Published online Oct 14, 2012. doi: 10.3748/wjg.v18.i38.5351
Revised: July 10, 2012
Accepted: July 18, 2012
Published online: October 14, 2012
AIM: To investigate the anti-inflammatory properties of Lacto-Wolfberry (LWB), both in vitro and using a mouse model of experimental colitis.
METHODS: The effects of LWB on lipopolysaccharide (LPS)-induced reactive oxygen species (ROS) and interleukin (IL)-6 secretion were assessed in a murine macrophage cell line. in vitro assessment also included characterizing the effects of LWB on the activation of NF-E2 related 2 pathway and inhibition of tumor necrosis factor-α (TNF-α)-induced nuclear factor-κB (NF-κB) activation, utilizing reporter cell lines. Following the in vitro assessment, the anti-inflammatory efficacy of an oral intervention with LWB was tested in vivo using a preclinical model of intestinal inflammation. Multiple outcomes including body weight, intestinal histology, colonic cytokine levels and anti-oxidative measures were investigated.
RESULTS: LWB reduced the LPS-mediated induction of ROS production [+LPS vs 1% LWB + LPS, 1590 ± 188.5 relative luminescence units (RLU) vs 389 ± 5.9 RLU, P < 0.001]. LWB was more effective than wolfberry alone in reducing LPS-induced IL-6 secretion in vitro (wolfberry vs 0.5% LWB, 15% ± 7.8% vs 64% ± 5%, P < 0.001). In addition, LWB increased reporter gene expression via the anti-oxidant response element activation (wolfberry vs LWB, 73% ± 6.9% vs 148% ± 28.3%, P < 0.001) and inhibited the TNF-α-induced activation of the NF-κB pathway (milk vs LWB, 10% ± 6.7% vs 35% ± 3.3%, P < 0.05). Furthermore, oral supplementation with LWB resulted in a reduction of macroscopic (-LWB vs +LWB, 5.39 ± 0.61 vs 3.66 ± 0.59, P = 0.0445) and histological scores (-LWB vs +LWB, 5.44 ± 0.32 vs 3.66 ± 0.59, P = 0.0087) in colitic mice. These effects were associated with a significant decrease in levels of inflammatory cytokines such as IL-1β (-LWB vs +LWB, 570 ± 245 μg/L vs 89 ± 38 μg/L, P = 0.0106), keratinocyte-derived chemokine/growth regulated protein-α (-LWB vs +LWB, 184 ± 49 μg/L vs 75 ± 20 μg/L, P = 0.0244), IL-6 (-LWB vs +LWB, 318 ± 99 μg/L vs 117 ± 18 μg/L, P = 0.0315) and other pro-inflammatory proteins such as cyclooxygenase-2 (-LWB vs +LWB, 0.95 ± 0.12 AU vs 0.36 ± 0.11 AU, P = 0.0036) and phosphorylated signal transducer and activator of transcription-3 (-LWB vs +LWB, 0.51 ± 0.15 AU vs 0.1 ± 0.04 AU, P = 0.057). Moreover, antioxidant biomarkers, including expression of gene encoding for the glutathione peroxidase, in the colon and the plasma anti-oxidant capacity were significantly increased by supplementation with LWB (-LWB vs +LWB, 1.2 ± 0.21 mmol/L vs 2.1 ± 0.19 mmol/L, P = 0.0095).
CONCLUSION: These results demonstrate the anti-inflammatory properties of LWB and suggest that the underlying mechanism is at least in part due to NF-κB inhibition and improved anti-oxidative capacity.
- Citation: Philippe D, Brahmbhatt V, Foata F, Saudan Y, Serrant P, Blum S, Benyacoub J, Vidal K. Anti-inflammatory effects of Lacto-Wolfberry in a mouse model of experimental colitis. World J Gastroenterol 2012; 18(38): 5351-5359
- URL: https://www.wjgnet.com/1007-9327/full/v18/i38/5351.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i38.5351
Inflammatory bowel disease (IBD), which includes Crohn’s disease (CD) and ulcerative colitis (UC), represents a group of chronic disorders characterized by inflammation of the gastrointestinal tract, typically with a relapsing and remitting clinical course. They affect between 0.5%-1% of the Western world’s population[1]. The primary focus of IBD therapy is to induce remission of acute inflammatory flare ups and to maintain the state of remission.
The innate immune system plays a central role in the acute inflammatory process. As part of the innate immune response, neutrophils are one of the early responders to local injury. Both, the circulating levels and activation of neutrophils, are increased in IBD patients with active disease[2,3]. Activated neutrophils and monocytes release a plethora of mediators including reactive oxygen species (ROS), eicosanoids and proinflammatory cytokines. In fact, the therapeutic benefit of depleting granulocytes in CD patients has been demonstrated[4]. Apart from neutrophils, monocytes and mucosal macrophages play an important role in the development of IBD as shown by an increase of the number of recruited monocytes and activated macrophages in the inflamed gut of patients with IBD[5]. Indeed during active inflammation, neutrophils recruit and activate monocytes which themselves secrete pro-inflammatory mediators such as tumor necrosis factor (TNF-α), interleukin (IL)-1β and IL-6[6].
A vast body of literature supports the role of nutritional therapy in IBD, particularly in CD (reviewed in[7,8]). While enteral nutrition is not as effective as steroid therapy in induction of remission in CD, the benefit to the patient is well established[9]. Thus, identification and characterization of novel anti-inflammatory foods may aid in improving the currently available nutritional formulations.
A variety of functional nutrients, such as glycosides[10], alkaloids[11] and black tea extracts[12], have been shown to exert their beneficial effects through inhibition of Nuclear factor-κB (NF-κB) activation. NF-κB is one of the most important regulators of pro-inflammatory cytokine expression and reducing its activity may have beneficial effects under acute inflammatory conditions[13]. Besides NF-κB, phytochemicals are also known to activate NF-E2 related 2 (Nrf2) pathway through the anti-oxidant response element causing an increase in the anti-oxidative enzymes such as catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase[14].
Wolfberry, the fruit of Lycium barbarum-also called as Goji (Gouqi or Gou QZ in Romanized Chinese), is a sweet red berry, which has been traditionally used in Chinese medicine. It is also one of the richest sources of zeaxanthin, an antioxidant that has been postulated to improve visual acuity[15]. Apart from antioxidant activity, wolfberry juice has also been demonstrated to have immunomodulatory effects[16]. However, a large part of the supporting evidence is derived either from in vitro experiments or animal studies wherein, wolfberry extracts were delivered parenterally. We believe that this might be due to reduced bioavailability of active ingredients when given enterally. Therefore, to improve bioavailability of its anti-oxidant components, wolfberry was processed with skimmed milk and freeze-dried to generate Lacto-Wolfberry (LWB), a water-dispersible powder[17]. This novel preparation, which contains approximately 50% wolfberry and 25% skimmed milk, has been clinically demonstrated to improve the bioavailability of zeaxanthin[17]. Subsequently, the immune-enhancing properties of LWB in both, young-adult and aged mice, have been characterized[18]. Recent studies have demonstrated that dietary supplementation with LWB enhances immune response to flu vaccine[19] and plasma oxidative capacity in elderly[20]. The aim of this study was to characterize the anti-inflammatory and anti-oxidative properties of LWB. We first demonstrate that LWB inhibits lipopolysaccharide (LPS)-induced ROS and IL-6 production in a murine macrophage cell line. Next, using reporter cell lines we show that LWB activates Nrf2 pathway, while inhibiting the NF-κB pathway. Finally, using a mice model of colitis, we demonstrate that LWB reduces the severity of colitis by mediating a reduction in pro-inflammatory cytokines, namely IL-6, IL1β and keratinocyte-derived chemokine/growth regulated protein-α (KC/GRO-α).
The murine macrophage cell line RAW 264.7 (ATCC, United States) was maintained in Dulbecco’s modified Eagle medium (DMEM, Amimed, Bioconcept, Switzerland) supplemented with 10% heat-inactivated fetal-calf serum (FCS, Amimed) at 37 °C in a 50 mL/L CO2/air incubator. Intracellular ROS was measured using a ROS-sensitive fluorescent dye, 2’,7’-dichlorofluorescin diacetate (DCFH2-DA, Sigma, United States). Cells (105 cells/well in 96 well plates) were incubated overnight with LPS, from Escherichia coli serotype 055:B5 (Sigma, United States) at 0.5 mg/L, either in the absence or presence of LWB (0.1% or 1% final concentration). Control cells in the absence of LPS were also included. Cells were then treated with 10 μmol DCFH2-DA for 30 min at 37 °C and washed twice with phosphate-buffered saline (PBS). Fluorescence was measured at 485 nm excitation and 538 nm emission by a Fluoroskan enzyme linked immunosorbent assay plate reader (Labsystems Oy, Finland) at the indicated time points. For experiments measuring IL-6, RAW 264.7 cells were seeded in 96 well plates at 104 cells/well. After 3 d (approximately 80% of confluence), cells were stimulated with LPS at 0.5 mg/L and incubated in the presence of either LWB (1% final concentration), wolfberry (0.5%) or skimmed milk (0.25%) for 24 h at 37 °C. Cell culture supernatants were then harvested and IL-6 secretion was quantified using commercial enzyme linked immunosorbant assay (ELISA) kit according to manufacturer’s protocol (Murine IL-6 Eli-pair, Diaclone, France). Cell viability was determined by CellTiter-Glow Luminescent assay (Promega, United States) according to manufacturer’s instructions. It should be noted that the same lots of wolfberry and skimmed milk that were used in the preparation of LWB were used for all experiments described.
The human colonic adenocarcinoma cell line, HT-29 (ATCC, United States), was stably transfected with the plasmid pNF-κB-SEAP-NPT. The plasmid was a kind gift from Prof. Kim (Natural Products Research Institute, Seoul). It contains a secreted alkaline phosphatase (SEAP) encoding sequence downstream of four tandem copies of NF-κB binding sites. Stably transfected cells (HT-29 clone 34) were maintained in high glucose (4.5 g/L) DMEM containing 1% L-glutamine, 10% heat-inactivated FCS, 1% penicillin/streptomycin, 500 mg/L G418 (Invitrogen, Switzerland) and 100 mg/L Normocin (Invivogen, France) at 37 °C in a 50 mL/L CO2/air incubator. For the NF-κB inhibition assay, HT-29 clone 34 cells were seeded at 104 cells/well in 96-flat bottom well plates. After 3-4 d of culture (approximately 80% confluence), cells were washed with PBS and stimulated with recombinant TNF-α (10 μg/L, RD systems, England) in the absence or the presence of either LWB (1% final concentration), wolfberry (0.5%) or skimmed milk (0.25%) for 24 h at 37 °C. SEAP release was assessed in the supernatants using the Phospha-LightTM System (Applied Biosystems, United States) according to manufacturer’s protocol.
AREc32 (CXR biosciences, United Kingdom) is a reporter cell line that stably expresses the anti-oxidant response element (ARE)-driven luciferase gene[21]. These cells were cultured at 12 000 cells/well in 96 well plates (Nunc) at 37 °C and 50 mL/L CO2/air incubator in DMEM supplemented with 10% FCS. After 1 d, cells were washed with PBS and treated, in the absence of serum, with either, LWB (1% final concentration), wolfberry (0.5%) or skimmed milk (0.25%) for 24 h at 37 °C. Luciferase activity was measured using the Luciferase Assay™ (Promega, United States) following manufacturer’s instructions.
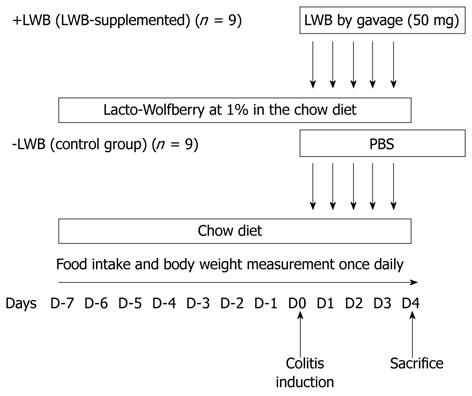
Male mice (C57BL/6J), aged 7 wk, were obtained from Charles River Laboratories Inc. (France) and housed five per cage in a temperature-controlled room with free access to food and water. The overall study design is provided in Figure 1. Mice (n = 9 per group) were randomly assigned to either the control (LWB, chow fed) or the LWB-supplemented group (+LWB, 1% in the diet) 7 d prior to colitis induction (D-7), which was induced on D0, as previously described[22]. Chemically induced colitis was performed as described earlier[23]. Briefly, colitis was induced with an intrarectal administration of 2,4,6-trinitrobenzene sulfonic acid (TNBS) at 125 mg/kg dissolved in 50% ethanol solution. In order to compensate for reduced food intake after colitis induction, mice were also supplemented by daily gavage with 50 mg of LWB from D0 to D4. Control mice were gavaged with an equal volume of PBS. Food intake and body weight measurements were taken once daily from D-7 to D4. Animals were sacrificed on D4. All experimental protocols were conducted in accordance with Swiss law and Nestlé policy on ethics and animal welfare.
The distal colon tissue was washed with PBS and macroscopic scoring was performed using the system of Wallace et al[24]. Samples of the inflamed tissues (1 cm above the anal canal) were collected for histological analysis. The tissues were fixed in 4% paraformaldehyde at 4 °C for 24 h. Sections were prepared, stained with hematoxylin and eosin, and graded according to Ameho et al[25].
Colon tissue homogenization, RNA extraction and reverse transcription were performed as described earlier[22]. Custom-made Low Density Array (LDA) cards were purchased from Applied Biosystems (United States) and used according to manufacturer’s instructions. Briefly, mixes (100 μL), containing 100 ng of cDNA, 2X TaqMan Mix and nuclease-free water, were prepared and loaded onto the LDA card. The LDA cards were then processed using an automated fluorometer ABI Prism 7900HT. Gene expression was calculated using the relative quantification method with SDS 2.2.2 software.
Colon tissue homogenization, protein extraction, electrophoresis and Western blotting analysis were performed as described earlier[22]. Briefly, after tissue homogenization, protein was quantified using the RC DC Protein Assay (Bio-Rad, United States). Proteins were loaded and separated on a 4%-12% bis-tris gel (Invitrogen). The blot was probed with antibodies against murine cyclooxygenase-2 (COX-2) (Cayman, United States), signal transducer and activator of transcription-3 (STAT-3) and phosphorylated STAT-3 (pSTAT-3, Cell Signalling Technology, United States) and β-Actin (Sigma, United States). Relative quantitation of bands was determined using Scion Image Densitometry System (Scion Corp., United States), with normalization to β-Actin.
Protein levels for myeloperoxidase (MPO) were measured in colon protein extracts by ELISA following the manufacturer’s instructions (Hycult biotechnology, The Netherlands).
IL-1β, IL-6, IL-10, IL-12p70, KC/GRO-α, interferon-γ (IFN-γ) and TNF-α were measured in the colon protein extracts using multiplex assay kits (Meso Scale Discovery, United States) according to manufacturer’s protocol. Cytokine concentrations were determined with Discovery Workbench 3.0 software, using curve 4-PL as suggested by the manufacturer.
Total anti-oxidant capacity of plasma was performed using an assay, which measures inhibition of 2,2’-azino-di-(3-ethylbenzthiazoline sulphonate) (ABTS®) to ABTS®+ by metmyoglobin as Trolox equivalents (Cayman, United States), according to manufacturer’s protocol.
Data were analyzed by means ± SE either the Mann-Whitney test or where appropriate, a two-way analysis of variance with a Bonferroni post test. P values of less than 5% were considered as significant.
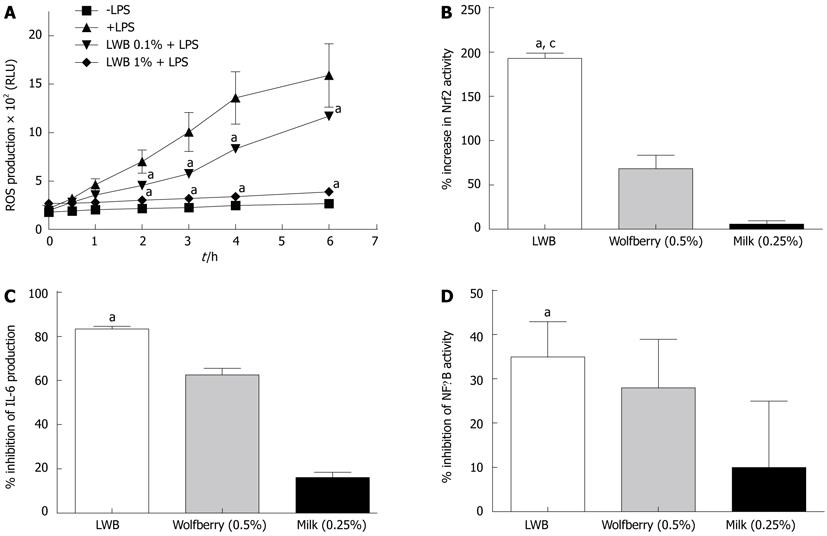
Anti-oxidant effects: ROS production from LPS-stimulated RAW 264.7 cells was evaluated in the presence and absence of LWB (Figure 2A). As expected, LPS increased ROS production, in a time dependent manner, as compared to the untreated controls (-LPS). Interestingly, LWB was able to reduce LPS-induced ROS production. The inhibitory effect of LWB was significant from the 2 h time point at both 0.1% and 1% final concentration. At the 6 h time point, LWB reduced the amount of LPS-induced ROS production by about 25% and 75%, at the concentrations of 0.1% and 1%, respectively (P < 0.001 for both concentrations). Next, the effects of LWB on Nrf2 activation, using a stable ARE-driven reporter gene expressing cell line, AREc32[21], were evaluated (Figure 2B). The data show that LWB at 1% final concentration increased Nrf2 activity by approximately 200%, whereas its individual components, i.e., wolfberry (0.5%) and milk (0.25%), induced only a mild or almost no increase in Nrf2 activity, respectively.
Anti-inflammatory effects: Finally, the effects of LWB (1%), wolfberry (0.5%) and milk (0.25%) on LPS-induced IL-6 production (Figure 2C) and TNF-α-induced NF-κB activity (Figure 2D) were assessed. As shown, LWB inhibited LPS-induced IL-6 production in RAW 264.7 cells by approximately 80% and TNF-α-induced NF-κB activity by approximately 35%. These were significantly different from the values obtained for milk, approximately 20% and 10%, respectively (P < 0.001 and P < 0.05, respectively). However, no significant differences were observed from wolfberry, which inhibited IL-6 production by approximately 65% and NF-κB activation by 10%.
The anti-inflammatory effects of LWB were characterized in chemically-induced colitis model as described in materials and methods section.
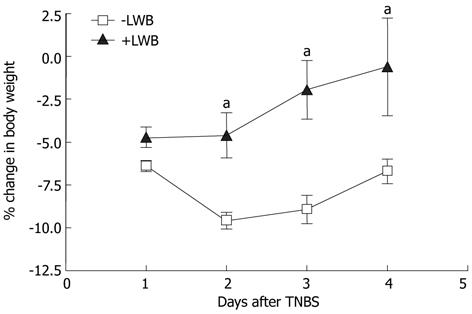
LWB attenuates colitis-induced body weight loss: Body weight and food intake of each mouse was monitored daily. Following colitis induction at D0, the percentage mean change in body weight of control mice was -6.4, -9.5, -8.9 and -7.6 at D1, D2, D3 and D4, respectively (Figure 3, LWB). The mice fed with LWB had a percentage mean change in body weight of -4.7, -4.6, -1.9 and -0.5 at D1, D2, D3 and D4, respectively (Figure 3, +LWB). Thus, while both mice have a reduction in body weight at D1, the reduction in body weight loss of the LWB fed mice were significantly lower from D2-D4 (P < 0.05 at D2 and P < 0.01 at D3 and D4). The total food intake between the two groups did not change (data not shown).
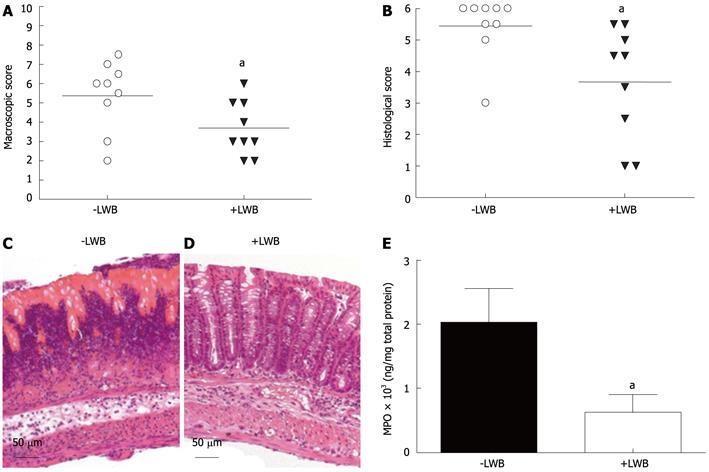
LWB reduces colonic inflammation: Supplementation with LWB significantly reduced the colonic inflammation as judged by macroscopic (Figure 4A) and histological (Figure 4B, C and D) evaluation of intestinal inflammation. Macroscopic lesions were assessed as delineated by Wallace et al[24]. Colons of control mice (-LWB) presented significantly higher scores compared to mice fed with LWB, 5.39 ± 0.61 and 3.66 ± 0.47, respectively (Figure 4A, P < 0.05). In agreement with the macroscopic assessment, histological evaluation showed lower inflammatory infiltrates and better mucosal integrity in mice fed with LWB (+LWB, Figure 4D) as compared to the control (-LWB, Figure 4C). This difference was reflected in the histological score, 5.44 ± 0.32 and 3.66 ± 0.59, for the control and LWB-fed mice, respectively, which was significantly different (Figure 4B, P < 0.01). The 70% reduction of MPO content in the LWB treated group (+LWB) as compared to control mice (-LWB) provides further support to reduced neutrophil infiltration in the LWB group (Figure 4E, P < 0.05).
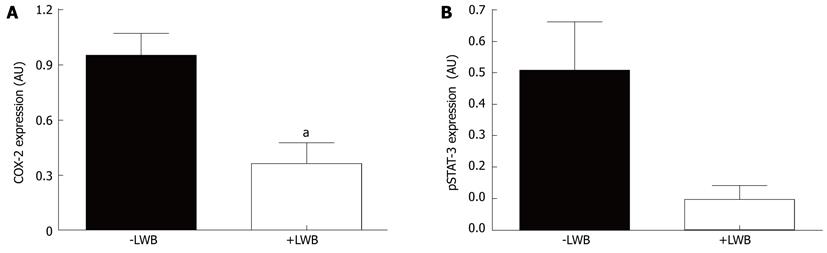
LWB reduces pro-inflammatory effector proteins: To delve deeper into the effect of LWB, levels of various effector proteins were measured in the colon tissue. As shown in Table 1, levels of IL-1β, IL-6 and KC/GRO-α were significantly reduced in colitic mice fed with LWB (+LWB) compared to the control (-LWB). Moreover IL-10, IFN-γ, TNF-α and IL-12p70 levels were also reduced by more than 50%, however these were not statistically significant. COX-2 and pSTAT3 levels were assessed semi- quantitatively by Western blotting analysis and densitometry. As shown in Figure 5A, colon of mice fed with LWB had approximately 65% reduction of COX-2 levels (P < 0.01). Mice fed with LWB also demonstrated an 80% reduction in pSTAT3 expression (Figure 5B) in the colon. However, this difference didn’t reach statistical significance (P = 0.057).
| Cytokines (μg/L) | -LWB | +LWB | Reduction (%) | P value |
| TNF-α | 80 ± 24 | 31 ± 4 | 61 | 0.0770 |
| IL-1β | 570 ± 245 | 89 ± 38 | 84 | 0.0106 |
| IL-6 | 318 ± 99 | 117 ± 18 | 63 | 0.0315 |
| KC/GRO-α | 184 ± 49 | 75 ± 20 | 59 | 0.0244 |
| IL-12p70 | 415 ± 129 | 89 ± 38 | 79 | 0.1135 |
| IFN-γ | 18 ± 6 | 7 ± 1 | 61 | 0.0770 |
| IL-10 | 428 ± 141 | 165 ± 27 | 61 | 0.0625 |
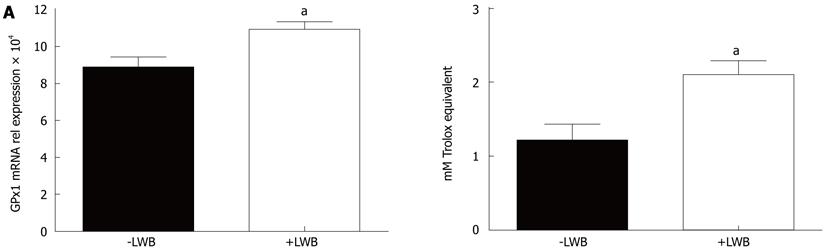
LWB improves anti-oxidative capacity: Finally, changes in mRNA expression of Nrf2 target genes, such as CAT, SOD2 and glutathione peroxidase (GPx1) were examined in the colon tissue. GPx1 mRNA expression was higher by more than 20% in mice fed with LWB (+LWB) compared to control (-LWB) (Figure 6A, P < 0.02). No difference of gene expression was detected for CAT and SOD2 (data not shown). Finally, the measures of anti-oxidant capacity in the plasma demonstrated that mice supplemented with LWB (+LWB) had more than 70% increase in anti-oxidative capacity compared to control mice (-LWB) (Figure 6B, P < 0.01).
The aim of the study was to investigate the anti-inflammatory properties of LWB in vitro and in an animal model of intestinal inflammation. Initial experiments showed that LWB reduces LPS-induced ROS generation. The anti-oxidant effects of wolfberry are well characterized[16]. Moreover, LWB has also been proposed to have ROS scavenging activity[26]. Thus, this finding was not surprising. However, phytochemicals have also been shown to activate the Nrf2 pathway[14]. Nrf2 is a redox-sensitive transcription factor, which regulates the expression of ARE-driven anti-oxidant enzymes[14]. Thus, the effect of LWB on Nrf2 activation was tested. Interestingly, LWB had a two fold increase in Nrf2 activation, however this effect was not fully replicated by its major components tested separately, i.e., either, wolfberry or milk. Hence, this could suggest a synergistic effect between the two major components of LWB resulting from the LWB manufacturing process. Finally, LWB demonstrated wolfberry-equivalent inhibition of LPS-induced IL-6 secretion and TNF-α-induced NF-κB activation. The beneficial effects observed in vitro prompted a further examination of LWB in an animal model of colitis.
The pro-inflammatory roles of ROS production, NF-κB activation and cytokines, such as IL-1β, IL-6, IL-8 and TNF-α, have been firmly established in IBD pathology[13,27,28]. Thus, the effects of LWB were tested in a murine model of colitis. Firstly, LWB supplementation attenuated colitis-induced body weight loss. Secondly, both colonic parameters, macroscopic and microscopic, confirmed a reduction in the severity of colitis after LWB intervention. In further support, supplementation with LWB reduced neutrophil infiltration in the colon tissue. In this experimental model, the secretion of Th1 cytokines, namely, IL-1β, IL-6, TNF-α and IFN-γ, play an important role in the propagation of colitis[29]. As NFκB activation controls expression of most of these genes[13] and considering the in vitro inhibitory effects of LWB on NF-κB activation, the cytokine levels were measured to gain mechanistic insight. Supplementation with LWB resulted in reduced levels of majority of these cytokines, while significantly reducing the levels of IL-1β and IL-6. Further, LWB supplementation reduced not just the levels of IL-6, but also the downstream signaling via STAT-3 activation. Interestingly however, there was only non significant trend observed in the reduction of TNF-α. It is possible that this could be either due to technical variability or due to different kinetics of this cytokine. Moreover, the significant reduction in KC/GRO-α, an established chemokine involved in neutrophil chemotaxis[30], can explain the reduction in neutrophil infiltration. Thus, modulation of cytokine levels, perhaps via a decrease in NF-κB activation, is responsible for the anti-inflammatory effects of LWB in this study. The increase of Nrf2 activation in vitro as well as an increase in the plasma anti-oxidative capacity and upregulation of GPx1 after LWB supplementation in colitic mice also suggests a possibility of an anti-oxidant mechanism underlying the anti-inflammatory effects of LWB. However, the lack of upregulation of other Nrf2 target genes, such as, CAT and SOD2 means that the anti-oxidative properties explains only part of the overall effects observed.
Wolfberry is believed to contain at least three different biologically active components: (1) Lycium Barbarum polysaccharides (LBP); (2) zeaxanthin dipalmitate; and (3) 2-O-β-D-glucopyranosyl-L-ascorbic acid (a Vitamin C analogue)[16]. The anti-oxidative properties of all three active ingredients of wolfberry are well documented[16]. LWB is prepared by a milk based extraction process of wolfberry, which is believed to increase bioavailability of its active ingredients, as demonstrated for zeaxanthin[17]. In addition, the in vitro assays suggest that the anti-inflammatory activity of LWB is attributable to wolfberry rather than its milk component. However, to the best of our knowledge, wolfberry has not been shown to reduce cytokine levels under inflammatory conditions, as observed in our present study. In fact, wolfberry has been shown to up-regulate cytokine expression[31] and both LWB and wolfberry have a demonstrated immune-enhancing effect[18,19,32-34]. On the other hand, the anti-inflammatory properties of milk components are well established[35,36]. Overall, it seems that depending on the physiological environment LWB may provide support to recover homeostasis and/or immune competence. In the present study, we can speculate that a synergistic effect between the anti-oxidative ingredients of wolfberry and the anti-inflammatory components in milk can also be a potential mechanism of the benefits observed in our model. In that respect, further studies are required to identify the active anti-inflammatory ingredients in LWB. Nutritional therapies are an effective and safe form of intervention to induce remission in active state of CD[7,8]. Despite this profile of effectiveness and safety, they have not gained widespread usage, particularly in the area of adult IBD. One of the reasons for this could be that while they are effective, a review of the clinical trials comparing the efficacy of nutritional therapies to steroids concluded that, they are not as effective as steroids in induction of remission[9]. Thus, clearly more needs to be done in this area. Our findings suggest that addition of LWB to enteral diet formulations might help improve disease outcomes in IBD patients. However, it should be noted that further work addressing efficacy in different colitis models and in-depth confirmation of mechanism of action is necessary before clinically relevant research can be undertaken.
Lacto-Wolfberry was kindly provided by Wang J (Nestlé Research Center, Beijing); the authors kindly acknowledge the technical assistance provided by Genevieve Perruisseau.
Inflammatory bowel disease (IBD) consists of a group of disorders, such as Crohn’s disease (CD) and ulcerative colitis (UC). The incidence of IBD is increasing throughout the world. Both, CD and UC are characterized by relapsing-remitting disease progression. Currently, there is no known cure for IBD and the various available therapies are only palliative. Herein, the authors have identified the anti-inflammatory properties of a nutritional ingredient in a preclinical model of colitis.
Due to the chronic nature of IBD and the adverse effects of existing therapies, nutritional ingredients with anti-inflammatory properties may benefit the patient in the long term. With this in mind, the characterization of anti-inflammatory properties of novel or traditional food ingredients is an important field of research.
Previously, the authors have characterized the benefits of Lacto-Wolfberry (LWB) on the adaptive immune system. In this article, they have characterized the anti-inflammatory properties of LWB. The authors first demonstrate the anti-inflammatory and anti-oxidative properties of LWB in cellular models and subsequently show that LWB can ameliorate chemically-induced colitis.
The identification of the anti-inflammatory properties of LWB raises new possibilities of developing novel nutritional solutions for patients with IBD.
LWB is a skimmed milk extract of the traditional Chinese ingredient, wolfberry, specifically developed to increase the bioavailability of its active ingredients.
In the original article, the authors examined the complex anti-inflammatory effect of LWB administration in 2,4,6-trinitrobenzene sulfonic acid induced colitis and in selected cell lines. The study is well designed and the results and conclusions are clear and logical.
| 1. | Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12 Suppl 1:S3-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 639] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 2. | Hanai H, Takeuchi K, Iida T, Kashiwagi N, Saniabadi AR, Matsushita I, Sato Y, Kasuga N, Nakamura T. Relationship between fecal calprotectin, intestinal inflammation, and peripheral blood neutrophils in patients with active ulcerative colitis. Dig Dis Sci. 2004;49:1438-1443. [PubMed] |
| 3. | McCarthy DA, Rampton DS, Liu YC. Peripheral blood neutrophils in inflammatory bowel disease: morphological evidence of in vivo activation in active disease. Clin Exp Immunol. 1991;86:489-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Fukuda Y, Matsui T, Suzuki Y, Kanke K, Matsumoto T, Takazoe M, Matsumoto T, Motoya S, Honma T, Sawada K. Adsorptive granulocyte and monocyte apheresis for refractory Crohn's disease: an open multicenter prospective study. J Gastroenterol. 2004;39:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Mahida YR, Patel S, Gionchetti P, Vaux D, Jewell DP. Macrophage subpopulations in lamina propria of normal and inflamed colon and terminal ileum. Gut. 1989;30:826-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 112] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 644] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 7. | Hartman C, Eliakim R, Shamir R. Nutritional status and nutritional therapy in inflammatory bowel diseases. World J Gastroenterol. 2009;15:2570-2578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 141] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Rajendran N, Kumar D. Role of diet in the management of inflammatory bowel disease. World J Gastroenterol. 2010;16:1442-1448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (2)] |
| 9. | Zachos M, Tondeur M, Griffiths AM. Enteral nutritional therapy for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2007;CD000542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Liu X, Wang JM. Iridoid glycosides fraction of Folium syringae leaves modulates NF-κB signal pathway and intestinal epithelial cells apoptosis in experimental colitis. PLoS One. 2011;6:e24740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Zhao WC, Song LJ, Deng HZ. Protective effect of total alkaloids of Sophora alopecuroides on dextran sulfate sodium-induced chronic colitis. Chin J Integr Med. 2011;17:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Song YA, Park YL, Kim KY, Chung CY, Lee GH, Cho DH, Ki HS, Park KJ, Cho SB, Lee WS. Black tea extract prevents lipopolysaccharide-induced NF-κB signaling and attenuates dextran sulfate sodium-induced experimental colitis. BMC Complement Altern Med. 2011;11:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Karrasch T, Jobin C. NF-kappaB and the intestine: friend or foe? Inflamm Bowel Dis. 2008;14:114-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74:1526-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 605] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 15. | Richer SP, Stiles W, Graham-Hoffman K, Levin M, Ruskin D, Wrobel J, Park DW, Thomas C. Randomized, double-blind, placebo-controlled study of zeaxanthin and visual function in patients with atrophic age-related macular degeneration: the Zeaxanthin and Visual Function Study (ZVF) FDA IND #78, 973. Optometry. 2011;82:667-680.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Potterat O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010;76:7-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 409] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 17. | Benzie IF, Chung WY, Wang J, Richelle M, Bucheli P. Enhanced bioavailability of zeaxanthin in a milk-based formulation of wolfberry (Gou Qi Zi; Fructus barbarum L.). Br J Nutr. 2006;96:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Vidal K, Benyacoub J, Sanchez-Garcia J, Foata F, Segura-Roggero I, Serrant P, Moser M, Blum S. Intake of a milk-based wolfberry formulation enhances the immune response of young-adult and aged mice. Rejuvenation Res. 2010;13:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Vidal K, Bucheli P, Gao Q, Moulin J, Shen LS, Wang J, Blum S, Benyacoub J. Immunomodulatory effects of dietary supplementation with a milk-based wolfberry formulation in healthy elderly: a randomized, double-blind, placebo-controlled trial. Rejuvenation Res. 2012;15:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Bucheli P, Vidal K, Shen L, Gu Z, Zhang C, Miller LE, Wang J. Goji berry effects on macular characteristics and plasma antioxidant levels. Optom Vis Sci. 2011;88:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Wang XJ, Hayes JD, Wolf CR. Generation of a stable antioxidant response element-driven reporter gene cell line and its use to show redox-dependent activation of nrf2 by cancer chemotherapeutic agents. Cancer Res. 2006;66:10983-10994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 263] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 22. | Philippe D, Favre L, Foata F, Adolfsson O, Perruisseau-Carrier G, Vidal K, Reuteler G, Dayer-Schneider J, Mueller C, Blum S. Bifidobacterium lactis attenuates onset of inflammation in a murine model of colitis. World J Gastroenterol. 2011;17:459-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Philippe D, Dubuquoy L, Groux H, Brun V, Chuoï-Mariot MT, Gaveriaux-Ruff C, Colombel JF, Kieffer BL, Desreumaux P. Anti-inflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammation. J Clin Invest. 2003;111:1329-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Wallace JL, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29-36. [PubMed] |
| 25. | Ameho CK, Adjei AA, Harrison EK, Takeshita K, Morioka T, Arakaki Y, Ito E, Suzuki I, Kulkarni AD, Kawajiri A. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut. 1997;41:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 199] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Feng Z, Jia H, Li X, Bai Z, Liu Z, Sun L, Zhu Z, Bucheli P, Ballèvre O, Wang J. A milk-based wolfberry preparation prevents prenatal stress-induced cognitive impairment of offspring rats, and inhibits oxidative damage and mitochondrial dysfunction in vitro. Neurochem Res. 2010;35:702-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Bouguen G, Chevaux JB, Peyrin-Biroulet L. Recent advances in cytokines: therapeutic implications for inflammatory bowel diseases. World J Gastroenterol. 2011;17:547-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 28. | Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 887] [Cited by in RCA: 873] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 29. | Boismenu R, Chen Y. Insights from mouse models of colitis. J Leukoc Biol. 2000;67:267-278. [PubMed] |
| 30. | Bozic CR, Gerard NP, von Uexkull-Guldenband C, Kolakowski LF, Conklyn MJ, Breslow R, Showell HJ, Gerard C. The murine interleukin 8 type B receptor homologue and its ligands. Expression and biological characterization. J Biol Chem. 1994;269:29355-29358. [PubMed] |
| 31. | Gan L, Zhang SH, Liu Q, Xu HB. A polysaccharide-protein complex from Lycium barbarum upregulates cytokine expression in human peripheral blood mononuclear cells. Eur J Pharmacol. 2003;471:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Chen Z, Kwong Huat Tan B, Chan SH. Activation of T lymphocytes by polysaccharide-protein complex from Lycium barbarum L. Int Immunopharmacol. 2008;8:1663-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Chen Z, Soo MY, Srinivasan N, Tan BK, Chan SH. Activation of macrophages by polysaccharide-protein complex from Lycium barbarum L. Phytother Res. 2009;23:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Chen Z, Lu J, Srinivasan N, Tan BK, Chan SH. Polysaccharide-protein complex from Lycium barbarum L. is a novel stimulus of dendritic cell immunogenicity. J Immunol. 2009;182:3503-3509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | de Medina FS, Daddaoua A, Requena P, Capitán-Cañadas F, Zarzuelo A, Dolores Suárez M, Martínez-Augustin O. New insights into the immunological effects of food bioactive peptides in animal models of intestinal inflammation. Proc Nutr Soc. 2010;69:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Walker A. Breast milk as the gold standard for protective nutrients. J Pediatr. 2010;156:S3-S7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 322] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
Peer reviewers: Dr. Ferenc Sipos, 2nd Department of Internal Medicine, Semmelweis University, Szentkirályi 46, 1088 Budapest, Hungary; Dr. Tedros Bezabeh, Institute for Biodiagnostics, National Research Council, 435 Ellice Avenue, Winnipeg R3B 1Y6, Canada; Jae Hee Cheon, Professor, Department of Internal Medicine, Yonsei University College of Medicine, 250 Seongsan-ro, Seodaemun-gu, Seoul 120-752, South Korea
S- Editor Gou SX L- Editor A E- Editor Zhang DN