Published online Oct 14, 2012. doi: 10.3748/wjg.v18.i38.5369
Revised: May 30, 2012
Accepted: June 8, 2012
Published online: October 14, 2012
AIM: To investigate the effects of Axl deglycosylation on tumor lymphatic metastases in mouse hepatocellular carcinoma cell lines.
METHODS: Western blotting was used to analyze the expression profile of Axl glycoprotein in mouse hepatocellular carcinoma cell line Hca-F treated with tunicamycin and PNGase F 3-(4,5)-dimethylthiazol(-zyl)-3,5-diphenyltetrazolium bromide (MTT) assay, extracellular matrix (ECM) invasion assay (in vitro) and tumor metastasis assay (in vivo) were utilized to evaluate the effect of Axl deglycosylation on the Hca-F cell proliferation, invasion and lymphatic metastasis.
RESULTS: Tunicamycin and PNGase F treatment markedly inhibited Axl glycoprotein synthesis and expression, proliferation, invasion, and lymphatic metastasis both in vitro and in vivo. In the MTT assay, proliferation was apparent in untreated Hca-F cells compared with treated Hca-F cells. In the ECM invasion assay (in vitro), treated cells passed through the ECMatrix gel in significantly smaller numbers than untreated cells (tunicamycin 5 μg/mL: 68 ± 8 vs 80 ± 9, P = 0.0222; 10 μg/mL: 50 ± 6 vs 80 ± 9, P = 0.0003; 20 μg/mL: 41 ± 4 vs 80 ± 9, P = 0.0001); (PNGase F 8 h: 66 ± 7 vs 82 ± 8, P = 0.0098; 16 h: 49 ± 4 vs 82 ± 8, P = 0.0001; 24 h: 34 ± 3 vs 82 ± 8, P = 0.0001). In the tumor metastasis assay (in vivo), average lymph node weights of the untreated Hca-F group compared with treated Hca-F groups (tunicamycin 5 μg/mL: 0.84 ± 0.21 g vs 0.72 ± 0.19 g, P = 0.3237; 10 μg/mL: 0.84 ± 0.21 g vs 0.54 ± 0.11 g, P = 0.0113; 20 μg/mL: 0.84 ± 0.21 g vs 0.42 ± 0.06 g, P = 0.0008); (PNGase F 8 h: 0.79 ± 0.15 g vs 0.63 ± 0.13 g, P = 0.0766; 16 h: 0.79 ± 0.15 g vs 0.49 ± 0.10 g, P = 0.0022; 24 h: 0.79 ± 0.15 g vs 0.39 ± 0.05 g, P = 0.0001). Also, average lymph node volumes of the untreated Hca-F group compared with treated Hca-F groups (tunicamycin 5 μg/mL: 815 ± 61 mm3vs 680 ± 59 mm3, P = 0.0613; 10 μg/mL: 815 ± 61 mm3vs 580 ± 29 mm3, P = 0.0001; 20 μg/mL: 815 ± 61 mm3vs 395 ± 12 mm3, P = 0.0001); (PNGase F 8 h: 670 ± 56 mm3vs 581 ± 48 mm3, P = 0.0532; 16 h: 670 ± 56 mm3vs 412 ± 22 mm3, P = 0.0001; 24 h: 670 ± 56 mm3vs 323 ± 11 mm3, P = 0.0001).
CONCLUSION: Alteration of Axl glycosylation can attenuate neoplastic lymphatic metastasis. Axl N-glycans may be a universal target for chemotherapy.
- Citation: Li J, Jia L, Ma ZH, Ma QH, Yang XH, Zhao YF. Axl glycosylation mediates tumor cell proliferation, invasion and lymphatic metastasis in murine hepatocellular carcinoma. World J Gastroenterol 2012; 18(38): 5369-5376
- URL: https://www.wjgnet.com/1007-9327/full/v18/i38/5369.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i38.5369
The receptor tyrosine kinases (RTKs) constitute a large family of transmembrane proteins that relay signals from extracellular growth factors into the cell[1,2]. The Tyro-Axl-Mer (TAM) subfamily shares the vitamin K-dependent ligand Gas6 (growth arrest specific 6). TAM receptors contain a combination of two immunoglobin-like domains and dual fibronectin type III repeats in the extracellular region, and a cytoplasmic kinase domain[3,4]. The TAM receptors regulate a diverse range of cellular responses including cell survival, proliferation, autophagy, migration, angiogenesis, platelet aggregation, and natural killer cell differentiation[4].
The Axl receptor (also called UFO, Tyro7, and Ark) is a RTK originally identified as a transforming gene in chronic myeloid leukemia[5,6]. Axl is expressed in various organs, including the brain, suggesting its involvement in mesenchymal and neural development[7,8]. Axl has been shown to have transforming potential when overexpressed during development. Axl overexpression is clearly associated with invasiveness and metastasis in several cancer cell types, including myeloid leukemia[6,9], esophageal[10], metastatic lung[11], metastatic colon[12], renal cell[13], prostate[14], breast[15], gastric[16], and thyroid[17] cancers. Axl also affects multiple pathways in angiogenesis[11]. Thus, Axl may play an important role in tumor progression, although its mechanism remains unknown.
Protein glycosylation is one of the major types of posttranslational modifications that has profound biological implications[18,19]. Specific changes in the glycosylation pattern of cell surface glycoproteins have been shown to correlate with metastatic efficiency in tumor cells[20]. In particular, protein N-glycosylation is one of the most prominent biochemical alterations in tumorigenesis and metastatic spread[21,22]. A cell surface transmembrane glycoprotein, little is known about the mechanism of Axl deglycosylation.
The mouse hepatocellular carcinoma cell line Hca-F is highly aggressive, with a metastasis rate over 80%. Hca-P, on the other hand, has a lymphatic metastasis rate of less than 30%. Both cell lines are derived from 615-mice ascites-type hepatocellular carcinoma cells. Hca-F and Hca-P cells metastasize only to lymph nodes, and not extrahepatic organs. However, the relationship between Axl glycosylation and lymphatic metastasis of mouse hepatocellular carcinoma cells remains unclear.
Our aim was to investigate whether Axl glycosylation regulates lymphatic metastasis. We demonstrated a possible correlation, based upon regulation of Axl glycosylation in mouse hepatocellular carcinoma cells.
Mouse hepatocellular carcinoma cell lines Hca-F and Hca-P, grown and stored in our institution (Department of Pathology, Dalian Medical University) were cultured in 90% Roswell Park Memorial Institute (RPMI)-1640 (Gibco) and supplemented with antibiotics (1 × penicillin/streptomycin 100 U/mL, Gibco) and 10% fetal bovine serum (FBS) (Gibco). Cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2. 615-mice (8 wk old males) were obtained from the Experimental Animal Center of Dalian Medical University.
107 cells were centrifuged at room temperature at 1000 ×g for 10 min. Cells were rinsed twice with phosphate buffered saline (PBS) at 1000 ×g for 5 min, and lysed with a protease inhibitor cocktail (whole protein extraction kit KGP2100, KeyGEN). Cells were suspended on a swing bed at 4 °C for 15 min, and centrifuged at 4 °C at 14 000 ×g for 15 min. Protein concentration of the whole cells was measured with a bicinchoninic acid protein assay kit (KGPBCA, KeyGEN).
Western blotting analysis was performed to evaluate Axl (with or without tunicamycin or PNGase F treatment) protein levels. Extracted proteins were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto polyvinylidene fluoride membranes (Pall Corporation). After blocking for 2 h with 5% skimmed milk in PBS containing 0.1% Tween 20 (PBST), membranes were incubated with rabbit anti-mouse Axl polyclonal antibody (Santa Cruz Biotech Inc., 1/200 diluted) overnight in 5% powdered skim milk buffer, washed thrice with PBS with 0.1% Tween 20, and then incubated with secondary antibody anti-rabbit-HRP (Santa Cruz Biotech Inc., 1/3000 diluted). Glyceraldehyde-3-phosphate dehydrogenase antibody (Santa Cruz Biotech Inc., 1/200 diluted) was used as controls. All blot analysis was performed with a ECL Western blotting kit (Amersham Biosciences, United Kingdom).
To inhibit N-linked glycosylation of newly synthesized proteins, Hca-F cells were washed once with PBS and cultivated for 12 h in fresh culture media (90% RPMI 1640 supplemented with antibiotics) with or without tunicamycin (Sigma Aldrich, St. Louis, MO) in a dose-dependent manner (0 μg/mL, 5 μg/mL, 10 μg/mL, or 20 μg/mL). Cells were washed with PBS and subjected to Western blotting analysis, 3-(4,5)-dimethylthiazol(-zyl)-3,5-diphenyltetrazolium bromide (MTT), migration in vitro, and tumor metastasis in vivo assays.
To remove N-glycans, protein fractions (100 μg) from Hca-F cells were deglycosylated with 25 units of PNGase F (Elizabethkingia meningoseptica; Sigma Aldrich, St. Louis, MO) in lysis buffer. Probes were incubated for 8 h, 16 h and 24 h at 37 °C. The reaction was terminated with Laemmlie’s sample buffer and proteins were separated on a gel as described earlier.
For deglycosylation of membrane proteins, intact Hca-F cells were incubated with 25 units of PNGase F for 24 h, washed, and treated as described for the MTT, migration in vitro, and tumor metastasis in vivo assays.
106 cells in 200 μL RPMI 1640 were seeded in duplicate into 96-well culture plates, and 100 μL MTT (5 mg/mL, Sigma) was added at 24 h, 48 h, 72 h, 96 h, and 120 h, respectively. After 4 h incubation at 37 °C in 5% CO2, 100 μL/well DMSO (final concentration 25%, Gibco) was pipetted to solubilize the formazan product for 30 min at room temperature. Absorbency (490 angstroms) was measured using a microplate reader (Bio-Rad).
Cell invasion in vitro was demonstrated using 24-well transwell units (Corning, NY, United States) with a 8 μm pore size polycarbonate filter coated with ECMatrix gel (Chemicon) to form a continuous thin layer[23]. Cells (3 × 105) were harvested in serum-free medium containing 0.1% BSA and added to the upper chamber. The lower chamber contained 500 μL RPMI 1640. Cells were incubated for 24 h at 37 °C, 5% CO2 incubator. At the end of incubation, cells on the upper surface of the filter were completely removed with a cotton swab. The filters were fixed in methanol and stained with Wright-Giemsa. Cells invading the matrigel that reached the lower surface of the filter were counted with light microscopy at a magnification of 400 ×. Samples were acquired in triplicate and data expressed as the average cell number in 5 fields.
Forty eight 615-mice were provided with sterilized food and water and equally divided into eight groups. 107 Hca-F cells (with or without tunicamycin or PNGase F treatment) were subcutaneously inoculated into the footpads. After 3 wk, mice were sacrificed and their axillary lymph nodes were isolated, weighed, and photographed.
Each assay was performed at least three times. Data were presented as the mean ± SD. Statistical differences between test groups was assessed by one-way analysis of variance and Scheffe’s test for post hoc analysis. A P-value of less than 0.05 was considered statistically significant. SPSS version 13.0 software was used for statistical analysis.
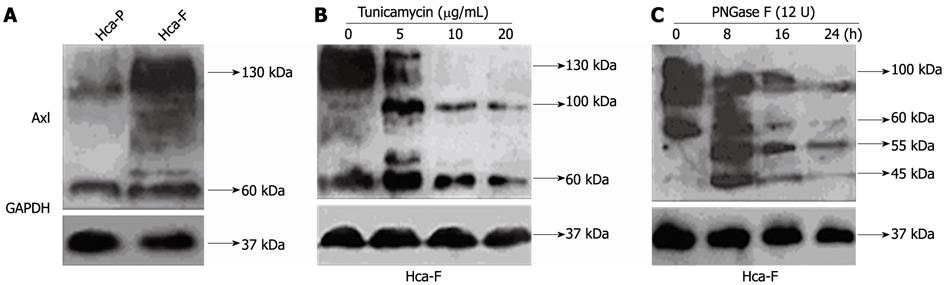
Axl glycoprotein relative expression was determined by Western blotting analysis using whole-cell extracts (Figure 1A). Axl expression varied among cell lines, with higher and lower levels in Hca-F and Hca-P cells, respectively (Figure 1A, P < 0.05).
Tunicamycin, an inhibitor of endogenous N-linked glycosylation of newly synthesized proteins, was used to inhibit Axl glycosylation of Hca-F cells. Treatment in a dose dependent manner (0 mg/mL, 5 mg/mL, 10 mg/mL, and 20 mg/mL) for 12 h showed N-linked glycosylation to be highly sensitive to tunicamycin inhibition (Figure 1B).
Axl appears as broad bands, with molecular weights ranging from 60 kDa to 140 kDa. With tunicamycin treatment, 130 kDa Axl band density decreased, 60 kDa band density increased, and a 100 kDa band appeared. As the dose of tunicamycin increased, the 130 kDa Axl band completely disappeared.
Whole protein aliquots extracted from Hca-F cells were exposed to exogenous PNGase F for deglycosylation (Figure 1C). 55 kDa and 45 kDa Axl bands appeared with PNGase F treatment. However, 60-140 kDa Axl band density significantly decreased. These results suggest that the N-glycosylation process for Hca-F cells responded to tunicamycin and PNGase F treatment.
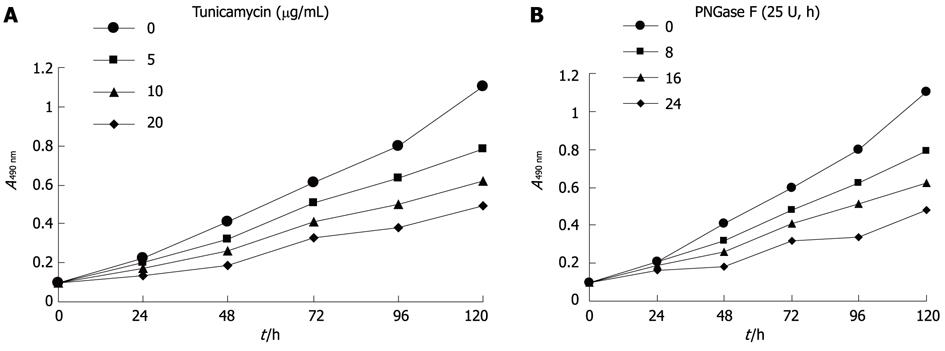
Hca-F cells treated with tunicamycin or PNGase F were measured for proliferative activity. Proliferation was apparent in untreated Hca-F cells compared with treated Hca-F cells (Figure 2A, B). Thus, Axl deglycosylation inhibited Hca-F cell proliferation in vitro.
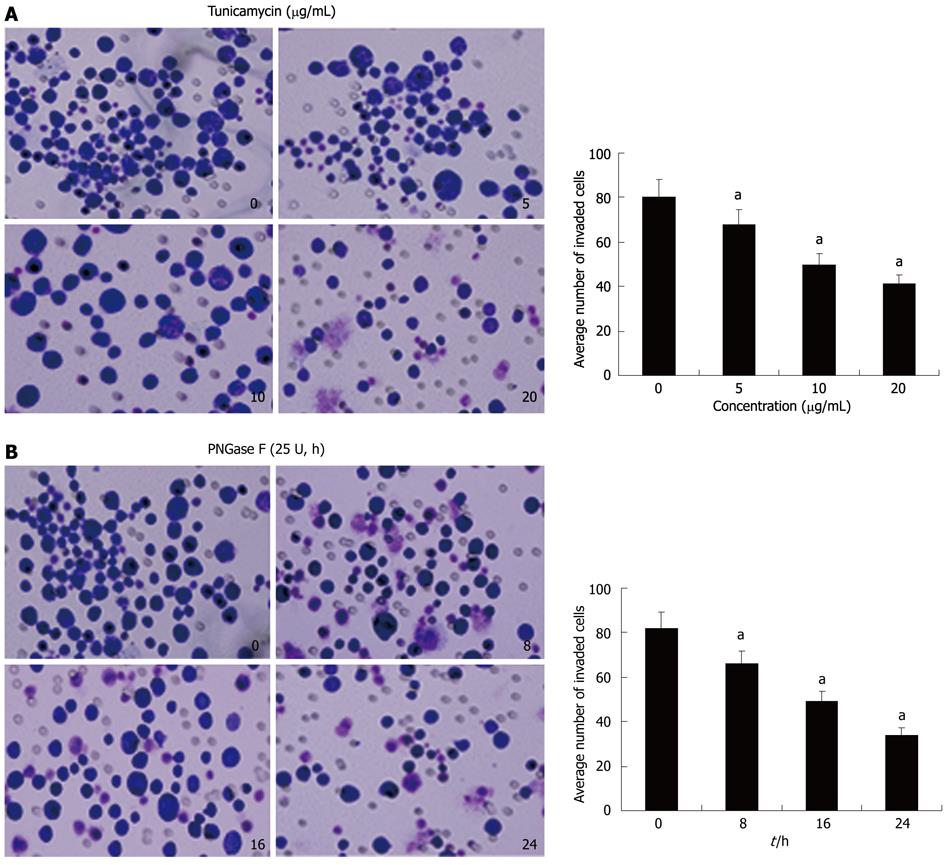
To examine whether Axl deglycosylation affects invasiveness of Hca-F cells, we performed in vitro ECMatrix gel analysis. We found that untreated and tunicamycin (Figure 3A) or PNGase F (Figure 3B) treated Hca-F cells or PNGase F had differing abilities to pass through an ECMatrix coated filter; therefore, the numbers of invading cells were unequal. Treated cells with tunicamycin passed through the ECMatrix gel in significantly smaller numbers than untreated cells (5 μg/mL: 68 ± 8 vs 80 ± 9, P = 0.0222; 10 μg/mL: 50 ± 6 vs 80 ± 9, P = 0.0003; 20 μg/mL: 41 ± 4 vs 80 ± 9, P = 0.0001). Similar results were shown with PNGase F treated Hca-F cells at 8 h, 16 h, and 24 h compared with untreated cells (66 ± 7 vs 82 ± 8, P = 0.0098; 49 ± 4 vs 82 ± 8, P = 0.0001; 34 ± 3 vs 82 ± 8, P = 0.0001). These results indicated that Axl deglycosylation reduced the invasiveness of Hca-F cells in vitro.
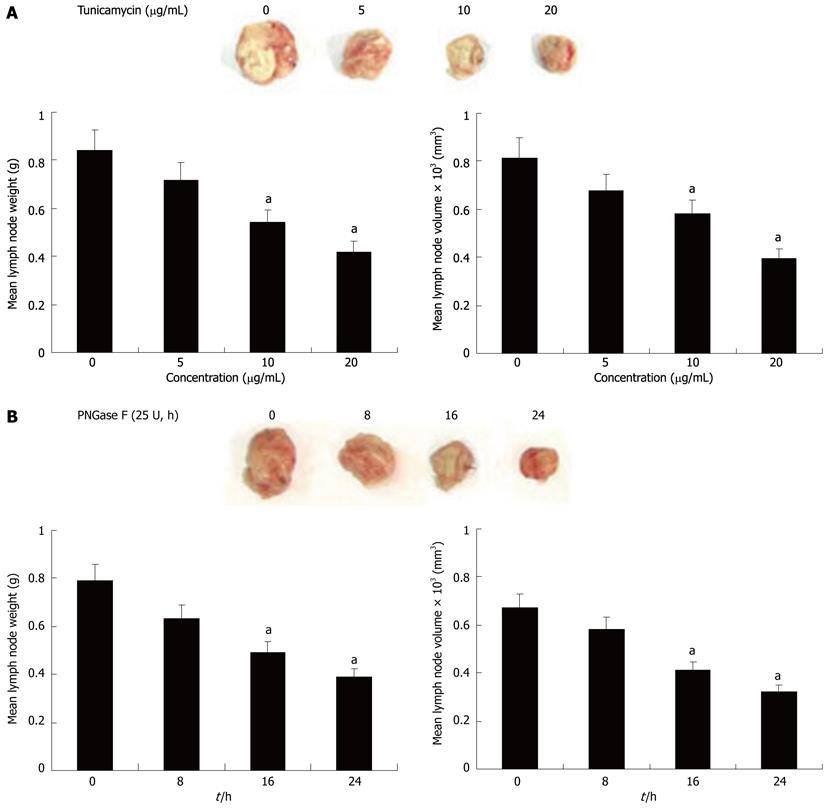
To further evaluate whether Axl deglycosylation was essential for tumor lymphatic metastasis in vivo, we tested the effect of Axl deglycosylation on the metastatic ability of Hca-F cells in mice peripheral lymph nodes. Treated and untreated Hca-F cells were injected in the footpads of 615-mice. After 3 wk’ inoculation, a significant reduction in positive lymph nodes in the deglycosylation groups was observed, compared with untreated controls (Figure 4). Average lymph node weights of the untreated Hca-F group compared with dose-adjusted tunicamycin treated Hca-F groups (5 μg/mL: 0.84 ± 0.21 g vs 0.72 ± 0.19 g, P = 0.3237; 10 μg/mL: 0.84 ± 0.21 g vs 0.54 ± 0.11 g, P = 0.0113; 20 μg/mL: 0.84 ± 0.21 g vs 0.42 ± 0.06 g, P = 0.0008) (Figure 4A left). The average lymph node volumes of these groups were 815 ± 61 mm3vs 680 ± 59 mm3, P = 0.0613; 815 ± 61 mm3vs 580 ± 29 mm3, P = 0.0001; 815 ± 61 mm3vs 395 ± 12 mm3, P = 0.0001 (Figure 4A right).
The average lymph node weights in the untreated Hca-F compared with PNGase F treated groups were: 8 h: 0.79 ± 0.15 g vs 0.63 ± 0.13 g, P = 0.0766; 16 h: 0.79 ± 0.15 g vs 0.49 ± 0.10 g, P = 0.0022; 24 h: 0.79 ± 0.15 g vs 0.39 ± 0.05 g, P = 0.0001 (Figure 4B left). The average lymph node volumes of these groups were: 670 ± 56 mm3vs 581 ± 48 mm3, P = 0.0532; 670 ± 56 mm3vs 412 ± 22 mm3, P = 0.0001; 670 ± 56 mm3vs 323 ± 11 mm3, P = 0.0001 (Figure 4B right). These results demonstrate Axl deglycosylation may reduce Hca-F cells to peripheral lymph nodes in vivo.
Axl has garnered attention because of its high expression in many tumor cells, and its key role in neoplastic invasion and metastasis. In this study, we demonstrated Axl protein expression varied based on antineoplastic treatment of mouse hepatocellular carcinoma cell lines Hca-F and Hca-P. We found Axl protein expression to be higher in Hca-F cells, which have high lymphatic metastasis potential compared with Hca-P cells, which have low lymphatic metastasis potential. This confirms previously reported findings of Axl overexpression in highly invasive lung adenocarcinoma cell lines, compared with their less invasive counterparts[11]. This suggests that high Axl expression may be associated with tumor lymphatic metastasis, and that Axl may be associated with tumor metastatic potential.
In our study, we achieved Hca-F deglycosylation with two methods. First, we inhibited N-glycan biosynthesis with tunicamycin; secondly, we extracted protein in the presence of PNGase F enzyme, which digests N-glycans. Both treatments resulted in significant effects on cell surface N-glycans by Western blotting assays.
Among post-translational modification reactions involving proteins, glycosylation is the most common; nearly 50% of all proteins are glycosylated[24]. Alterations of glycan structures are frequently observed in various cancer cells[25]; and this appears to be one association in cancer invasion and metastasis. We found Axl deglycosylation to be a possible factor in tumor progression, including cell proliferation, invasion, and lymphatic metastasis. In this study, we detected a significant inhibition of proliferation and invasion in Axl deglycosylated Hca-F cells in vitro, by both MTT and extracellular matrix assays. These results confirmed prior reports that cell proliferation requires growth factors signalling through cell surface glycoprotein receptors, which may be inactive when underglycosylated[26]. Although our findings support the role of Axl deglycosylation in reducing cell proliferation and invasion in vitro, its mechanism had not been elucidated. Further experiments showed that Axl deglycosylation led to a significant reduction in metastatic lymph node burden in vivo. These results were consistent previous reports of changes in N-linked oligosaccharide branching associated with malignancy and metastasis[27].
Some authors reported that the addition of exogenous Gas6 mediated the migration and invasion of Hca-F cells both in vitro and in vivo through the Axl pathway[28]. RNAi-mediated knockdown of Axl expression decreased the ability of YAP-expressing MIHA cells and of the primary HCC cell line to proliferate and invade[29]. In our study, we were unable to elucidate the mechanism by which Axl deglycosylation inhibits lymphatic metastasis in murine Hca-F cells. However, in many glycoproteins, N-linked oligosaccharides contribute to the folding, stability, and biological function of adhesion molecules and growth factor receptors on cell surfaces[30-32]. An increasing body of evidence indicates that glycoprotein glycans are involved in the regulation of cellular functions, including cell-cell communication and signal transduction[33,34]. The products of N-acetylglucosaminyltransferase (GnT)-IV, GnT-V and 1,6-fucosyltransferase (1,6-FucT) are all increased in hepatocellular carcinoma[35]. The presence of 1,6-GlcNAc structures in N-glycans and the expression of GnT-V, which catalyzes the addition of the 1,6-branching, were shown to promote metastasis[36-39]. At the very least, these reports demonstrate the relationship between metastasis and N-glycans to be extremely complicated. This area requires additional research.
In conclusion, we have found a role of Axl glycosylation in mediating tumor cell proliferation and invasion, and have provided the first evidence that Axl deglycosylation is required for lymphatic metastasis in murine hepatocellular carcinoma cell lines. These results may at least partially explain the role of Axl glycosylation in the promotion of lymphatic metastasis. This study may provide new insights into regulatory mechanisms of mouse hepatocellular carcinoma with lymphatic metastasis.
Axl has been shown to have transforming capability when overexpressed. Prior studies have revealed Axl overexpression to be clearly associated with cancer invasiveness and metastasis. Axl also has multiple effects in angiogenesis. While Axl may play an important role in tumor progression, its mechanisms of action have not been understood.
The authors investigated the potential effect of Axl deglycosylation the regulation of tumor lymphatic metastasis in mouse hepatocellular carcinoma cell lines. The authors evaluated the expression profile of Axl glycoprotein in the mouse hepatocellular carcinoma cell line Hca-F, which was treated with tunicamycin and PNGase F. Furthermore, the authors analyzed the effect of Axl glycosylation by tunicamycin and PNGase F treatment in Hca-F cells with regards to proliferation, invasion, and lymphatic metastasis both in vitro and in vivo.
Protein N-glycosylation is increasingly being recognized as one of the most prominent biochemical alterations in tumorigenesis and metastatic spread. However, as a cell surface transmembrane glycoprotein, little is known about Axl deglycosylation and its mechanism of action. Axl glycosylation was attenuated by tunicamycin and PNGase F to determine the effect on Hca-F cell proliferation, invasion, and lymphatic metastasis.
The authors have found the role of Axl glycosylation in mediating tumor cells proliferation, invasion and provided the first evidence that deglycosylation of Axl is required for metastasis of hepatocellular carcinoma cells to lymph nodes. This study may provide new insights into regulatory mechanisms of mouse hepatocellular carcinoma with lymphatic metastasis.
The Axl receptor (also named UFO, Tyro7, and Ark) is a receptor tyrosine kinase (RTK) originally identified as a transforming gene in chronic myeloid leukemia. The RTKs constitute a large family of transmembrane proteins that relay signals from extracellular growth factors into the cell.
In this study, the effect of Axl deglycosylation on lymphatic metastasis was investigated in mouse hepatocellular carcinoma cell lines. Differing Axl expression levels were found in mouse Hca-F and Hca-P cell lines, which are characterized by high and low metastatic potential, respectively. A decrease in Axl glycosylation by tunicamycin or PNGase F treatment resulted in a reduced proliferation, invasion, and lymphatic metastasis, both in vitro and in vivo. This work is potentially relevant in understanding hepatocellular carcinoma.
| 1. | Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3110] [Cited by in RCA: 3124] [Article Influence: 120.2] [Reference Citation Analysis (1)] |
| 2. | Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548-5557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Hafizi S, Dahlbäck B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev. 2006;17:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35-83. [PubMed] |
| 5. | Janssen JW, Schulz AS, Steenvoorden AC, Schmidberger M, Strehl S, Ambros PF, Bartram CR. A novel putative tyrosine kinase receptor with oncogenic potential. Oncogene. 1991;6:2113-2120. [PubMed] |
| 6. | O'Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, Espinosa R, Le Beau MM, Earp HS, Liu ET. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016-5031. [PubMed] |
| 7. | Faust M, Ebensperger C, Schulz AS, Schleithoff L, Hameister H, Bartram CR, Janssen JW. The murine ufo receptor: molecular cloning, chromosomal localization and in situ expression analysis. Oncogene. 1992;7:1287-1293. [PubMed] |
| 8. | Prieto AL, Weber JL, Lai C. Expression of the receptor protein-tyrosine kinases Tyro-3, Axl, and mer in the developing rat central nervous system. J Comp Neurol. 2000;425:295-314. [PubMed] |
| 9. | Rochlitz C, Lohri A, Bacchi M, Schmidt M, Nagel S, Fopp M, Fey MF, Herrmann R, Neubauer A. Axl expression is associated with adverse prognosis and with expression of Bcl-2 and CD34 in de novo acute myeloid leukemia (AML): results from a multicenter trial of the Swiss Group for Clinical Cancer Research (SAKK). Leukemia. 1999;13:1352-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Nemoto T, Ohashi K, Akashi T, Johnson JD, Hirokawa K. Overexpression of protein tyrosine kinases in human esophageal cancer. Pathobiology. 1997;65:195-203. [PubMed] |
| 11. | Shieh YS, Lai CY, Kao YR, Shiah SG, Chu YW, Lee HS, Wu CW. Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia. 2005;7:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 224] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 12. | Craven RJ, Xu LH, Weiner TM, Fridell YW, Dent GA, Srivastava S, Varnum B, Liu ET, Cance WG. Receptor tyrosine kinases expressed in metastatic colon cancer. Int J Cancer. 1995;60:791-797. [PubMed] |
| 13. | Chung BI, Malkowicz SB, Nguyen TB, Libertino JA, McGarvey TW. Expression of the proto-oncogene Axl in renal cell carcinoma. DNA Cell Biol. 2003;22:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Sainaghi PP, Castello L, Bergamasco L, Galletti M, Bellosta P, Avanzi GC. Gas6 induces proliferation in prostate carcinoma cell lines expressing the Axl receptor. J Cell Physiol. 2005;204:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 164] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Meric F, Lee WP, Sahin A, Zhang H, Kung HJ, Hung MC. Expression profile of tyrosine kinases in breast cancer. Clin Cancer Res. 2002;8:361-367. [PubMed] |
| 16. | Wu CW, Li AF, Chi CW, Lai CH, Huang CL, Lo SS, Lui WY, Lin WC. Clinical significance of AXL kinase family in gastric cancer. Anticancer Res. 2002;22:1071-1078. [PubMed] |
| 17. | Ito M, Nakashima M, Nakayama T, Ohtsuru A, Nagayama Y, Takamura N, Demedchik EP, Sekine I, Yamashita S. Expression of receptor-type tyrosine kinase, Axl, and its ligand, Gas6, in pediatric thyroid carcinomas around chernobyl. Thyroid. 2002;12:971-975. [PubMed] |
| 18. | Bertozzi CR, Kiessling LL. Chemical glycobiology. Science. 2001;291:2357-2364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1561] [Cited by in RCA: 1584] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 19. | Solís D, Jiménez-Barbero J, Kaltner H, Romero A, Siebert HC, von der Lieth CW, Gabius HJ. Towards defining the role of glycans as hardware in information storage and transfer: basic principles, experimental approaches and recent progress. Cells Tissues Organs. 2001;168:5-23. [PubMed] |
| 20. | Dennis JW, Granovsky M, Warren CE. Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta. 1999;1473:21-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 507] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 21. | Dwek RA. Glycobiology: Toward Understanding the Function of Sugars. Chem Rev. 1996;96:683-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2320] [Cited by in RCA: 2232] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 22. | Ihara S, Miyoshi E, Ko JH, Murata K, Nakahara S, Honke K, Dickson RB, Lin CY, Taniguchi N. Prometastatic effect of N-acetylglucosaminyltransferase V is due to modification and stabilization of active matriptase by adding beta 1-6 GlcNAc branching. J Biol Chem. 2002;277:16960-16967. [PubMed] |
| 23. | Zhu P, Ding J, Zhou J, Dong WJ, Fan CM, Chen ZN. Expression of CD147 on monocytes/macrophages in rheumatoid arthritis: its potential role in monocyte accumulation and matrix metalloproteinase production. Arthritis Res Ther. 2005;7:R1023-R1033. [PubMed] |
| 24. | Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1421] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 25. | Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309-5318. [PubMed] |
| 26. | Ding DX, Vera JC, Heaney ML, Golde DW. N-glycosylation of the human granulocyte-macrophage colony-stimulating factor receptor alpha subunit is essential for ligand binding and signal transduction. J Biol Chem. 1995;270:24580-24584. [PubMed] |
| 27. | Kudo T, Nakagawa H, Takahashi M, Hamaguchi J, Kamiyama N, Yokoo H, Nakanishi K, Nakagawa T, Kamiyama T, Deguchi K. N-glycan alterations are associated with drug resistance in human hepatocellular carcinoma. Mol Cancer. 2007;6:32. [PubMed] |
| 28. | He L, Zhang J, Jiang L, Jin C, Zhao Y, Yang G, Jia L. Differential expression of Axl in hepatocellular carcinoma and correlation with tumor lymphatic metastasis. Mol Carcinog. 2010;49:882-891. [PubMed] |
| 29. | Xu MZ, Chan SW, Liu AM, Wong KF, Fan ST, Chen J, Poon RT, Zender L, Lowe SW, Hong W. AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene. 2011;30:1229-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 198] [Article Influence: 13.2] [Reference Citation Analysis (1)] |
| 30. | Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res. 1989;52:257-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 821] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 31. | Dwek RA. Glycobiology: more functions for oligosaccharides. Science. 1995;269:1234-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Wyss DF, Choi JS, Li J, Knoppers MH, Willis KJ, Arulanandam AR, Smolyar A, Reinherz EL, Wagner G. Conformation and function of the N-linked glycan in the adhesion domain of human CD2. Science. 1995;269:1273-1278. [PubMed] |
| 33. | Saxon E, Bertozzi CR. Chemical and biological strategies for engineering cell surface glycosylation. Annu Rev Cell Dev Biol. 2001;17:1-23. [PubMed] |
| 34. | Taniguchi N, Miyoshi E, Gu J, Honke K, Matsumoto A. Decoding sugar functions by identifying target glycoproteins. Curr Opin Struct Biol. 2006;16:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Yamashita K, Koide N, Endo T, Iwaki Y, Kobata A. Altered glycosylation of serum transferrin of patients with hepatocellular carcinoma. J Biol Chem. 1989;264:2415-2423. [PubMed] |
| 36. | Dennis JW, Laferté S, Waghorne C, Breitman ML, Kerbel RS. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987;236:582-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 740] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 37. | Yoshimura M, Nishikawa A, Ihara Y, Taniguchi S, Taniguchi N. Suppression of lung metastasis of B16 mouse melanoma by N-acetylglucosaminyltransferase III gene transfection. Proc Natl Acad Sci USA. 1995;92:8754-8758. [PubMed] |
| 38. | Granovsky M, Fata J, Pawling J, Muller WJ, Khokha R, Dennis JW. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat Med. 2000;6:306-312. [PubMed] |
| 39. | Murata K, Miyoshi E, Kameyama M, Ishikawa O, Kabuto T, Sasaki Y, Hiratsuka M, Ohigashi H, Ishiguro S, Ito S. Expression of N-acetylglucosaminyltransferase V in colorectal cancer correlates with metastasis and poor prognosis. Clin Cancer Res. 2000;6:1772-1777. [PubMed] |
Peer reviewer: Francesco Feo, Professor, Department of Biomedical Sciences, Section of Experimental Pathology and Oncology, University of Sassari, Via P, Manzella 4, 07100 Sassari, Italy
S- Editor Lv S L- Editor A E- Editor Zhang DN