED) objective function[14]. In another study of gastric cancer patients that used Cox’s proportional hazards model to compare survival rates, a significant difference in survival was observed in patients with tumors < 10 cm and ≥ 10 cm[15]. We utilized ROC curve analysis to determine the appropriate tumor size cutoff value predicting 5-year OS rate in patients with advanced gastric cancer in the lower third of the stomach. In clinical and epidemiological fields, ROC analysis is frequently used to determine the cutoff values and reflect the correctness of a method of evaluation. According to the basic principle of ROC curves[16], when the Youden index is maximum, and the sensitivity is maximum, the corresponding tumor size is the appropriate cutoff value; besides, the method of evaluation can reflect predicted efficiency only when AUCs range between 0.7 and 0.9. From our ROC curves, a maximum sensitivity (0.800) at a tumor size cutoff value of 4.8 cm; our finding, of an AUC of 0.795 (P = 0.000), suggests that tumor size can reliably predict postoperative outcomes in patients with gastric cancer. Tumor size has shown positive associations with histological type, depth of tumor invasion, lymph node metastasis, peritoneal metastasis, blood vessel invasion and perineural invasion[17-20]. We found that LSTs were highly aggressive and malignant, with high disease stages. Compared with SSTs, LSTs showed deeper infiltration and were associated with more extensive lymph node metastasis, as well as having a significantly lower 5-year OS rate (37.1% vs 63.3%, P < 0.05), indicating that a cutoff of 4.8 cm could be used as a size criterion for gastric cancers.
The prognostic role of tumor size in gastric cancer remains unclear. An examination of 697 patients with gastric cancer who had undergone gastrectomy with curative intent found that tumor size was a predictor of survival in univariate analysis, but not in multivariate analysis[21]. In contrast, other researchers found that tumor size was an independent predictor of prognosis. For example, when patients were divided by tumor size into three subgroups, ≤ 4 cm, ≤ 10 cm, and > 10 cm in diameter, tumor size independently predicted patient survival[20]. Similarly, using a cutoff of 8 cm, tumor size was independently prognostic of survival[22]. We found that tumor size was significantly correlated with patient prognosis in both univariate and multivariate analysis, as were depth of tumor invasion and lymph node metastasis. Another study hypothesized that it was difficult to identify the most important variables associated with prognosis, and that the precise evaluation of the impact of tumor size on prognosis was feasible only when depth of invasion was specified[23]. Therefore, that study evaluated survival in patients with pT3 stage gastric cancer relative to pN stage in patients with LSTs and SSTs, finding that tumor size significantly influenced prognosis in pT3N2-3 stage tumors (P = 0.004). To eliminate depth of tumor invasion and lymph node metastasis as factors, we compared survival in patients in the LST and SST groups according to pT and pN stages. We observed no significant differences in 5-year OS of patients with pT2-3N0-2 stage tumors classified as LSTs and SSTs. In contrast, the 5-year OS rates were significantly lower in LST than in SST patients with pN3 stage or pT4a stage tumors (P < 0.05). In patients with pT2-3N0-2 stage tumors, the tumors likely did not infiltrate the serosa and had less extensive lymph node metastasis, reducing the likelihood of free cancer cells in the peritoneal cavity and decreasing the possibility of peritoneal recurrence. Tumor size, therefore, did not significantly affect postoperative survival in these patients. In patients with pN3 stage or pT4a stage LSTs, however, the interactions between tumors and lymphatic tissue were enhanced, thus increasing the likelihood of lymph node micrometastasis and diffusion to lymphatic vessels; the larger the area of the serosa invaded by tumor, the greater the likelihood for intraperitoneal dissemination, and the poorer the prognosis[24-26]. Tumor size was therefore correlated with survival of patients with pN3 stage or pT4a stage gastric cancer.
In conclusion, using a cutoff value of 4.8 cm, tumor size may be a prognostic factor in patients with pN3 stage or pT4a stage advanced gastric cancer located in the lower third of the stomach.
In addition to lymph node metastasis and depth of tumor invasion, tumor size is an important clinicopathological feature of gastric cancer because it can be measured easily before or during surgery. To date, however, the prognostic role of tumor size on survival in patients with gastric cancer remains unclear, with no consensus formula to calculate an appropriate cutoff value.
Tumor size is included in the staging systems of many malignant diseases such as lung and breast cancer. Although tumor size has been reported to independently influence prognosis in patients with gastric cancer, an appropriate cutoff size has not been determined, especially for advanced gastric cancers located in the lower third of the stomach.
The authors utilized receiver-operating characteristic (ROC) curve analysis to determine the appropriate tumor size cutoff value and assessed the relationship between tumor size and overall survival rate.
The authors found that, using a cutoff of 4.8 cm, tumor size was a prognostic factor in patients with pN3 stage or pT4a stage advanced gastric cancers located in the lower third of the stomach. That is, patients with pN3 stage or pT4a stage tumors ≥ 4.8 cm had a poorer prognosis than those with tumors < 4.8 cm.
In assessing the effect of tumor size in a large number of patients (430) with advanced gastric cancer located in the lower third of the stomach, the authors found that tumor size, using a cutoff of 4.8 cm, was prognostic in patients with pN3 stage or pT4a stage tumors. This finding has important clinical implications for gastrointestinal surgeons and for patient prognosis.
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8283] [Cited by in RCA: 8236] [Article Influence: 457.6] [Reference Citation Analysis (11)] |
| 2. | Desai AM, Pareek M, Nightingale PG, Fielding JW. Improving outcomes in gastric cancer over 20 years. Gastric Cancer. 2004;7:196-201; discussion 201-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 803] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 4. | Yokota T, Ishiyama S, Saito T, Teshima S, Narushima Y, Murata K, Iwamoto K, Yashima R, Yamauchi H, Kikuchi S. Lymph node metastasis as a significant prognostic factor in gastric cancer: a multiple logistic regression analysis. Scand J Gastroenterol. 2004;39:380-384. [PubMed] |
| 5. | Saito H, Fukumoto Y, Osaki T, Fukuda K, Tatebe S, Tsujitani S, Ikeguchi M. Prognostic significance of level and number of lymph node metastases in patients with gastric cancer. Ann Surg Oncol. 2007;14:1688-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Kim JP, Lee JH, Kim SJ, Yu HJ, Yang HK. Clinicopathologic characteristics and prognostic factors in 10 783 patients with gastric cancer. Gastric Cancer. 1998;1:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 196] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Yasuda K, Shiraishi N, Inomata M, Shiroshita H, Izumi K, Kitano S. Prognostic significance of macroscopic serosal invasion in advanced gastric cancer. Hepatogastroenterology. 2007;54:2028-2031. [PubMed] |
| 8. | Sheen-Chen SM, Chou CW, Chen MC, Chen FC, Chen YS, Chen JJ. Adenocarcinoma in the middle third of the stomach--an evaluation for the prognostic significance of clinicopathological features. Hepatogastroenterology. 1997;44:1488-1494. [PubMed] |
| 9. | Mohri Y, Tanaka K, Ohi M, Yokoe T, Miki C, Kusunoki M. Prognostic significance of host- and tumor-related factors in patients with gastric cancer. World J Surg. 2010;34:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Li C, Oh SJ, Kim S, Hyung WJ, Yan M, Zhu ZG, Noh SH. Risk factors of survival and surgical treatment for advanced gastric cancer with large tumor size. J Gastrointest Surg. 2009;13:881-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Jun KH, Jung H, Baek JM, Chin HM, Park WB. Does tumor size have an impact on gastric cancer? A single institute experience. Langenbecks Arch Surg. 2009;394:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [PubMed] |
| 13. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 820] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 14. | Wang X, Wan F, Pan J, Yu GZ, Chen Y, Wang JJ. Tumor size: a non-neglectable independent prognostic factor for gastric cancer. J Surg Oncol. 2008;97:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Kunisaki C, Makino H, Takagawa R, Oshima T, Nagano Y, Kosaka T, Ono HA, Otsuka Y, Akiyama H, Ichikawa Y. Tumor diameter as a prognostic factor in patients with gastric cancer. Ann Surg Oncol. 2008;15:1959-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Gallop GR, Crits-Christoph P, Muenz LR, Tu XM. Determination and interpretation of the Optimal Operating Point for ROC Curves derived through generalized linear models.. Understanding Statistics. 2003;2:219-242. [RCA] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Saito H, Osaki T, Murakami D, Sakamoto T, Kanaji S, Oro S, Tatebe S, Tsujitani S, Ikeguchi M. Macroscopic tumor size as a simple prognostic indicator in patients with gastric cancer. Am J Surg. 2006;192:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Giuliani A, Caporale A, Di Bari M, Demoro M, Gozzo P, Corona M, Miccini M, Ricciardulli T, Tocchi A. Maximum gastric cancer diameter as a prognostic indicator: univariate and multivariate analysis. J Exp Clin Cancer Res. 2003;22:531-538. [PubMed] |
| 19. | Xu CY, Shen JG, Shen JY, Chen WJ, Wang LB. Ulcer size as a novel indicator marker is correlated with prognosis of ulcerative gastric cancer. Dig Surg. 2009;26:312-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Adachi Y, Oshiro T, Mori M, Maehara Y, Sugimachi K. Tumor size as a simple prognostic indicator for gastric carcinoma. Ann Surg Oncol. 1997;4:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Yokota T, Ishiyama S, Saito T, Teshima S, Yamada Y, Iwamoto K, Takahashi M, Murata K, Yamauchi H. Is tumor size a prognostic indicator for gastric carcinoma? Anticancer Res. 2002;22:3673-3677. [PubMed] |
| 22. | Bilici A, Uygun K, Seker M, Ustaalioglu BB, Aliustaoglu M, Temiz S, Aksu G, Gezen C, Yavuzer D, Kaya S. The effect of tumor size on overall survival in patients with pT3 gastric cancer: experiences from 3 centers. Onkologie. 2010;33:676-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Chi Z, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lin JX. [Prognostic significance of tumor size in T3 gastric cancer]. Zhonghua Wei Chang Wai Ke Zazhi. 2011;14:114-116. [PubMed] |
| 24. | Ishii K, Kinami S, Funaki K, Fujita H, Ninomiya I, Fushida S, Fujimura T, Nishimura G, Kayahara M. Detection of sentinel and non-sentinel lymph node micrometastases by complete serial sectioning and immunohistochemical analysis for gastric cancer. J Exp Clin Cancer Res. 2008;27:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Kikuchi S, Katada N, Sakuramoto S, Koabayashi N, Shimao H, Sakakibara Y, Kakita A. Factors associated with pN3 stage tumors according to the TNM classification in advanced gastric cancer. Hepatogastroenterology. 2003;50:1723-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Boku T, Nakane Y, Minoura T, Takada H, Yamamura M, Hioki K, Yamamoto M. Prognostic significance of serosal invasion and free intraperitoneal cancer cells in gastric cancer. Br J Surg. 1990;77:436-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 163] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
Peer reviewers: Mario M D’Elios, Professor, University of Florence, viale Morgagni 85, Florence, 50134, Italy; Dr. Wei-Dong Tong, MD, MS, BS, Associate Professor, Department of General Surgery, Veterans Affairs Medical Center, Medical College of Wisconsin, Milwaukee, WI 53295, United States
S- Editor Lv S L- Editor Kerr C E- Editor Lu YJ