Published online Sep 21, 2012. doi: 10.3748/wjg.v18.i35.4925
Revised: May 16, 2012
Accepted: May 26, 2012
Published online: September 21, 2012
AIM: To investigate the role of bone marrow-derived endothelial progenitor cells (EPCs) in the angiogenesis of hepatocellular carcinoma (HCC).
METHODS: The bone marrow of HCC mice was reconstructed by transplanting green fluorescent protein (GFP) + bone marrow cells. The concentration of circulating EPCs was determined by colony-forming assays and fluorescence-activated cell sorting. Serum and tissue levels of vascular endothelial growth factor (VEGF) and colony-stimulating factor (CSF) were quantified by enzyme-linked immunosorbent assay. The distribution of EPCs in tumor and tumor-free tissues was detected by immunohistochemistry and real-time polymerase chain reaction. The incorporation of EPCs into hepatic vessels was examined by immunofluorescence and immunohistochemistry. The proportion of EPCs in vessels was then calculated.
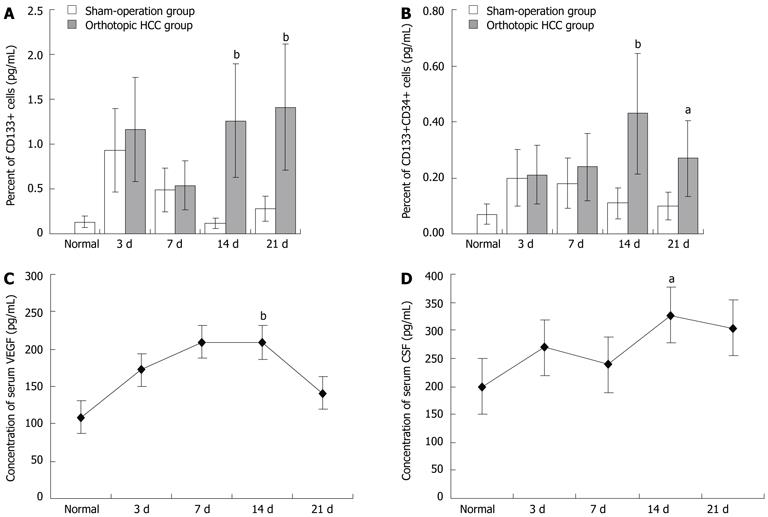
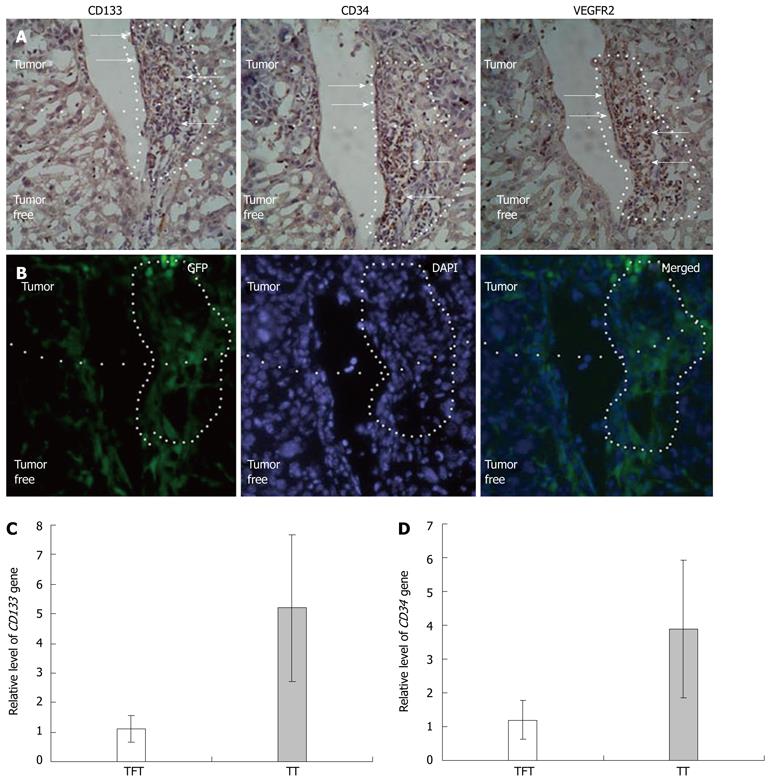
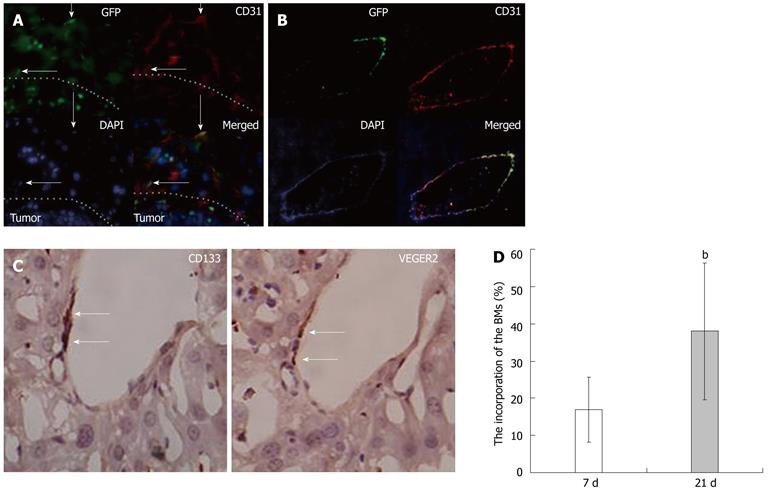
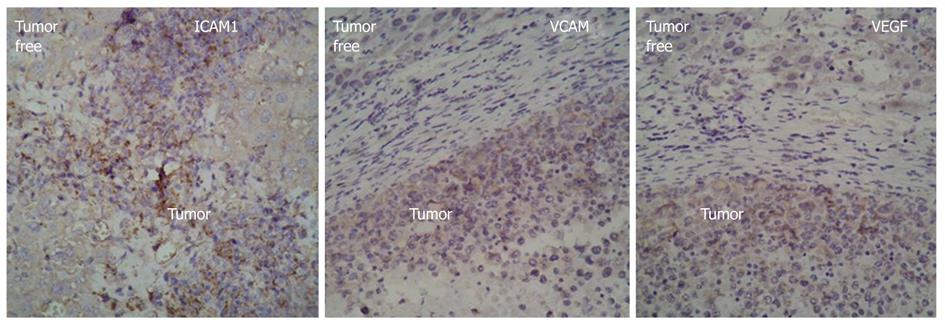
RESULTS: The HCC model was successful established. The flow cytometry analysis showed the mean percentage of CD133CD34 and CD133VEGFR2 double positive cells in HCC mice was 0.45% ± 0.16% and 0.20% ± 0.09% respectively. These values are much higher than in the sham-operation group (0.11% ± 0.13%, 0.05% ± 0.11%, n = 9) at 14 d after modeling. At 21 d, the mean percentage of circulating CD133CD34 and CD133VEGFR2 cells is 0.23% ± 0.19%, 0.25% ± 0.15% in HCC model vs 0.05% ± 0.04%, 0.12% ± 0.11% in control. Compared to the transient increase observed in controls, the higher level of circulating EPCs were induced by HCC. In addition, the level of serum VEGF and CSF increased gradually in HCC, reaching its peak 14 d after modeling, then slowly decreased. Consecutive sections stained for the CD133 and CD34 antigens showed that the CD133+ and CD34+ VEGFR2 cells were mostly recruited to HCC tissue and concentrated in tumor microvessels. Under fluorescence microscopy, the bone-marrow (BM)-derived cells labeled with GFP were concentrated in the same area. The relative levels of CD133 and CD34 gene expression were elevated in tumors, around 5.0 and 3.8 times that of the tumor free area. In frozen liver sections from HCC mice, cells co-expressing CD133 and VEGFR2 were identified by immunohistochemical staining using anti-CD133 and VEGFR2 antibodies. In tumor tissue, the double-positive cells were incorporated into vessel walls. In immunofluorescent staining. These CD31 and GFP double positive cells are direct evidence that tumor vascular endothelial cells (VECs) come partly from BM-derived EPCs. The proportion of GFP CD31 double positive VECs (out of all VECs) on day 21 was around 35.3% ± 21.2%. This is much higher than the value recorded on day 7 group (17.1% ± 8.9%). The expression of intercellular adhesion molecule 1, vascular adhesion molecule 1, and VEGF was higher in tumor areas than in tumor-free tissues.
CONCLUSION: Mobilized EPCs were found to participate in tumor vasculogenesis of HCC. Inhibiting EPC mobilization or recruitment to tumor tissue may be an efficient strategy for treating HCC.
- Citation: Sun XT, Yuan XW, Zhu HT, Deng ZM, Yu DC, Zhou X, Ding YT. Endothelial precursor cells promote angiogenesis in hepatocellular carcinoma. World J Gastroenterol 2012; 18(35): 4925-4933
- URL: https://www.wjgnet.com/1007-9327/full/v18/i35/4925.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i35.4925
Hepatocellular carcinoma (HCC) is one of the most vascular solid tumors, and neovascularization plays an important role in HCC development. In HCC, neovascularization status is correlated with disease progression and patient prognosis. Endothelial progenitor cells (EPCs) have been proved to be the main source of adult neovascularization. Until now, there has not been any data regarding the role of EPCs in HCC neovascularization. This study is the first to prove that EPCs incorporate into vessels directly and play an important role in HCC neovascularization.
It has been confirmed that neovascularization is vital to further multiplication, metastasis, and recurrence in malignant tumors. Without exogenous blood vessels, tumors stop growing and enter a state of suspended animation at about 2-3 cubic millimeters. The acquisition of angiogenic capacity is considered a sign of tumor development and of metastasis[1].
Recently, increasing amounts of data have revealed that bone-marrow (BM)-derived EPCs can foster the initiation and maintenance of angiogenic processes by integrating into developing vasculature under hypoxic conditions and into tumors. In 1997, EPCs were first isolated using anti-CD34 monoclonal antibodies. CD34-antigen, however, could not distinguish immature EPCs from mature circulating endothelial cells (CECs) because CD34 is expressed on both. With the discovery of CD133, an antigen restricted to immature EPCs, distinguishing between EPC and CECs has become easier[2]. VEFGR2 is another marker of EPCs. VEFGR2 signaling causes EPCs to differentiate. EPCs resemble embryonic angioblasts, which characteristically migrate, proliferate, and differentiate into mature endothelial cells[3]. In general, EPCs can be identified as cells that simultaneously express the cell surface markers CD133, CD34, and VEGFR2[4,5]. Santarelli et al[6] used mouse tumor models to demonstrate that BM-derived EPCs are greatly mobilized into the bloodstream, and home specifically to tumor tissues. In the tumor microenvironment, the recruited EPCs merged with the walls of a growing blood vessel, where they differentiated into endothelial cells and so promoted tumor growth. It has been reported that, during cancer proliferation, the number of circulating BM-derived progenitor cells increases dramatically. Circulating BM-derived EPCs have been used to determine the optimal dose of anti-angiogenic drugs, most successfully in breast cancer[7]. In addition, Taylor proved that high levels of circulating BM-derived progenitor cells are correlated with tumor metastatic status. Recently, the contribution of EPCs to tumor angiogenesis has also been reported in clinical tumor samples from 6 patients who received bone marrow transplants from donors of the opposite sex. The endothelial cells within the tumor vessels were of donor origin, as shown by the mismatched sex chromosomes[8].
At present, the significance of circulating EPCs and the contribution of EPCs to tumor vessels have been confirmed in only a few solid malignancies, such as malignant glioma[6], lung cancer[9], and mammal tumors[10]. However, the extent to which EPCs contribute to the generation of the tumor vessels varies significantly across different studies, from substantial to zero[11,12]. The role of EPCs in neovascularization has been the subject of great debate.
Tumor microvessel density is closely related to tumor growth, invasion, and metastasis and to patient prognosis[13,14]. It has been confirmed that the formation of new capillaries is essential to tumor development, and the status of angiogenesis is an important prognostic indicator in hepatocellular carcinoma[15,16]. There are only three papers in PUBMED, including our previous study[17], that compare the circulating EPC levels of HCC patients to those of healthy controls or those of liver cirrhosis patients[18,19]. We established an orthotropic hepatic cancer model, observed the dynamic changes of circulating EPCs, and determined whether BM-derived EPCs contributed to tumor vessels directly. We also calculated the incorporation rate of the EPCs in vessels. All this information is extremely important to evaluating the role of EPCs in neovascularization in HCC.
For BM reconstruction, C57BL/6 male mice aged 8-10 wk were lethally irradiated (10 Gy) and transplanted with BM cells collected from green fluorescent protein (GFP)-C57BL/6 transgenic mice[6]. Four weeks later, the HCC model showed a GFP positive rate of circulating nucleated cells of over 90%, suggesting full BM recovery. The mice were then used to construct an orthotropic hepatic cancer mice model by intra-hepatic injection of 1 × 105-2 × 105 H22 hepatoma cells (given to us by Dr. Ru-Tian Li of the Nanjing University Institute of Oncology, China). Sterile extract H22 hepatoma cells, normal saline to adjust the number of cancer cells to 106/mL for use. Intraperitoneal injection of sodium pentobarbital anesthesia C57BL/6 mice, disinfection shop towel, in the middle upper abdomen about the xiphoid mainly as a 1 cm incision into the abdominal cavity. Gently press out part of the lobe with bilateral costal arch, wet gauze pad in the bottom lobe to protect, and then with 1 mL medical syringe needles inserted along the long axis of lobe about 0.5 cm, the slow injection of H22 hepatoma cells 0.1 mL (cell number 1 × 105-2 × 105). Pull out the needle at the same time, rapidly at the pressure in the eye of a needle, and gently press on it with a cotton for 1 min to stop bleeding. Carefully place the liver back into the abdominal cavity. The control group received same volume of phosphate buffered solution (PBS). All mice were from the national genetically engineered mouse resource bank of China.
Flow cytometry (FCM) was used to evaluate the degree of EPC mobilization. Three hundred microliters of peripheral blood was obtained from the retroorbital plexus at 3 d, 7 d, 14 d, and 21 d after the establishment of the orthotropic HCC model. Erythrocytes were split by red blood cell lysis buffer. Mononuclear cells were incubated for 30 min at 4 °C with Alexa Fluor488 conjugated anti-CD133, Alexa Fluor647 conjugated anti-CD34, PE-conjugated anti-VEGFR2 (eBioscience, United States). Appropriate fluorochrome-conjugated isotypes were used as controls. In addition, serum concentration of vascular endothelial growth factor (VEGF), platelet-derived growth factor [colony-stimulating factor (CSF)] was tested by enzyme-linked immunosorbent assay kit (ADL, United States) following the collection of serum at the same times.
Liver tissue was obtained from the GFP labeled BM-HCC model on day 14. After paraformaldehyde perfusion and gradient dehydration, consecutive frozen liver tissue sections 2 μm in thickness were cut in a cryostat, fixed, blocked, and incubated at 4 °C overnight with primary antibody rabbit anti-mouse CD133 (1:200), VEGFR2 (1:100, Abcam, United States), rat anti-mouse CD34 (1:250, ebioscience, United States) respectively. Secondary antibody was horseradish peroxidase. Positive reactions were visualized with diaminobenzidine solution followed by counterstaining with hematoxylin. Negative controls were obtained by substituting the primary antibodies with PBS.
Consecutive 2 μm frozen liver tissue sections were cut as stated above. Sections were blocked and then incubated overnight with rat anti-mouse CD31(1:800, eBiosicence, United States). After washing in PBS, slices were incubated with secondary antibodies Alexa Fluor 488-conjugated rabbit anti-rat IgG antibody (1:1000, molecular probes). 4',6'-diamidino-2-phenylindole hydrochloride was used to dry the nucleus. Normal mice given PBS-substituted for antibodies as a negative control. Double-labeled immunofluorescence staining sections were evaluated using fluorescence microscopy.
Mouse livers were obtained at 7 d or 21 d after the establishment of the GFP-BM HCC model. Two-microliter frozen anti-CD31 immunofluorescence staining sections were created as stated above. Specimens were analyzed microscopically by two independent observers who counted the number of BM-derived vascular ECs (VECs) co-expressing GFP, CD31, and total VECs in 15 random high-power fields. The quantitative contribution of EPCs to total VECs is expressed as percentages[20].
Liver tissue was obtained from the GFP-labeled BM-HCC model on day 14. Conventionally processed and embedded sections cut at a thickness of 3 μm were deparaffinized, blocked, and incubated at 4 °C overnight with primary antibody: rabbit anti-mouse intercellular adhesion molecule 1 (ICAM1) (1:50, Protein Tech Group, United States), vascular adhesion molecule 1 (VCAM1) (1:200 Santa Cruz Biotech, Santa Cruz, CA, United States), VEGF (1:1000 Abcam, City, State, United States). The secondary antibody was horseradish peroxidase. Positive reactions were visualized with diaminobenzidine solution followed by counterstaining with hematoxylin. Negative controls were obtained by substituting the primary antibodies with PBS.
The levels of actin and CD133 mRNAs in the tumor and tumor-free areas were measured by real-time quantitative polymerase chain reaction (PCR). RNA was extracted from the livers with Trizol (Invitrogen, United States). cDNA was generated using Takara Primer ScriptTM 1st stand cDNA synthesis kits (JP). The sequences of primers were designed and synthesized by Takara. Primers for CD133 (5’-AACGTGGTCCAGCCGAATG-3’, 5’-TCCCAGGATGGCGCAGATA-3’), CD34 (5’-ACCCACCGAGCCATATGCTTAC-3’, 5’-GATACCCTGGGCCAACCTCA-3’), β-actin (5’-CAT CCG TAA AGA CCT CTA TGC CAA C-3’, 5’-ATG GAG CCA CCG ATC CAC A-3’). The PCR reactions were performed in the light of instruction of SYBR Premix Ex TaqTM Kit (Takara, Japan) using the Stratagene Mx3000P QPCR System (Bioscience Corporation). Each sample was measured in duplicate. The relative level of expression was determined by DDCt normalized to endogenous control (β-actin).
Statistical comparisons were performed using the t-test and analysis of variance when data were normally distributed or with separate variance estimation t-test or rank sum test. All statistical procedures were performed using SPSS 15.0. All data are presented as mean ± SD. Values of P < 0.05 were considered statistically significant.
The successful establishment of the HCC model was confirmed by a pathological exam confirming classic clinicopathological characteristics of HCC, intratumor hemorrhage, intrahepatic metastasis, and tumor neovascularization.
The FCM analysis showed the mean percentage of circulating CD133CD34 and CD133VEGFR2 double positive cells in HCC mice to be 0.45% ± 0.16% and 0.20% ± 0.09% (n = 7), respectively. These values are much higher than in the sham-operation group (0.11% ± 0.13%, 0.05% ± 0.11%, n = 9) at 14 d after modeling. At 21 d, the mean percentage of circulating CD133CD34 and CD133VEGFR2 cells is 0.23% ± 0.19%, 0.25% ± 0.15% in HCC model vs 0.05% ± 0.04%, 0.12% ± 0.11% in control. The mobilization of BM cells may have been a response to the operation. Compared to the transient increase observed in controls, the higher level of circulating EPCs were induced by HCC (Figure 1A, B). In addition, the level of serum VEGF and CSF increased gradually in HCC, reaching its peak 14 d after modeling, then slowly decreased (Figure 1C, D).
Consecutive sections stained for the CD133 and CD34 antigens showed that the CD133+ and CD34+ VEGFR2 cells were mostly recruited to HCC tissue and concentrated in tumor microvessels (Figure 2A). Under fluorescence microscopy, the BM-derived cells labeled with GFP were concentrated in the same area (Figure 2B). The relative levels of CD133 and CD34 gene expression were elevated in tumors, around 5.0 and 3.8 times that of the tumor free area (Figure 2C, D). As determined by protein and gene expression levels, EPCs in HCC mice were recruited to tumors and tumor microvessels. The results of each three trials were largely consistent with each other.
In frozen liver sections from HCC mice, cells co-expressing CD133 and VEGFR2 were identified by immunohistochemical staining using anti-CD133 and VEGFR2 antibodies. In tumor tissue, the double-positive cells were incorporated into vessel walls (Figure 3C). In immunofluorescent staining, an antigen specific to mature endothelial cells CD31[21] was used to identify VECs. The image data show that cells co-expressing CD31 and GFP existed not only in sinusoid endothelial cells (Figure 3A) but also in hepatic veins (Figure 3B). These CD31 and GFP double positive cells are direct evidence that tumor VECs come partly from BM-derived EPCs. The proportion of GFP CD31 double positive VECs (out of all VECs) on day 21 was around 35.3% ± 21.2%. This is much higher than the value recorded on day 7 group (17.1% ± 8.9%) (Figure 3D).
For further understanding the mechanism by which BM-derived EPCs home to tumors, we detected the expression of ICAM1, VCAM1, and VEGF in tumor tissues and tumor-free areas using immunohistochemistry. The results showed that the expression of ICAM1, VCAM1, and VEGF was higher in tumor areas than in tumor-free tissues (Figure 4).
Enhanced neovascularization is the most obvious clinicopathological characteristic of HCC, and tumor microvascular density is closely related to HCC progression and patient prognosis[2,22]. Although studies in some tumor models have shown that EPCs are mobilized into peripheral blood and contribute to tumor vascularization, to date, only Hong Kong University, our hepatic cancer institute, and the Medical University of Vienna have successively demonstrated that levels of circulating EPCs increase significantly and are correlated to clinical outcome in patients with HCC. The role of EPCs in HCC neovascularization is not currently clear. Based on our previous clinical findings[17,23], we established an orthotopic HCC model and used GFP as tracer to evaluate the role of EPCs in HCC-related angiogenesis[24,25]. According to PUBMED search, this is the first article to show direct visual evidence proving that EPCs promote angiogenesis by differentiating into vascular endothelia cells in HCC.
Thus far, the clinical significance of circulating EPCs has been confirmed in only a few solid malignancies, such as neurospongioma[25], hepatic cancer[17-19], breast cancer[26,27] and ovarian cancer[28]. Levels of circulating BM-derived stem cells have been used to determine the optimal dose of anti-angiogenic drugs, most successfully in breast cancer patients[7]. In the current investigation, which, unlike our previous clinical study, does not involve any interference from cirrhosis, it is much clearer that the mobilization of EPCs and the up-regulation of the BM-mobilization-related factors VEGF and CSF are the results of the development of HCC (Figure 1). Though wounds and injuries also can mobilize EPCs into the blood[29], the controls in this study showed only transient increases in, VEGF and CSF, and levels of circulating EPCs remained relatively low[30]. In terminal stage of the tumor, the decline in levels of serum cytokines and circulating EPCs may be the result of a disordered environment and systemic failure.
Characterization of EPCs, proof-of-principle strategies design to study the importance of vasculogenesis in tumors are current impediments. CD34-antigen could not distinguish immature EPCs from mature CECs. Recently, scientists discovered an antigen restricted to immature EPCs named CD133 which can distinguish between EPC and CECs. VEFGR2 is another marker of EPCs. VEFGR2 signaling causes EPCs to differentiate. EPCs resemble embryonic angioblasts, which characteristically migrate, proliferate, and differentiate into mature endothelial cells. Generally, EPCs can be identified as cells that simultaneously express the cell surface markers CD133, CD34, and VEGFR2.
In this study, we used GFP as a tracer because that tumor growth is accompanied by the formation of tumor blood vessels, we hypothesized that EPCs could home to liver tumors specifically. To test this hypothesis. The immunohistochemical image data of HCC tissue serial frozen sections showed many cells expressing EPC-specific antigens CD133, CD34, and VEGFR2, and these were concentrated in tumor tissues and tumor vessels (Figure 2A). Relative to tumor-free tissues, many BM-derived cells labeled by GFP specifically homed to tumor tissues and tumor vessels occupying the same positions as the CD133-, CD34-, and VEGFR2-positive cells (Figure 2B). This indicates that these antigen-positive cells come from bone marrow and co-express the specific antigens CD133, CD34, and VEGFR2. Real-time PCR analysis showed the levels of mRNA for stem cell markers CD133 and CD34 were around 5 and 3 times higher, respectively, than in tumor free tissues (Figure 2C). These results may be due to the fact that CD34 is expressed in both mature and immature cells, while CD133 is limited to immature cells (Figure 3B). EPCs’ tropism for malignant tissues can also be observed in other cancer models, in which ICAM-1 is considered the main endothelial surface receptor responsible for recruitment of EPCs[31]. VEGF is believed to be the most important and essential mobilization factor in the induction of matrix metalloproteinase-9 (MMP-9) expression in bone marrow cells (BMCs). MMP-9, in turn, cleaves and activates the VEGF receptor of BMCs, permitting the transfer of BMCs and mobilizing EPCs into circulation[32]. In our HCC model, besides up-regulated VEGF, the levels of VEGF and ICAM1 in tumor tissue also increased significantly. This is consistent with our previously published clinical results[19]. From this, we can reasonably infer that, during tumor growth, HCC tissue releases mobilization factors, such as VEGF, into circulation. As the matrix degrades, BM-derived EPCs migrate into the liver through the bloodstream. Under the chemoattractive influence of locally high VEGF content VEGF and cellular adhesive molecules, the EPCs become come to rest in the hepatic tumor area.
Until now, the contribution of EPCs to blood vessel formation has been proved in only a few types of cancer, including malignant glioma[26] and Ewing’s sarcoma[33,34]. We have identified and confirmed the clinical significance of mobilized EPCs in HCC[17]. Here, we directly prove that EPCs contribute to the formation of new HCC vessels not only in hepatic sinusoids (Figure 3A) but also in the veins of the liver (Figure 3C). Moreover, immunohistochemical image data has directly shown that cells co-expressing CD133 and VEGFR2 incorporate into live vessels (Figure 3D). This indicates that BM-derived EPCs stimulate tumor growth by promoting neovascularization. In addition, the incorporation rate of EPCs on day 21 was found to be much higher than on day 7. In this sense, the role of EPCs in neovascularization seem to become more important as HCC growth progresses (Figure 4C). Mature ECs have relatively limited proliferative capacity. In the current body of literature, the proportion of EPCs to VECs has been found to vary widely, from zero to more than 90%[11,12]. Other studies have concluded that EPCs do not contribute to the vascular endothelium and are not needed for tumor growth[35]. The fact that these studies used different tumor models may be the source of these different results.
Ever since 1971, anti-angiogenesis and anti-vascular treatments have emerged as a clinically promising anti-cancer strategy in HCC therapy. However, though anti-angiogenic therapies to HCC have been used clinically for a long time, the actual effect is not as satisfying as would be expected. Damaging tumor vasculature or blood embolization does lead to local metabolic disturbance, acidosis, hypoxia, and eventually to necrosis. This causes the release of soluble factor into the bloodstream by tumors directly and by the responding system indirectly. These soluble factors mobilize more EPCs into the blood and promote the formation of new blood vessels to compensate for the lost blood supply. In fact, it has been found that tumor necrosis, hypoxia, and ischemia can all cause dramatic increases in serum levels of VEGF and in the number of EPCs in circulation EPCs[36-38]. These cells home to the vasculature of treated tumors and promote tumor neovascularization. In such cases, even if tumor vasculature is damaged and local angiogenesis is inhibited, BM-derived EPCs independently form the blood vessels necessary for tumor regrowth. The existence of compensatory cellular and molecular mechanisms inhibits the efficacy current targeted anti-angiogenic therapies. We suggest inhibiting the recruitment of circulating EPCs in concert with anti-vascular treatment. We have demonstrated previously that the number of circulating EPCs rises dramatically in patients with HCC. Now we further display that, during the course of HCC, large numbers of EPCs are mobilized and recruited to tumor areas, where they promote HCC angiogenesis by integrating directly into the tumor vessels directly. As HCC develops, the proportion of EPCs in vessels seems to rise gradually.
In view of current data, we have reason to believe that the BM is the important source of cells for neovascularization in HCC. We infer that blockage of EPC mobilization or recruitment to tumors may slow down hepatic cancer growth and improve the efficacy of therapies.
Neovascularization is the key process for tumor development, invasiveness and metastasis. It has been accepted that tumor-neovascularization raised in two ways, one is endothelial sprouting, another is bone marrow derived cells differentiating into mature vascular endothelial cells. Hepatocellular carcinoma (HCC) is one of the most vascular solid tumors, in which neovascularization plays an important role. The status of neovascularization in HCC correlates with the disease progression and patient’s prognosis.
At present, the significance of circulating endothelial progenitor cells (EPCs) and contribution of EPCs to tumor vessels have been confirmed only in a few solid malignancies, such as malignant glioma, lung caner and mammal tumor, however, the extent to which EPCs contribute to the generation of the tumor vessels is highly variable in different studies, from substantial to zero. So the role of EPCs on neovascularization has been argued a lot. Till now, there is no any data to evaluate the role of bone marrow cells (BMCs) in HCC neovascularization yet.
Ever since Professor Folkman put forward the dependence of tumor growth on angiogenesis in 1971, anti-angiogenesis or anti-vascular treatment has emerged as a clinically promising anti-cancer strategy in HCC therapies. However, though anti-angiogenic therapies to HCC such as chemotherapy and embolization have been in clinical application for a long time, the actual effect is not as satisfying as expected yet. It is well known HCC is a highly vascularized solid tumor. The authors have demonstrated that circulation BMCs/bone marrow-derived stem cells rise dramatically in patients with HCC previously in clinical cancer research, now the authors further display during the course of HCC, large number of BMCs was mobilized and recruited to tumor area, then promote HCC angiogenesis by integrating into tumor vessels directly.
The study results suggest that the BMCs play a prominent role in HCC neovascularization, and inhibiting EPC mobilization or recruitment to tumor tissue may be an efficient strategy for treating HCC.
EPCs: Endothelial progenitor cells can differentiate into mature vascular endothelial cells for formation of new vessels (vasculogenesis).
This is a good descriptive study to investigate the role of bone marrow-derived EPC in the angiogenesis of HCC. The results are interesting and suggest that mobilized EPCs were found to participate in tumor vasculogenesis of HCC and inhibiting EPC mobilization or recruitment to tumor tissue may be an efficient strategy for treating HCC.
| 1. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5115] [Cited by in RCA: 5984] [Article Influence: 108.8] [Reference Citation Analysis (1)] |
| 2. | Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952-958. [PubMed] |
| 3. | Gao D, Mittal V. The role of bone-marrow-derived cells in tumor growth, metastasis initiation and progression. Trends Mol Med. 2009;15:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 263] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Massa M, Rosti V, Ramajoli I, Campanelli R, Pecci A, Viarengo G, Meli V, Marchetti M, Hoffman R, Barosi G. Circulating CD34+, CD133+, and vascular endothelial growth factor receptor 2-positive endothelial progenitor cells in myelofibrosis with myeloid metaplasia. J Clin Oncol. 2005;23:5688-5695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Santarelli JG, Udani V, Yung YC, Cheshier S, Wagers A, Brekken RA, Weissman I, Tse V. Incorporation of bone marrow-derived Flk-1-expressing CD34+ cells in the endothelium of tumor vessels in the mouse brain. Neurosurgery. 2006;59:374-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Fürstenberger G, von Moos R, Lucas R, Thürlimann B, Senn HJ, Hamacher J, Boneberg EM. Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy of primary breast cancer. Br J Cancer. 2006;94:524-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 169] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, Antin JH, Myerson D, Hamilton SR, Vogelstein B. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11:261-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 356] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 9. | Pircher A, Kähler CM, Skvortsov S, Dlaska M, Kawaguchi G, Schmid T, Gunsilius E, Hilbe W. Increased numbers of endothelial progenitor cells in peripheral blood and tumor specimens in non-small cell lung cancer: a methodological challenge and an ongoing debate on the clinical relevance. Oncol Rep. 2008;19:345-352. [PubMed] |
| 10. | Bagley RG, Weber W, Rouleau C, Teicher BA. Pericytes and endothelial precursor cells: cellular interactions and contributions to malignancy. Cancer Res. 2005;65:9741-9750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1457] [Cited by in RCA: 1376] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 12. | Wickersheim A, Kerber M, de Miguel LS, Plate KH, Machein MR. Endothelial progenitor cells do not contribute to tumor endothelium in primary and metastatic tumors. Int J Cancer. 2009;125:1771-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Mann CD, Neal CP, Garcea G, Manson MM, Dennison AR, Berry DP. Prognostic molecular markers in hepatocellular carcinoma: a systematic review. Eur J Cancer. 2007;43:979-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Morita R, Sato K, Nakano M, Miura H, Odaka H, Nobori K, Kosaka T, Sano M, Watanabe H, Shioya T. Endothelial progenitor cells are associated with response to chemotherapy in human non-small-cell lung cancer. J Cancer Res Clin Oncol. 2011;137:1849-1857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Gao D, Nolan D, McDonnell K, Vahdat L, Benezra R, Altorki N, Mittal V. Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochim Biophys Acta. 2009;1796:33-40. [PubMed] |
| 16. | Ganslmayer M, Zimmermann A, Zopf S, Herold C. Combined inhibitors of angiogenesis and histone deacetylase: efficacy in rat hepatoma. World J Gastroenterol. 2011;17:3623-3629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Yu D, Sun X, Qiu Y, Zhou J, Wu Y, Zhuang L, Chen J, Ding Y. Identification and clinical significance of mobilized endothelial progenitor cells in tumor vasculogenesis of hepatocellular carcinoma. Clin Cancer Res. 2007;13:3814-3824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Sieghart W, Fellner S, Reiberger T, Ulbrich G, Ferlitsch A, Wacheck V, Peck-Radosavljevic M. Differential role of circulating endothelial progenitor cells in cirrhotic patients with or without hepatocellular carcinoma. Dig Liver Dis. 2009;41:902-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Ho JW, Pang RW, Lau C, Sun CK, Yu WC, Fan ST, Poon RT. Significance of circulating endothelial progenitor cells in hepatocellular carcinoma. Hepatology. 2006;44:836-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Murayama T, Tepper OM, Silver M, Ma H, Losordo DW, Isner JM, Asahara T, Kalka C. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp Hematol. 2002;30:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 200] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, Gurtner GC. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood. 2005;105:1068-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 325] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 22. | Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, Walsh K, Isner JM, Asahara T. HMG-CoA reductase inhibitor mobilizes bone marrow--derived endothelial progenitor cells. J Clin Invest. 2001;108:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 499] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 23. | Yu DC, Chen J, Sun XT, Zhuang LY, Jiang CP, Ding YT. Mechanism of endothelial progenitor cell recruitment into neo-vessels in adjacent non-tumor tissues in hepatocellular carcinoma. BMC Cancer. 2010;10:435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Finney MR, Greco NJ, Haynesworth SE, Martin JM, Hedrick DP, Swan JZ, Winter DG, Kadereit S, Joseph ME, Fu P. Direct comparison of umbilical cord blood versus bone marrow-derived endothelial precursor cells in mediating neovascularization in response to vascular ischemia. Biol Blood Marrow Transplant. 2006;12:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Greenfield JP, Jin DK, Young LM, Christos PJ, Abrey L, Rafii S, Gutin PH. Surrogate markers predict angiogenic potential and survival in patients with glioblastoma multiforme. Neurosurgery. 2009;64:819-826; discussion 826-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Rafat N, Beck GCh, Schulte J, Tuettenberg J, Vajkoczy P. Circulating endothelial progenitor cells in malignant gliomas. J Neurosurg. 2010;112:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Naik RP, Jin D, Chuang E, Gold EG, Tousimis EA, Moore AL, Christos PJ, de Dalmas T, Donovan D, Rafii S. Circulating endothelial progenitor cells correlate to stage in patients with invasive breast cancer. Breast Cancer Res Treat. 2008;107:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Su Y, Zheng L, Wang Q, Li W, Cai Z, Xiong S, Bao J. Quantity and clinical relevance of circulating endothelial progenitor cells in human ovarian cancer. J Exp Clin Cancer Res. 2010;29:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Kienstra KA, Jackson KA, Hirschi KK. Injury mechanism dictates contribution of bone marrow-derived cells to murine hepatic vascular regeneration. Pediatr Res. 2008;63:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Goon PK, Lip GY, Stonelake PS, Blann AD. Circulating endothelial cells and circulating progenitor cells in breast cancer: relationship to endothelial damage/dysfunction/apoptosis, clinicopathologic factors, and the Nottingham Prognostic Index. Neoplasia. 2009;11:771-779. [PubMed] |
| 31. | Kasselman LJ, Kintner J, Sideris A, Pasnikowski E, Krellman JW, Shah S, Rudge JS, Yancopoulos GD, Wiegand SJ, Croll SD. Dexamethasone treatment and ICAM-1 deficiency impair VEGF-induced angiogenesis in adult brain. J Vasc Res. 2007;44:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Ebrahem Q, Chaurasia SS, Vasanji A, Qi JH, Klenotic PA, Cutler A, Asosingh K, Erzurum S, Anand-Apte B. Cross-talk between vascular endothelial growth factor and matrix metalloproteinases in the induction of neovascularization in vivo. Am J Pathol. 2010;176:496-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Reddy K, Cao Y, Zhou Z, Yu L, Jia SF, Kleinerman ES. VEGF165 expression in the tumor microenvironment influences the differentiation of bone marrow-derived pericytes that contribute to the Ewing's sarcoma vasculature. Angiogenesis. 2008;11:257-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Zhou Z, Stewart KS, Yu L, Kleinerman ES. Bone marrow cells participate in tumor vessel formation that supports the growth of Ewing's sarcoma in the lung. Angiogenesis. 2011;14:125-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Purhonen S, Palm J, Rossi D, Kaskenpää N, Rajantie I, Ylä-Herttuala S, Alitalo K, Weissman IL, Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci USA. 2008;105:6620-6625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 302] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 36. | Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 241] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 37. | Shaked Y, Kerbel RS. Antiangiogenic strategies on defense: on the possibility of blocking rebounds by the tumor vasculature after chemotherapy. Cancer Res. 2007;67:7055-7058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Xiao EH, Guo D, Bian DJ. Effect of preoperative transcatheter arterial chemoembolization on angiogenesis of hepatocellular carcinoma cells. World J Gastroenterol. 2009;15:4582-4586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
Peer reviewer: Pradyumna Kumar Mishra, Professor, Translational Research, Tata Memorial Centre, Kharghar, Navi Mumbai 410210, India
S- Editor Gou SX L- Editor A E- Editor Zhang DN