Published online Oct 7, 2012. doi: 10.3748/wjg.v18.i37.5205
Revised: July 4, 2012
Accepted: July 18, 2012
Published online: October 7, 2012
AIM: To introduce and evaluate a new method to repair bile duct defect with a degradable stent and autologous tissues.
METHODS: Eight Ba-Ma mini-pigs were used in this study. Experimental models with common bile duct (CBD) defect (0.5-1.0 cm segment of CBD resected) were established and then CBD was reconstructed by duct to duct anastomosis with a novel degradable stent made of poly [sebacic acid-co-(1,3-propanediol)-co-(1,2-propanediol)]. In addition, a vascularized greater omentum was placed around the stent and both ends of CBD. Cholangiography via gall bladder was performed for each pig at postoperative months 1 and 3 to rule out stent translocation and bile duct stricture. Complete blood count was examined pre- and post-operatively to estimate the inflammatory reaction. Liver enzymes and serum bilirubin were examined pre- and post-operatively to evaluate the liver function. Five pigs were sacrificed at month 3 to evaluate the healing of anastomosis. The other three pigs were raised for one year for long-term observation.
RESULTS: All the animals underwent surgery successfully. There was no intraoperative mortality and no bile leakage during the observation period. The white blood cell counts were only slightly increased on day 14 and month 3 postoperatively compared with that before operation, the difference was not statistically significant (P = 0.652). The plasma level of alanine aminotransferase on day 14 and month 3 postoperatively was also not significantly elevated compared with that before operation (P = 0.810). Nevertheless, the plasma level of γ-glutamyl transferase was increased after operation in both groups (P = 0.004), especially 2 wk after operation. The level of serum total bilirubin after operation was not significantly elevated compared with that before operation (P = 0.227), so did the serum direct bilirubin (P = 0.759). By cholangiography via gall bladder, we found that the stent maintained its integrity of shape and was still in situ at month 1, and it disappeared completely at month 3. No severe CBD dilation and stricture were observed at both months 1 and 3. No pig died during the 3-mo postoperative observation period. No sign of necrosis, bile duct stricture, bile leakage or abdominal abscess was found at reoperation at month 3 postoperatively. Pigs had neither fragments of stent nor stones formed in the CBD. Collagen deposit was observed in the anastomosis by hematoxylin and eosin (HE) and Masson’s trichrome stains. No severe cholestasis was observed in liver parenchyma by HE staining. Intestinal obstruction was found in a pig 4 mo after operation, and no bile leakage, bile duct stricture or biliary obstruction were observed in laparotomy. No sign of bile duct stricture or bile leakage was observed in the other two pigs.
CONCLUSION: The novel method for repairing bile duct defect yielded a good short-term effect without postoperative bile duct stricture. However, the long-term effect should be further studied.
- Citation: Liang YL, Yu YC, Liu K, Wang WJ, Ying JB, Wang YF, Cai XJ. Repair of bile duct defect with degradable stent and autologous tissue in a porcine model. World J Gastroenterol 2012; 18(37): 5205-5210
- URL: https://www.wjgnet.com/1007-9327/full/v18/i37/5205.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i37.5205
Laparoscopic cholecystectomy (LC) has been the gold standard procedure for benign gall bladder diseases. Unfortunately, the widespread application of LC has led to a concurrent rise in the incidence of major bile duct injuries (BDI) ranging from 0.3% to 0.65%[1-5]. Occurrence of BDI results in difficult reconstruction, even for experienced hepatobiliary surgeons, and a prolonged hospital stay and a high risk of long-term complications. Roux-en-Y hepaticojejunostomy has been the most commonly used approach for biliary reconstruction, especially in cases of duct transection injury[1,6-9]. But its long-term outcome is still far from satisfied due to the high incidence of reflux cholangitis, choledocholithiasis, anastomotic stenosis caused by scar contracture[10-12] and even canceration[13-15]. In recent years, primary duct-to-duct reconstruction has been used in living-donor liver transplantation and has gained good effects[16-18]. It preserves the function of Oddi’s sphincter, which provides a barrier to prevent any reflux into the bile duct[13]. However, major drawbacks, including early ischemic necrosis, leakage, and late anastomotic stricture, cannot be overcome so far. In this research, we created a novel method for bile duct defect repair, and proved its feasibility and safety.
Eight experimental Ba-Ma mini-pigs of either sex, weighing 15-20 kg, were provided by Shanghai Multi-Bio-Sci-Tech Co., Ltd., China (license No: SCXK 2005-0002). The animals were housed one per cage at the Experimental Animal Center at Zhejiang University. They were allowed to be accustomed to the laboratory environment for more than one week before the start of the experiment. All animals had free access to water and standard food until the day before surgery. The study was approved by the Ethics Committee of Zhejiang University.
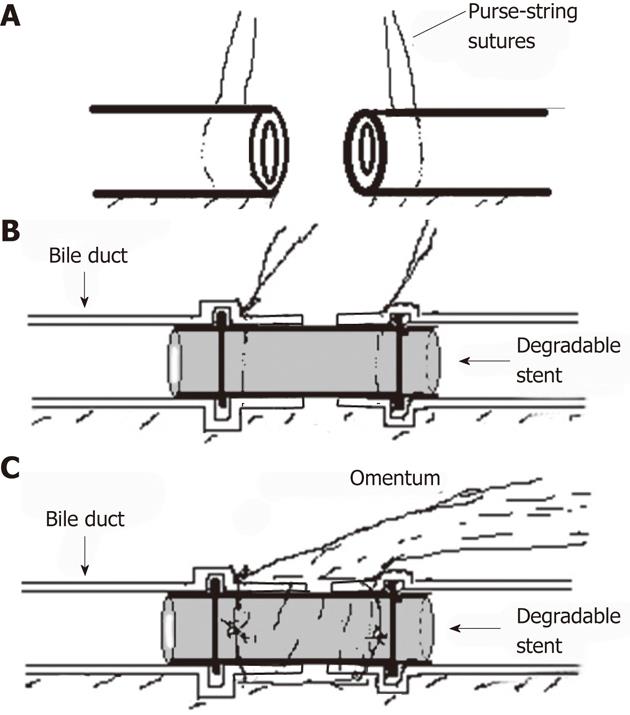
The stent (Figure 1) is made of a novel biodegradable elastomer which is manufactured by the Institute of Polymer Science of Zhejiang University. The elastomer is synthesized with 1,3-propanediol, 1,2-propanediol, and sebacic acid, and then shaped into hollow tube by injecting molding, with a hump ring in both ends. The stent used in the research is 6 mm in external diameter, 1 mm in thickness, and 4 mm in inner diameter. The distance between two hump rings is 10 mm. The stent can completely degrade into carbon dioxide and water in about 3 mo when submerged in fresh human bile in vitro. The biocompatible quality of the novel elastomer was approved by the State Food and Drug Administration of China (No. G20090993).
A total of eight Ba-Ma mini-pigs (15-20 kg) were included in this study. The pigs were fasted for 24 h before surgery. Experimental models with common bile duct (CBD) defect (0.5-1.0 cm segment of CBD resected) were established and CBD was reconstructed by duct to duct anastomosis with the novel biodegradable stent. In addition, a vascularized greater omentum was placed around the stent and both ends of CBD. The incidence of jaundice and bile leakage was evaluated. Five pigs were subjected to the examination of complete blood count before operation and on day 14 and month 3 after operation to estimate the inflammatory reaction. Alanine aminotransferase (ALT), γ-glutamyl transferase (γ-GT), serum total bilirubin (TBIL) and direct bilirubin test were also examined pre- and post-operatively to evaluate the liver function. Cholangiography was performed for each pig on months 1 and 3 postoperatively to rule out stent translocation and bile duct stricture. The five pigs were reoperated on to observe anastomosis three months after operation and were sacrificed immediately after that. The peritoneal cavity was observed for signs of bile leakage and stricture. The anastomosis was evaluated pathologically, including hematoxylin and eosin (HE) stain and Masson trichrome stain. Liver tissue slides with HE staining were also observed. The other three pigs were raised for one year for long-term observation.
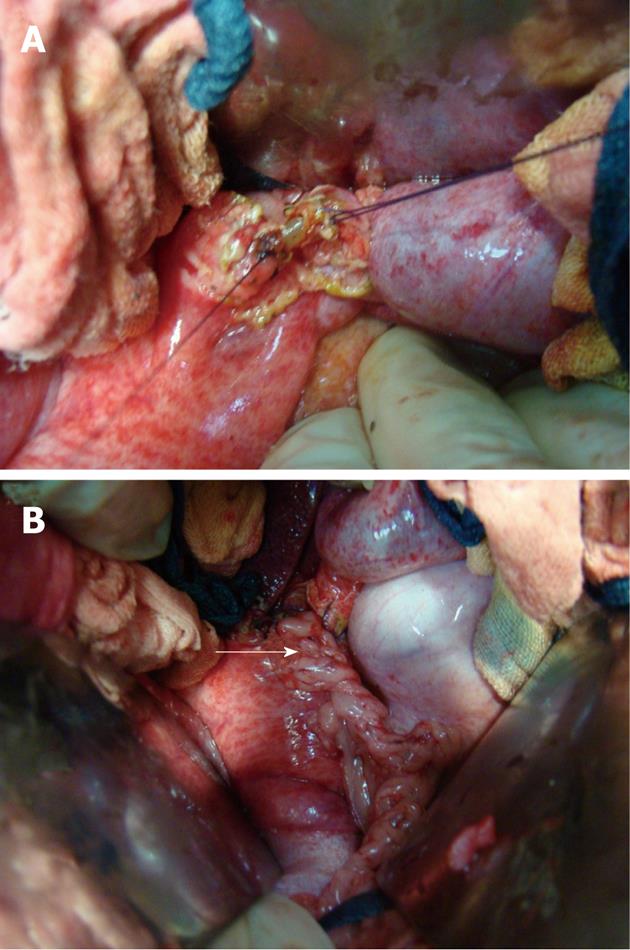
Pigs were anesthetized by intramuscular injection of pentobarbital sodium solution (20 mg/mL) with 0.1 mL/kg. Laparotomy was performed via a midline incision. Hepatic hilar was dissected firstly to free the CBD. Then a 0.5-1.0 cm segment of CBD under junction of cystic duct was resected. The CBD was reconstructed by duct to duct anastomosis with a stent: purse-string sutures with 4-0 Vicryl (Ethicon, Somerville, NJ, United States) were made on both ends of bile duct (Figure 2A). The stent was inserted into the both ends of bile duct as shown in Figure 2B and Figure 3A. Two ends of bile duct were slightly closed and the purse-string sutures were tied to fix both ends of bile duct on the stent to reconstruct the CBD. A vascularized greater omentum was placed around the stent and both ends of CBD (Figure 2C and 3B).
All pigs were given free access to water, but without food for 24 h postoperatively. They were given half of their normal diet on postoperative day 2. Normal diet was resumed on postoperative day 3.
For statistical analysis, the Kruskal-Wallis test was used. P < 0.05 was considered statistically significant. Statistical analysis was performed using the SPSS statistical software package (Version 13.0, SPSS Inc, Chicago, IL).
Surgery was successful in all the cases. There was neither intraoperative death nor bile leakage detected during the observation period. The white blood cell counts were only slightly increased on day 14 and month 3 postoperatively compared with that before operation, the difference was not statistically significant. The plasma level of ALT on day 14 and month 3 postoperatively was also not significantly elevated compared with that before operation. Nevertheless, the plasma level of γ-GT was increased after operation in both groups, especially 2 wk after operation. The plasma level of TBIL after operation was not significantly increased compared with that before operation, so did the plasma level of serum direct bilirubin (Table 1).
| Before operation | After day 14 | Operation month 3 | P value | |
| WBC | 16.62 | 18.5 | 18.34 | 0.652 |
| ALT | 51.2 | 57.2 | 54.4 | 0.810 |
| γ-GT | 50.2 | 125.8 | 72.8 | 0.004 |
| DBIl | 1.1 | 1.78 | 1.44 | 0.227 |
| TBIl | 1.76 | 2.2 | 2.06 | 0.759 |
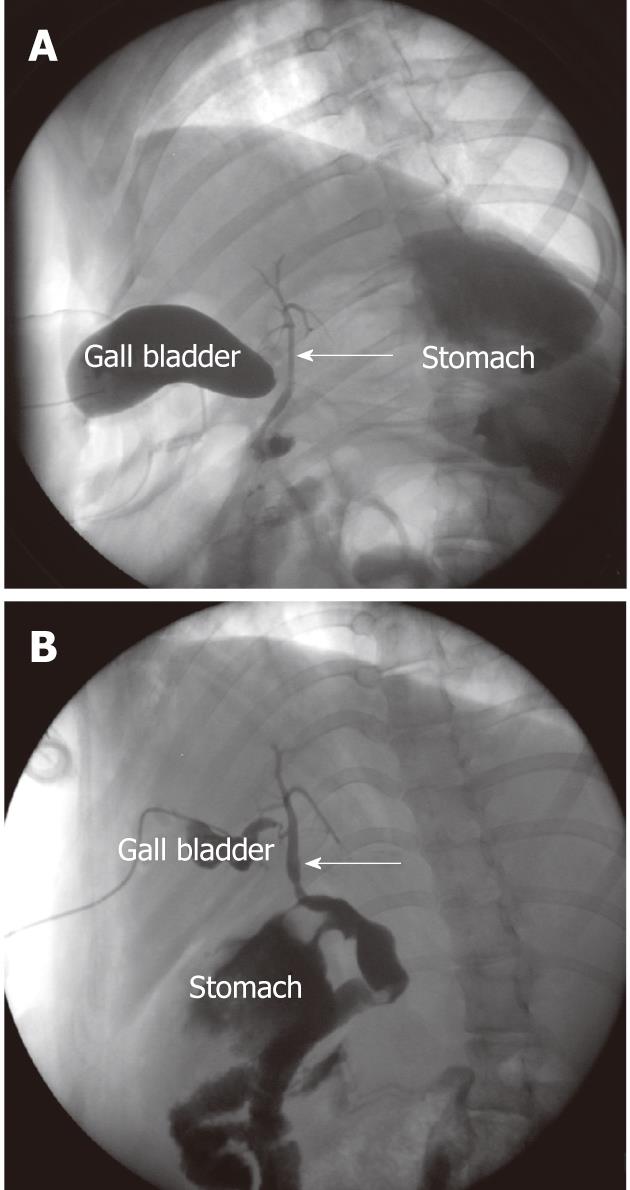
By cholangiography via gall bladder, we found that the stent maintained its integrity of shape and was still in situ on month 1, and it disappeared completely on month 3. No severe CBD dilation and stricture were observed both on months 1 and 3 (Figure 4).
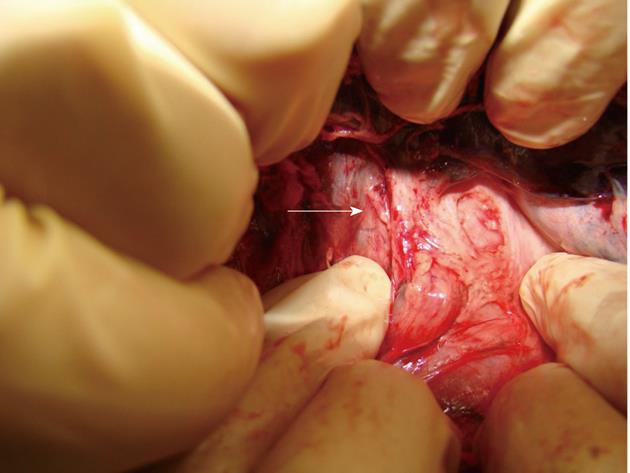
No pig died during the 3-mo postoperative observation period. No sign of necrosis, bile duct stricture, bile leakage or abdominal abscess was found when the animals were reoperated on month 3 postoperatively, and none of the pigs had fragment of stents, and stones formed in the CBD (Figure 5).
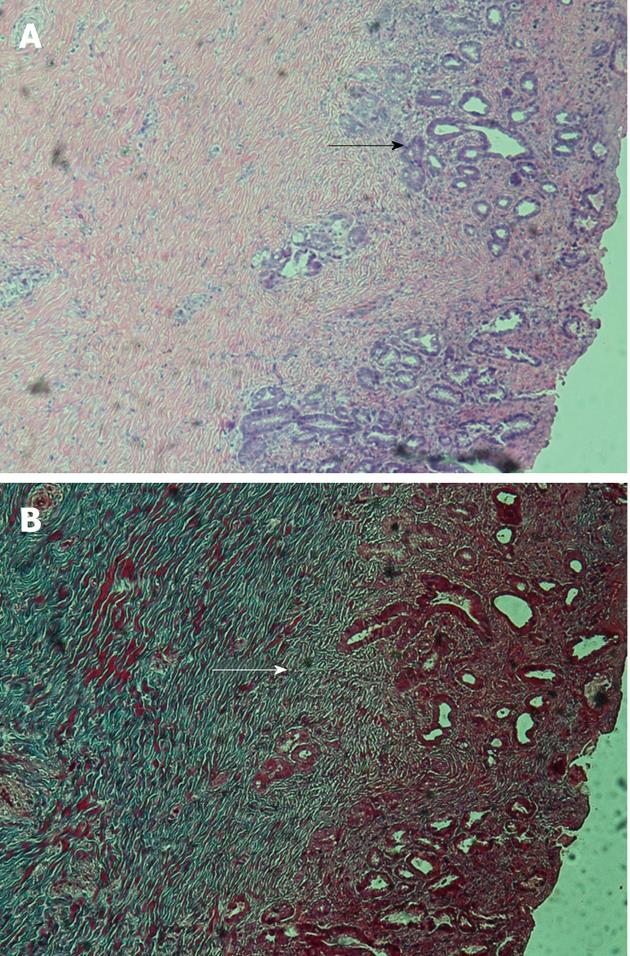
Histologically, collagen deposit and bile duct glands proliferation were observed in the anastomosis by hematoxylin and eosin and Masson’s trichrome stains (Figure 6). No severe cholestasis was found in liver parenchyma by HE staining.
In long-term observation, a pig had intestinal obstruction 4 mo after operation, but no bile leakage, bile duct stricture or biliary obstruction were observed in laparotomy. There was no sign of bile duct stricture or bile leakage in the other two pigs.
Bile duct injury is a major complication in biliary surgery such as LC. It can be classified by several classification systems according to the injured site, e.g., Bismuth classification[19] and Strasberg classification[20]. In some cases, bile duct was partially resected and reconstruction with end to end suture was almost impossible for the high incidence of bile leakage and postoperative anastomosis stricture[21,22].
To keep the biliary continuity and integrality of function is an essential principle for biliary reconstruction. A Roux-en-Y hepaticojejunostomy has been the most commonly used procedure for CBD defect. However, the function of Oddi’s sphincter was lost in patients undergoing this procedure, leading to a high incidence of postoperative reflux cholangitis[13,23]. In this research, we aimed to create a new method to repair bile duct defect without sacrificing the function of Oddi’s sphincter.
In this procedure, the stent played an important role. The stent was made of degradable material named poly [sebacic acid-co-(1,3-propanediol)-co-(1,2-propanediol)] (PSPP)[24-26]. Degradable stent had a number of advantages, especially in eliminating the need for stent removal[27,28]. The PSPP belongs to polyester elastomer and possesses a good biocompatibility which is essential for medical applications. The stent connected the two ends of bile duct to maintain the continuity of biliary structure, and it was also used as an inner stent to prevent anastomosis restenoses[22,29,30]. Its degradation time in bile duct is set between 2 and 3 mo, conforming to the healing process of bile duct.
According to the previous reports, the defect of bile duct could be replaced by autologous veins and stents (both silicone stents and biodegradable stents) and they concluded that construction of bile duct appeared to take place by tissue migration[31-34]. Blood supply is important for tissue migration and repair of bile duct defect[35], so vascularized large omentum may be a better autologous tissue compared with veins for temporary repair before bile duct tissue migration was completed. With the assistance of the degradable stent and omentum, we do not need to free much residual bile duct or to mobilize the duodenum to make sure that the anastomosis is tension-free and properly vascularized[36].
Taking into account that bile leakage and bile duct stricture were the two major complications, postoperative cholangiography through gall bladder was repeated to rule out these complications, and liver function was also examined repeatedly to rule out the complication of biliary stricture. The postoperative level of bilirubin and ALT was not significantly elevated compared with that before operation. No obvious bile duct dilatation or stenosis was observed in any pigs by cholangiography. As a result, there was no sign of bile leakage or biliary stricture.
Histologically, collagen deposit and bile duct gland proliferation were observed in the anastomosis three months after operation by HE and Masson’s trichrome stains. The neonatal biliary tissue had completely replaced the omentum. Liver parenchyma was also observed by HE stain to rule out cholestasis, and positive result was found. Those results proved that the method is feasible and the biliary tissue can migrate along the surface of stent.
The stent degraded in 3 mo in this research, and the presence of stricture after degradation of the stent is essential to the success of this method, so three pigs were raised for one year to observe its long-term effect. One pig was excluded from long-term observation due to the complication of intestinal obstruction occurring in month 4 after operation and no sign of biliary stricture was found in laparotomy.
This is also a simple method. Only two purse-string sutures are needed without end to end suture and dissection of vein. Vascularized large omentum is easy to mobilize and cover around defect and anastomosis. In addition, two sizes of the stent are available for different situations; the stent would be easily placed into the bile duct if a proper size is selected.
These results suggested that it is a feasible method for repairing bile duct defect, however, the long-term effect (more than one year) should be further observed.
By the early 1990s, laparoscopic cholecystectomy (LC) had replaced open cholecystectomy as the gold standard procedure for benign gall bladder diseases. Nevertheless, the widespread application of LC has led to a concurrent rise in the incidence of major bile duct injuries. The management of major bile duct injury is a surgical challenge even for experienced hepatobiliary surgeons at tertiary referral centers. Hepaticojejunostomy and duct to duct anastomosis are two commonly used methods for repairing bile duct injury. Despite their advantages, both methods have their critical defects which are difficult to overcome.
Bile duct injury is a severe complication encountered by almost every hepatobiliary surgeon. The management of major bile duct injury has become a hotspot in clinical studies. With the development of tissue engineering, several researches on biodegradable materials for biliary duct have been conducted.
In this research, the authors created a new method for repairing the bile duct defect with satisfactory effect in a three-month observation period. A novel anastomotic stent and omentum are involved in the new method. By this method, a larger proportion of patients can undergo end-to-end anastomosis and avoid the need for a hepaticojejunostomy. It is also of importance to preserve the function of Oddi’s sphincter.
The study results suggest that repairing bile duct defect by this new method yielded a good short-term effect without postoperative bile duct stricture. It might be applicable in clinical settings in the near future.
Elastomer is a class of polymers with a good elasticity. Poly [sebacic acid-co-(1,3-propanediol)-co-(1,2-propanediol)] belongs to polyester elastomer which is considered to have a good biocompatibility.
The authors studied the efficacy of the new degradable stent for the bile duct repair in pig experiment. And they concluded that it is a feasible method for repairing bile duct defect. The idea and research of this study are very interesting and applicable for clinical settings in the near future.
| 1. | Machado NO. Biliary complications postlaparoscopic cholecystectomy: mechanism, preventive measures, and approach to management: a review. Diagn Ther Endosc. 2011;2011:967017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Diamantis T, Tsigris C, Kiriakopoulos A, Papalambros E, Bramis J, Michail P, Felekouras E, Griniatsos J, Rosenberg T, Kalahanis N. Bile duct injuries associated with laparoscopic and open cholecystectomy: an 11-year experience in one institute. Surg Today. 2005;35:841-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Waage A, Nilsson M. Iatrogenic bile duct injury: a population-based study of 152 776 cholecystectomies in the Swedish Inpatient Registry. Arch Surg. 2006;141:1207-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 4. | Misawa T, Saito R, Shiba H, Son K, Futagawa Y, Nojiri T, Kitajima K, Uwagawa T, Ishida Y, Ishii Y. Analysis of bile duct injuries (Stewart-Way classification) during laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg. 2006;13:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Tantia O, Jain M, Khanna S, Sen B. Iatrogenic biliary injury: 13,305 cholecystectomies experienced by a single surgical team over more than 13 years. Surg Endosc. 2008;22:1077-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Mercado MA, Domínguez I. Classification and management of bile duct injuries. World J Gastrointest Surg. 2011;3:43-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Sicklick JK, Camp MS, Lillemoe KD, Melton GB, Yeo CJ, Campbell KA, Talamini MA, Pitt HA, Coleman J, Sauter PA. Surgical management of bile duct injuries sustained during laparoscopic cholecystectomy: perioperative results in 200 patients. Ann Surg. 2005;241:786-792; discussion 793-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 299] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 8. | Schmidt SC, Langrehr JM, Hintze RE, Neuhaus P. Long-term results and risk factors influencing outcome of major bile duct injuries following cholecystectomy. Br J Surg. 2005;92:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Tocchi A, Costa G, Lepre L, Liotta G, Mazzoni G, Sita A. The long-term outcome of hepaticojejunostomy in the treatment of benign bile duct strictures. Ann Surg. 1996;224:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 10. | Al-Ghnaniem R, Benjamin IS. Long-term outcome of hepaticojejunostomy with routine access loop formation following iatrogenic bile duct injury. Br J Surg. 2002;89:1118-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Röthlin MA, Löpfe M, Schlumpf R, Largiadèr F. Long-term results of hepaticojejunostomy for benign lesions of the bile ducts. Am J Surg. 1998;175:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Yan JQ, Peng CH, Ding JZ, Yang WP, Zhou GW, Chen YJ, Tao ZY, Li HW. Surgical management in biliary restricture after Roux-en-Y hepaticojejunostomy for bile duct injury. World J Gastroenterol. 2007;13:6598-6602. [PubMed] [DOI] [Full Text] |
| 13. | Tocchi A, Mazzoni G, Liotta G, Lepre L, Cassini D, Miccini M. Late development of bile duct cancer in patients who had biliary-enteric drainage for benign disease: a follow-up study of more than 1,000 patients. Ann Surg. 2001;234:210-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 178] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 14. | Bettschart V, Clayton RA, Parks RW, Garden OJ, Bellamy CO. Cholangiocarcinoma arising after biliary-enteric drainage procedures for benign disease. Gut. 2002;51:128-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (3)] |
| 15. | Strong RW. Late bile duct cancer complicating biliary-enteric anastomosis for benign disease. Am J Surg. 1999;177:472-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Ishiko T, Egawa H, Kasahara M, Nakamura T, Oike F, Kaihara S, Kiuchi T, Uemoto S, Inomata Y, Tanaka K. Duct-to-duct biliary reconstruction in living donor liver transplantation utilizing right lobe graft. Ann Surg. 2002;236:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Yamamoto S, Sato Y, Oya H, Nakatsuka H, Kobayashi T, Hara Y, Watanabe T, Kurosaki I, Hatakeyama K. Risk factors and prevention of biliary anastomotic complications in adult living donor liver transplantation. World J Gastroenterol. 2007;13:4236-4241. [PubMed] |
| 18. | Kasahara M, Egawa H, Takada Y, Oike F, Sakamoto S, Kiuchi T, Yazumi S, Shibata T, Tanaka K. Biliary reconstruction in right lobe living-donor liver transplantation: Comparison of different techniques in 321 recipients. Ann Surg. 2006;243:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | McMahon AJ, Fullarton G, Baxter JN, O'Dwyer PJ. Bile duct injury and bile leakage in laparoscopic cholecystectomy. Br J Surg. 1995;82:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 148] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180:101-125. [PubMed] |
| 21. | Stewart L, Way LW. Bile duct injuries during laparoscopic cholecystectomy. Factors that influence the results of treatment. Arch Surg. 1995;130:1123-1128; discussion 1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | de Reuver PR, Busch OR, Rauws EA, Lameris JS, van Gulik TM, Gouma DJ. Long-term results of a primary end-to-end anastomosis in peroperative detected bile duct injury. J Gastrointest Surg. 2007;11:296-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Miyazawa M, Torii T, Toshimitsu Y, Okada K, Koyama I, Ikada Y. A tissue-engineered artificial bile duct grown to resemble the native bile duct. Am J Transplant. 2005;5:1541-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Cai X, Jin R, Liang Y, Huang D, Peng S. Experimental study of primary repair of colonic leakage with a degradable stent in a porcine model. J Gastrointest Surg. 2011;15:1995-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Wang Y, Cai X, Cai H, Liang Y, Huang D, Liang X. Experimental study of colonic anastomosis with a degradable stent in a porcine model. Am J Surg. 2010;199:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Liu K, Yu H, Zhang M, Yu Y, Wang Y, Cai X. Sutureless primary repair of colonic perforation with a degradable stent in a porcine model of fecal peritonitis. Int J Colorectal Dis. 2012;Epub ahead of print. [PubMed] |
| 27. | Xu X, Liu T, Liu S, Zhang K, Shen Z, Li Y, Jing X. Feasibility of biodegradable PLGA common bile duct stents: an in vitro and in vivo study. J Mater Sci Mater Med. 2009;20:1167-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Tashiro H, Ogawa T, Itamoto T, Ushitora Y, Tanimoto Y, Oshita A, Amano H, Asahara T. Synthetic bioabsorbable stent material for duct-to-duct biliary reconstruction. J Surg Res. 2009;151:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Wu JS, Soper NJ. Comparison of laparoscopic choledochotomy closure techniques. Surg Endosc. 2002;16:1309-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Isla AM, Griniatsos J, Wan A. A technique for safe placement of a biliary endoprosthesis after laparoscopic choledochotomy. J Laparoendosc Adv Surg Tech A. 2002;12:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Cushieri A, Baker PR, Anderson RJ, Holley MP. Total and subtotal replacement of the common bile duct: effect of transhepatic silicone tube stenting. Gut. 1983;24:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Heistermann HP, Palmes D, Stratmann U, Hohlbach G, Hierlemann H, Langer M, Spiegel HU. A new technique for reconstruction of the common bile duct by an autologous vein graft and a biodegradable endoluminal stent. J Invest Surg. 2006;19:57-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Capitanich P, Herrera J, Iovaldi ML, Balbuena R, Casas G, Malizia P, Bun M, Mezzadri N. Bile duct replacement using an autologous femoral vein graft: an experimental study. Preliminary results. J Gastrointest Surg. 2005;9:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Heistermann HP, Palmes D, Hierlemann H, Ebsen M, Horstmann R, Hohlbach G, Spiegel HU. [Reconstruction of bile duct lesions by an autologous vein graft and a bio-degradable endoluminal stent in an animal model: technique and clinical impact]. Zentralbl Chir. 2003;128:952-957. [PubMed] |
| 35. | van Hattum AH, James J, Klopper PJ, Muller JH. Healing of a superficial lesion in the common bile duct of the rabbit. Neth J Surg. 1981;33:25-31. [PubMed] |
| 36. | Jabłońska B, Lampe P. Iatrogenic bile duct injuries: etiology, diagnosis and management. World J Gastroenterol. 2009;15:4097-4104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (2)] |
Peer reviewers: Atsushi Irisawa, Professor, Department of Gastroenterology, Fukushima Medical University Aizu Medical Center, 10-75, Shiromae, Aizuwakamatsu 960-8555, Japan; Tokihiko Sawada, Professor, Second Department of Surgery, Dokkyo University School of Medicine, Kitakobayashi 880, Mibu, Shimotsuga, Tochigi 321-0293, Japan; Nobuhiro Ohkohchi, Professor, Department of Surgery, Institute of Clinical Medicine, University of Tsukuba, Tenoudai 1-1-1, Tsukuba 305-8575, Japan
S- Editor Wu X L- Editor Ma JY E- Editor Xiong L