Published online Sep 21, 2012. doi: 10.3748/wjg.v18.i35.4866
Revised: May 17, 2012
Accepted: June 15, 2012
Published online: September 21, 2012
AIM: To evaluate the effects of indomethacin [dual cyclooxygenase (COX)-1/COX-2 inhibitor] and 3-(3,4-difluorophenyl)-4-(4-(methylsulfonyl) phenyl)-2-(5H)-furanone (MF-tricyclic) (COX-2 selective inhibitor) in a rat experimental model of Barrett’s esophagus and esophageal adenocarcinoma.
METHODS: A total of 112 surviving post-surgery rats were randomly divided into three groups: the control group (n = 48), which did not receive any treatment; the indomethacin group (n = 32), which were given 2 mg/kg per day of the COX-1/COX-2 inhibitor; and the MF-tricyclic group (n = 32), which received 10 mg/kg per day of the selective COX-2 inhibitor. Randomly selected rats were killed either 8 wk or 16 wk after surgery. The timing of the deaths was in accordance with a previous study performed in our group. Only rats that were killed at the times designated by the protocol were included in the study. We then assessed the histology and prostaglandin E2 (PGE2) expression levels in the rat esophagi. An additional group of eight animals that did not undergo esophagojejunostomy were included in order to obtain normal esophageal tissue as a control.
RESULTS: Compared to a control group with no treatment (vehicle-treated rats), indomethacin treatment was associated with decreases in ulcerated esophageal mucosa (16% vs 35% and 14% vs 17%, 2 mo and 4 mo after surgery, respectively; P = 0.021), length of intestinal metaplasia in continuity with anastomosis (2 ± 1.17 mm vs 2.29 ± 0.75 mm and 1.25 ± 0.42 mm vs 3.5 ± 1.54 mm, 2 mo and 4 mo after surgery, respectively; P = 0.007), presence of intestinal metaplasia beyond anastomosis (20% vs 71.4% and 0% vs 60%, 2 mo and 4 mo after surgery, respectively; P = 0.009), severity of dysplasia (0% vs 71.4% and 20% vs 85.7% high-grade dysplasia, 2 mo and 4 mo after surgery, respectively; P = 0.002), and adenocarcinoma incidence (0% vs 57.1% and 0% vs 60%, 2 mo and 4 mo after surgery, respectively; P < 0.0001). Treatment with the selective COX-2 inhibitor, MF-tricyclic, did not prevent development of intestinal metaplasia or adenocarcinoma. In parallel, we observed a significant decrease in PGE2 levels in indomethacin-treated rats, but not in those treated with MF-tricyclic, at both 2 mo and 4 mo. Compared to control rats that did not undergo surgery (68 ± 8 ng/g, P = 0.0022 Kruskal-Wallis test) there was a significant increase in PGE2 levels in the esophageal tissue of the rats that underwent surgery either 2 mo (1332 ± 656 ng/g) or 4 mo (1121 ± 1015 ng/g) after esophagojejunostomy. However, no differences were found when esophageal PGE2 levels were compared 2 mo vs 4 mo post-esophagojejunostomy. At both the 2- and 4-mo timepoints, we observed a significant decrease in PGE2 levels in indomethacin-treated rat esophagi compared to those in either the control or MF-tricyclic groups (P = 0.049 and P = 0.017, respectively). No differences in PGE2 levels were found when we compared levels in rats treated with MF-tricyclic to not-treated rats.
CONCLUSION: In this rat model of gastrointestinal reflux, indomethacin was associated with a decrease in the severity of esophagitis and reduced development of esophageal intestinal metaplasia and adenocarcinoma.
- Citation: Esquivias P, Morandeira A, Escartín A, Cebrián C, Santander S, Esteva F, García-González MA, Ortego J, Lanas A, Piazuelo E. Indomethacin but not a selective cyclooxygenase-2 inhibitor inhibits esophageal adenocarcinogenesis in rats. World J Gastroenterol 2012; 18(35): 4866-4874
- URL: https://www.wjgnet.com/1007-9327/full/v18/i35/4866.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i35.4866
The incidence of esophageal adenocarcinoma (EA) in the United States and Western Europe has risen dramatically in the last few decades[1-3]. The reasons for this substantial increase are not entirely understood, but it has been linked to an increase in the incidence of gastroesophageal reflux (GERD) and Barrett’s esophagus (BE), which is a recognized risk factor for the development of EA. BE is a consequence of chronic GERD, which represents the starting point of the sequence: GERD, inflammation, BE, low-grade dysplasia, high-grade dysplasia, carcinoma in situ, and invasive adenocarcinoma[4]. The molecular changes in the progression from GERD to BE and then to EA are not entirely understood[5], but in the last few years, cyclooxygenase-2 (COX-2) has emerged as a key player in carcinogenesis arising from BE; therefore, it is a potential therapeutic target in the sequence leading to EA. Evidence supporting the involvement of COX-2 in this sequence includes epidemiological studies that have demonstrated a protective effect of nonsteroidal antiinflammatory drugs (NSAIDs) against EA and in vivo animal studies in experimental models of chronic esophagitis and EA, in which administration of COX-2 inhibitors induced a reduction in the incidence of esophageal cancer. In addition, upregulation of COX-2 has been described at both mRNA and protein levels in humans with GERD, BE, or EA[6]. COX-1, the other cyclooxygenase isoenzyme, was traditionally considered to be a constitutively expressed isoform that generates prostaglandins for normal physiological functions such as platelet aggregation, gastric mucosal protection, and renal electrolyte metabolism; it was thought not be involved in carcinogenesis. Recent evidence, however, has demonstrated that COX-1 may be important in the development of different neoplasias, because elevated levels of COX-1 expression have been found in prostate, breast, and cervical cancers[7]. Furthermore, studies in Min+/- mice showed that homologous disruption of either COX-2 or COX-1 genes is followed by a reduction in polyp formation of approximately 80%[7]. In the esophagus, the role of COX-1 in the development of EA has not been investigated extensively. In a previous study, expression of both COX-1 and COX-2 were analyzed at the mRNA level in 123 primary human esophageal adenocarcinomas; mRNA levels of both isoenzymes were increased. Interestingly, RNA expression levels of COX-1 and COX-2 correlated significantly with one other, and expression of both isoenzymes correlated with the expression of vascular endothelial growth factors A and C (VEGF-A and VEGF-C)[8]. Another study reported an increase in COX-1 expression in a rat model of esophagitis induced by acid reflux[9]. Therefore, to analyze the effects of COX inhibitors in the development of EA due to gastrointestinal reflux, we assessed the effect of a dual COX-1/COX-2 inhibitor (indomethacin) in a rat model of esophageal adenocarcinoma induced by gastroenteroesophageal reflux, and compared it with the effect of a selective COX-2 inhibitor (3-(3,4-difluorophenyl)-4-(4-(methylsulfonyl) phenyl)-2-(5H)-furanone) (MF-tricyclic).
All of the animal studies were carried out in the Biomedicine and Biomaterials group of the University of Zaragoza, which is officially recorded as a research establishment that practices adequate husbandry and use of all research animals in accordance with Good Laboratory Practices guidelines. All procedures were approved by the in-house Ethics Committee for Animal Experiments from the University of Zaragoza. The care and use of animals were performed according to the Spanish Policy for Animal Protection RD1201/05, which meets the European Union Directive 86/609 on the protection of animals used for experimental and other scientific purposes. Throughout the experiment, all rats were housed in specifically designed cages for this type of animal (EU Dim; IFFA Credo) and kept in computer-controlled conditions of light (12-h light/dark cycle), noise (45-50 dB), temperature (21 ± 2 °C), and humidity (60%-70%). The animals were cared for by veterinary staff. A total of 128 five-week-old female Wistar rats (Harlan, Barcelona, Spain) weighing 220-300 g were used for this study.
Solid food was withdrawn for 24 h and water for 6-8 h before surgery. Anesthesia consisted of 75 mg/kg weight ketamine (50 mg/mL, Ketolar; Parke-Davis, Madrid, Spain) and 0.5 mg/kg weight medetomidine (1 mg/mL, Domtor; Pfizer, Madrid, Spain), which were given as a mixture in a single intramuscular injection. Before surgery, an intramuscular injection of 100 mg/kg weight cefazolin (Kefol; Lilly, Madrid, Spain) was administered as antibiotic prophylaxis. Rats underwent esophagojejunostomy with gastric preservation, allowing the gastroduodenal content to flow back into the esophagus. This surgery was performed according to a previously described protocol[10]. After surgery, the rats were housed in hanging cages to prevent bed ingestion and allowed to drink water ad libitum, with fasting until day 3. Water with 0.3 mg/mL buprenorphine (Buprex; Esteve, Barcelona, Spain) was provided during the first 72 postoperative hours. An additional intramuscular injection of cefazolin was administered 24 h after surgery. Rats received 50 mg/kg iron dextran (Difortin; Econatura, Madrid, Spain) intramuscularly once every 4 wk until the end of the experiment. Rats were killed by carbon dioxide inhalation either 2 mo or 4 mo after surgery. A blood sample was obtained for drug analysis from all of the animals immediately before death. Immediately after death, the esophagus was removed and opened longitudinally. Two-thirds of the esophageal specimen was prepared for pathological study. The rest of the esophagus was cut into two slices: the lower and the upper halves of the esophagus. The lower half samples were frozen immediately in liquid nitrogen and then kept at -80 °C until biochemical studies were performed.
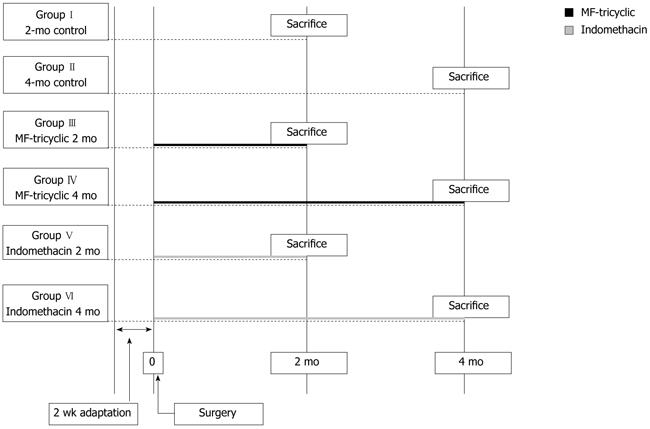
Two weeks after surgery, the 112 surviving post-surgery rats were randomly divided into three groups: the control group (n = 48), which did not receive any treatment; the indomethacin group (n = 32), which were given the COX-1/COX-2 inhibitor; and the MF-tricyclic group (n = 32), which received the selective COX-2 inhibitor. Randomly selected rats were killed either 8 or 16 wk after surgery. The timing of the deaths was in accordance with a previous study[10]. Only rats that were killed at the times designated by the protocol were included in the study. A scheme of the study design is shown in Figure 1. An additional group of eight animals that did not undergo esophagojejunostomy were included in order to obtain normal esophageal tissue.
MF-tricyclic was administered at a dose of 10 mg/kg body weight/d. This dose was selected according to a previous study of esophageal adenocarcinoma prevention in the same model[11]. The drug was kindly provided by Merck Frost (Quebec, Canada), which produced a premixed chow containing the drug. The concentration of MF-tricyclic in the chow was calculated using the average weight of Wistar rats and a daily consumption of 16-20 g of chow/d. This rate of chow consumption was evaluated in previous experiments[10]. Indomethacin (Sigma, Madrid, Spain) was given at a dose of 2 mg/kg body weight/d. This dose was selected according to a previous study of the inhibition of colorectal cancer[12]. The drug was added to drinking water. Indomethacin was replaced on a daily basis and administered in dark bottles in order to avoid light degradation.
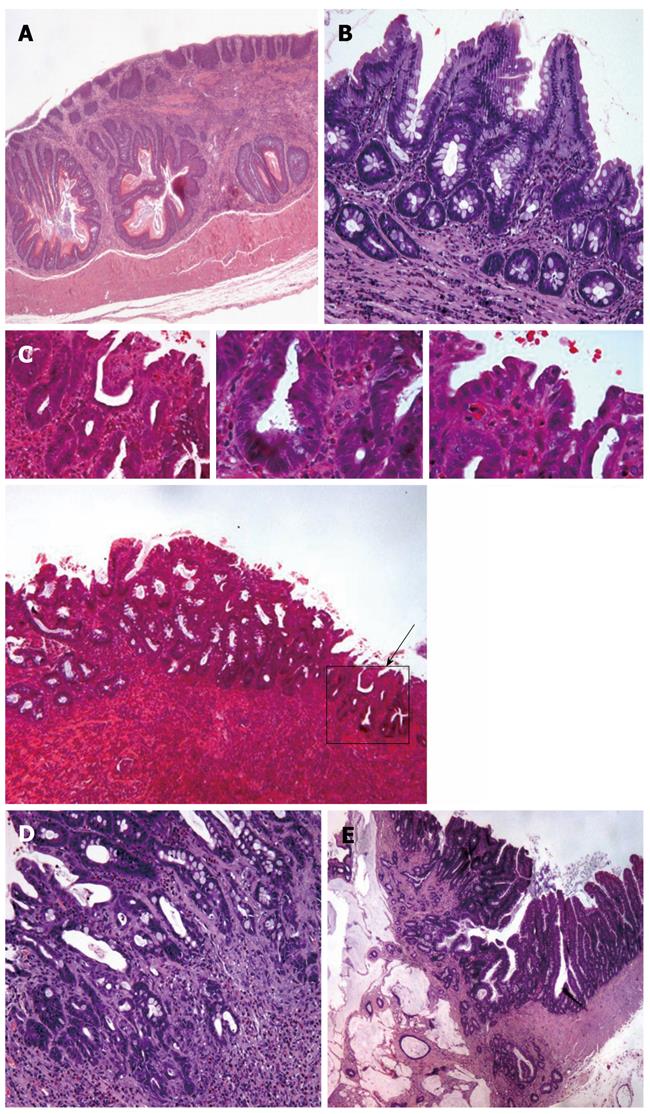
Rat esophagi were examined by two independent pathologists who were unaware of the experimental conditions. Changes in the squamous epithelium were classified into the following five categories: (1) Reactive changes: characterized by the presence of basal cell hyperplasia, increased length of papillae, and hyperkeratosis with areas of inflammation and ulceration; (2) Columnar-lined metaplasia (intestinal metaplasia): squamous epithelium was replaced with columnar-lined epithelium comprising occasional and incomplete differentiation of goblet cells; the length of the epithelial transformation was greater than 3 mm starting from the anastomosis site or was intercalated, or it could be seen beyond the surgical anastomosis, and was surrounded by squamous epithelium; (3) Dysplasia: characterized by nuclear atypia, partial loss of mucosecretory function and cell polarity, and an increase in mitotic figures. Dysplasia was classified as low-grade dysplasia (LGD) and high-grade dysplasia (HGD) according to the criteria proposed by Haggitt[13], as previously described in this experimental model[14]. In LGD, the crypt architecture is preserved or minimally distorted; the nuclei may be stratified but without reaching the apical surface of glands, nuclei are enlarged, crowded and hyperchromatic, mitotic figures may be found in the upper portion of the crypt; goblet and columnar cell mucus is usually diminished or absent, but goblet cells in which the mucous droplet does not communicate with the luminal surface may be observed. The abnormalities extend to the mucosal surface. In HGD, distortion of crypt architecture usually is present and may be marked. It is composed of branching and lateral budding of crypts, a villiform configuration of the mucosal surface or intraglandular bridging of epithelium to form a cribriform pattern of “back-to-back” glands. Nuclear abnormalities are present as in LGD, and stratification reaches the crypt luminal surface. There may be a loss of nuclear polarity, and nuclei often vary markedly in size, shape, and staining characteristics. Goblet and columnar cell mucus is usually absent. The abnormalities extend to the mucosal surface; (4) Adenocarcinoma: glandular structures of dysplastic columnar epithelial with stromal invasion and deep infiltration; and (5) Squamous carcinoma: characterized by accumulation of atypical cells with nuclear hyperchromasia, abnormally clumped chromatin, and loss of polarity. The two pathologists showed a high concordance (kappa coefficient > 0.8). However, in cases where there was disagreement, a third pathologist was required to resolve the discrepancy.
Prostaglandin E2 (PGE2) analysis was performed in the lower half of the esophagus. Tissue samples were added to 1 mL of cold PBS containing 10 μmol indomethacin. They were then homogenized (DIAX 900, Heidolph, Kelheim, Germany) on ice and centrifuged for 15 min at 1200 rpm (4 °C). Supernatants were collected in 2 mL tubes. Prostaglandin extraction was performed as previously described by Powell[15]. PGE2 was measured by radioimmunoassay [prostaglandin E2 (I125); Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom].
MF-tricyclic concentration analysis in rat plasma was performed by Merck Frost Canada; the results were disclosed to us after the study was complete. Indomethacin levels were determined as follows: 50 μg (10 μL of a 5 mg/mL methanolic solution) of the internal standard probenecid was added to 1 mL of serum before each analysis. Afterwards, samples were extracted with 2 mL of ethyl acetate and an aliquot of the organic phase was withdrawn, evaporated to dryness under a nitrogen stream at room temperature, and redissolved in 300 μL of methanol. Fifty microliters of these solutions were analyzed by high-performance liquid chromatography (Waters 600 Controller, 996 Photodiode Array Detector). A method capable of quantifying indomethacin was developed using a C-18 column (150 mm × 3.9 mm, 4 μm particle size; Waters, Barcelona, Spain) and probenecid as the internal standard. The column was equilibrated at a flow rate of 1 mL/min with a mobile phase consisting of acetonitrile in water (51:49, v/v) and 0.1% trifluoroacetic acid. The eluate was monitored at 257 nm[16].
Data management and statistical analysis were performed using SPSS software. Results from biochemical assays were expressed as mean ± SE. Data were compared between groups by nonparametric tests (Kruskall-Wallis and Mann-Whitney); P < 0.05 was considered to be statistically significant. Covariance analysis was used to analyze the quantitative variables of the drug, introducing the number of months as a covariant. Multiple comparisons were corrected by means of the Bonferroni method. Logistic models were adjusted for qualitative variables, evaluating in turn the effect of the drug and the number of months.
Macroscopically, all of the rats that underwent esophagojejunostomy showed dilatation and thickening of the middle and lower esophagus. The distal esophagus showed ulceration. Macroscopically, we did not identify any esophageal tumors. The esophagi of all of the rats that were operated on presented inflammatory infiltrate. In most of the animals, the upper and middle parts of the esophagus showed squamous hyperplasia. The esophagi of all of the rats that were operated had columnar-lined metaplasia in continuity with the jejunal epithelium. Dysplasia had developed in all of the rats that underwent surgery. Neoplastic transformation of the epithelium was always found near the anastomosis, surrounded by columnar epithelium. Histologically, 87.5% of tumors were adenocarcinomas, and 12.5% were squamous carcinoma (Table 1). Microscopically, in some animals, a second type of metaplasia was found far above the anastomosis. This metaplasia contained isolated or grouped goblet cells, which formed authentic mucous glands in some cases. However, in any case, these metaplastic foci progressed to adenocarcinoma.
| Group | I (n = 14) | II (n = 15) | III (n = 11) | IV (n = 6) | V (n = 5) | VI (n = 6) |
| Inflammation | 14 | 15 | 11 | 6 | 5 | 6 |
| Ulcerated mucosa,% | 35.7 | 37.3 | 33.18 | 39.58 | 16 | 14.17 |
| Squamous hyperplasia,% | 10 | 14 | 2 | 5 | 2 | 2 |
| Intestinal metaplasia in continuity with anastomosis, mm | 2.29 | 3.5 | 2.32 | 2.83 | 2 | 1.25 |
| Intestinal metaplasia beyond anastomosis,% | 10 | 9 | 5 | 4 | 1 | 0 |
| Dysplasia | 14 | 14 | 10 | 6 | 5 | 5 |
| High-grade dysplasia/low-to-medium grade dysplasia | 10/4 | 12/2 | 4/6 | 4/2 | 0/5 | 1/4 |
| Neoplasia | 8 | 9 | 4 | 3 | - | - |
| Mucinous adenocarcinoma | 7 | 9 | 3 | 2 | - | - |
| Squamous adenocarcinoma | 1 | - | 1 | 1 | - | - |
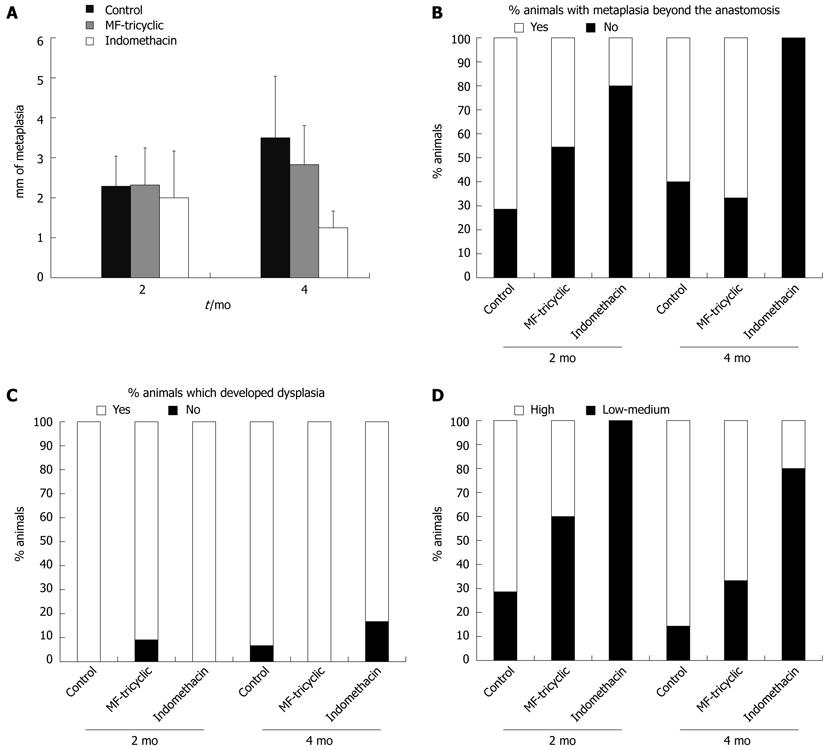
Inflammation, ulceration, intestinal metaplasia, dysplasia, and neoplasias were present in all of the rats that underwent esophagojejunostomy. When the percentage of ulcerated surface was analyzed, there was a significant decrease in the groups treated with indomethacin compared to the control and MF-tricyclic groups. The indomethacin treatment groups showed a significant reduction in the length of metaplasia; we also observed a significant decrease in the percentage of metaplasia beyond the anastomosis (Figures 2, 3A and B).
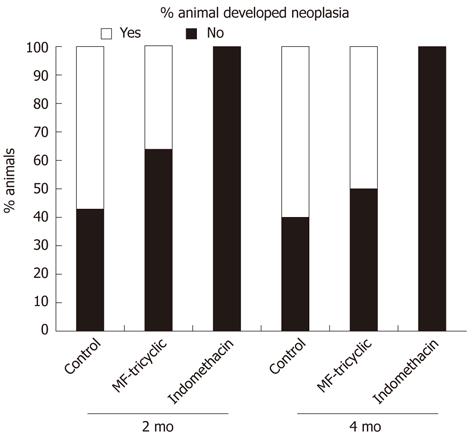
Most of the rats developed some degree of dysplasia. When we analyzed the occurrence of low-grade vs high-grade dysplasia, we found that the percentage of rats that developed high-grade dysplasia was significantly lower in rats treated with indomethacin compared to the MF-tricyclic and control groups (Figures 3C, D). The absence of neoplasias in the indomethacin-treated group (0% vs 36.4% in the MF-tricyclic group and 57.1% in the control group 2 mo after surgery, and 0% vs 50% in the MF-tricyclic group and 60% in the control group 4 mo after surgery), did not allow us to estimate the risk effect as odds ratio. Nevertheless, when we applied a log-linear model, we concluded that time was a nonsignificant factor in the presence of neoplasia. When we analyzed the effect of treatment, we observed a lower incidence of neoplasia in the indomethacin group (P≤ 0.001) (Figure 4).
There was a significant increase in PGE2 levels in the esophageal tissue of the rats that underwent surgery either 2 mo (1332 ± 656 ng/g) or 4 mo (1121 ± 1 015 ng/g) after esophagojejunostomy compared to control rats that did not undergo surgery (68 ± 8 ng/g; P = 0.0022 Kruskal-Wallis test). However, no differences were found when esophageal PGE2 levels were compared 2 mo vs 4 mo post-esophagojejunostomy.
At both the 2- and 4-mo timepoints, we observed a significant decrease in PGE2 levels in indomethacin-treated rat esophagi compared to those in either the control or MF-tricyclic groups (P = 0.049 and P = 0.017, respectively). Rats treated with MF-tricyclic did not show differences in PGE2 levels compared to non-treated animals (Table 2).
| 2 mo | 4 mo | |
| Without treatment | 1332.7 ± 656.7 | 1121.6 ± 1015.6 |
| MF-tricyclic | 1776.8 ± 1403.3 | 1914.9 ± 1905.5 |
| Indomethacin | 480.3 ± 333.4 | 243.9 ± 85.7 |
All of the rats treated with either MF-tricyclic or indomethacin had detectable levels of the drugs in their plasma. The serum drug concentration in rats receiving 10 mg/kg body weight MF-tricyclic was 1.62 ± 0.19 μg/mL. The rats receiving 2 mg/kg indomethacin had plasma levels of 1.97 ± 0.43 μg/mL. As expected, the rats in the untreated control group did not have detectable MF-tricyclic or indomethacin levels.
In the present study, we have used an enteroesophageal reflux model in rats to evaluate the impact of dual COX-1/COX-2 vs selective COX-2 inhibition in the neoplastic transformation of esophageal epithelium. Although in this experimental model the pH of the refluxate increases with increasing distance from the stomach to the anastomosis, non-acidic refluxate has been shown to enhance the occurrence of intestinal metaplasia with dysplasia and esophageal adenocarcinoma[17]. In addition, these models are widely used because they reproduce the sequence of histological changes that take place in human esophageal adenocarcinogenesis and show morphological and phenotypic characteristics similar to those observed in human Barrett’s esophagus and associated adenocarcinoma, including mucin features and expression of differentiation markers and markers of neoplastic progression[14,18].
Our results showed that treatment with the dual COX-1/COX-2 inhibitor indomethacin induced a significant reduction in the area of ulcerated mucosa, the length of metaplasia in continuity with anastomosis, the incidence of metaplasia beyond the anastomosis, the severity of dysplasia, and the incidence of adenocarcinoma in this rat model of chronic gastrointestinal reflux. In contrast, treatment with the selective COX-2 inhibitor, MF-tricyclic, did not prevent the development of these lesions in this model. Our results are consistent with those reported by Heath et al[19] in a human clinical trial, in which treatment with 200 mg/d of celecoxib, another selective inhibitor of COX-2, did not prevent the progression of Barrett’s dysplasia to cancer. In parallel with our histopathological results, we found a significant decrease in PGE2 levels in indomethacin-treated rats, but not in the MF-tricyclic group, which suggests that PGE2 inhibition is also linked to the effect observed with these two agents. Increased levels of PGE2 during chronic inflammation were previously correlated with carcinogenesis. In an esophagitis rabbit model, Lanas et al[20] observed high PGE2 levels associated with increased activity of both COX-1 and COX-2. The levels decreased when rabbits were treated with the nonselective COX inhibitor, indomethacin.
In an experimental model, Buttar et al[11] showed that treatment with sulindac had a preventive effect against EA. In contrast with our findings, they observed a similar, although numerically lower, beneficial effect with MF-tricyclic, although they also did not see significant mucosal PGE2 inhibition with this agent. In our study, we used the same dose of MF-tricyclic as that reported in Buttar’s experiment, but the serum drug levels found in our rats were higher. Therefore, we suggest that the apparent differences between these results could be due to the different experimental periods of treatment (28 and 16 wk in Buttar’s and our study, respectively). In a 40-wk experimental study, Oyama et al[21] showed that pathological changes associated with accelerated cell proliferation, such as regenerative thickening and basal cell hyperplasia, were significantly suppressed in the treated group (celecoxib 500 ppm, 100 mg/kg/d) throughout the experiment. However, the incidence of columnar lined epithelium and EA were significantly suppressed only at the end of the study (40 wk). Similarly, Chen et al[22] showed beneficial effects in animals treated with celecoxib over a 40-wk experimental period. We selected the endpoint of the present study according to previous research performed in our group, in which rats under esophagojejunostomy showed a high incidence of esophageal adenocarcinoma and intestinal metaplasia 4 mo after surgery. It is possible that this period of time is sufficient to see a positive effect with profound inhibition of both types of COX activity as with indomethacin, but is not long enough to see the results of weaker or partial COX inhibition with a selective COX-2 agent.
Most studies, including ours, showed a greater decrease in tissue PGE2 levels with a nonselective COX inhibitor compared to COX-2 selective inhibitors[11,20,23]. This effect suggests that PGE2-derived COX-1 inhibition may play a role in the progression from gastrointestinal reflux to EA. In addition, additional mechanisms not linked to either COX-1 or COX-2 inhibition may be involved in this process. Orido et al[24] observed that treatment with indomethacin, but not sulindac, decreases arachidonic acid uptake in a human colorectal cell line. They suggested that the anticancer effect of indomethacin could be independent of COX-2 inhibition. Moreover, Kim et al[25] evaluated the induction of NAG-1 and apoptosis by various NSAIDs, such indomethacin, sulindac, and diclofenac. Indomethacin was the most potent inducer of both NAG-1 expression (P < 0.05) and apoptosis (P < 0.05). Kopp et al[26] and Castro et al[27] reported that sodium salicylate, acetylsalicylic acid, and indomethacin inhibit activation of NF-κB[26,27]. Hanif et al[28] observed that NSAIDs inhibit the proliferation rate of two colon cancer cell lines independent of their ability to inhibit prostaglandin synthesis.
In addition to the above observations, it is important to note that much higher doses of NSAIDs are required for inhibition of proliferation and angiogenesis and induction of apoptosis, some of the mechanisms that have been suggested to contribute to antitumor activity, than for inhibition of prostaglandin synthesis[29].
In conclusion, treatment with indomethacin, a dual COX-1/COX-2 inhibitor, in our experimental model of EA, prevented the development of intestinal metaplasia and esophageal adenocarcinoma and reduced PGE2 production. In contrast, treatment with MF-tricyclic, a selective COX-2 inhibitor, was not associated with these effects. The profound inhibition of mucosal PGE2 with indomethacin, but not with MF-tricyclic, might partly explain the different effects, but other reasons, such as the length of therapy, activity of COX-1, and involvement of COX-independent mechanisms, could also explain the results, and deserve further investigation.
We thank Sara Serrano, Lidia Floria and Pilar Pina from the Service of Pathology for their invaluable technical assistance.
Over the last decades, the incidence of esophageal adenocarcinoma in the United States and Western Europe has dramatically risen. Epidemiological studies have reported that consumption of cyclooxygenase (COX) inhibitors leads to a decrease in the chance of developing esophageal cancer.
COX-2 has become a target for the prevention of esophageal adenocarcinoma. There are several studies in which administration of COX-2 inhibitors showed a reduction in the incidence of esophageal adenocarcinoma in rat reflux-induced models. In contrast to previous studies, the research showed no effect when a selective COX-2 inhibitor was administered, while a non-selective COX inhibitor, indomethacin, inhibited the complete carcinogenic sequence in the rat esophagus.
Recent evidence has demonstrated that COX-1 may be important in the development of different neoplasias, because elevated levels of COX-1 expression were found in prostate, breast, and cervical cancers. In the study, treatment with indomethacin, a dual COX-1/COX-2 inhibitor, prevented the development of intestinal metaplasia and esophageal adenocarcinoma and reduced prostaglandin E2 (PGE2) production. In contrast, treatment with 3-(3, 4-difluorophenyl)-4-(4-(methylsulfonyl)phenyl)-2-(5H)-furanone (MF-tricyclic), a selective COX-2 inhibitor, was not associated with these effects. These results suggest that COX-1 also plays an important role in the development of esophageal adenocarcinoma.
The inhibitory effect of the complete carcinogenic sequence observed with indomethacin in this study suggests two new aspects that should be further investigated; firstly, the potential role of COX-1 in esophageal adenocarcinogenesis and secondly, the involvement of cyclooxygenase-independent anticancer actions of indomethacin.
The authors evaluated the effect of indomethacin, a COX non-selective inhibitor, and MF-tricyclic, a COX-2 selective inhibitor, in an experimental model of Barrett’s esophagus and EA in rats. The study showed that indomethacin but not MF-tricyclic prevented the development of intestinal metaplasia and esophageal adenocarcinoma and reduced PGE2 production. The results suggest that COX-1 plays an important role in the development of esophageal adenocarcinoma.
| 1. | Pera M, Manterola C, Vidal O, Grande L. Epidemiology of esophageal adenocarcinoma. J Surg Oncol. 2005;92:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Blot WJ, Devesa SS, Kneller RW, Fraumeni JF. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1155] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 3. | Devesa SS, Blot WJ, Fraumeni JF. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049-2053. [PubMed] |
| 4. | Mutoh M, Watanabe K, Kitamura T, Shoji Y, Takahashi M, Kawamori T, Tani K, Kobayashi M, Maruyama T, Kobayashi K. Involvement of prostaglandin E receptor subtype EP(4) in colon carcinogenesis. Cancer Res. 2002;62:28-32. [PubMed] |
| 5. | Chang SH, Ai Y, Breyer RM, Lane TF, Hla T. The prostaglandin E2 receptor EP2 is required for cyclooxygenase 2-mediated mammary hyperplasia. Cancer Res. 2005;65:4496-4499. [PubMed] |
| 6. | Piazuelo E, Jimenez P, Lanas A. COX-2 inhibition in esophagitis, Barrett's esophagus and esophageal cancer. Curr Pharm Des. 2003;9:2267-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Jiménez P, García A, Santander S, Piazuelo E. Prevention of cancer in the upper gastrointestinal tract with COX-inhibition. Still an option? Curr Pharm Des. 2007;13:2261-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | von Rahden BH, Stein HJ, Pühringer F, Koch I, Langer R, Piontek G, Siewert JR, Höfler H, Sarbia M. Coexpression of cyclooxygenases (COX-1, COX-2) and vascular endothelial growth factors (VEGF-A, VEGF-C) in esophageal adenocarcinoma. Cancer Res. 2005;65:5038-5044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Hayakawa T, Fujiwara Y, Hamaguchi M, Sugawa T, Okuyama M, Sasaki E, Watanabe T, Tominaga K, Oshitani N, Higuchi K. Roles of cyclooxygenase 2 and microsomal prostaglandin E synthase 1 in rat acid reflux oesophagitis. Gut. 2006;55:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Piazuelo E, Cebrián C, Escartín A, Jiménez P, Soteras F, Ortego J, Lanas A. Superoxide dismutase prevents development of adenocarcinoma in a rat model of Barrett's esophagus. World J Gastroenterol. 2005;11:7436-7443. [PubMed] |
| 11. | Buttar NS, Wang KK, Leontovich O, Westcott JY, Pacifico RJ, Anderson MA, Krishnadath KK, Lutzke LS, Burgart LJ. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett's esophagus. Gastroenterology. 2002;122:1101-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 262] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 12. | Brown WA, Skinner SA, Malcontenti-Wilson C, Vogiagis D, O'Brien PE. Non-steroidal anti-inflammatory drugs with activity against either cyclooxygenase 1 or cyclooxygenase 2 inhibit colorectal cancer in a DMH rodent model by inducing apoptosis and inhibiting cell proliferation. Gut. 2001;48:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Haggitt RC. Barrett's esophagus, dysplasia, and adenocarcinoma. Hum Pathol. 1994;25:982-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 441] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Su Y, Chen X, Klein M, Fang M, Wang S, Yang CS, Goyal RK. Phenotype of columnar-lined esophagus in rats with esophagogastroduodenal anastomosis: similarity to human Barrett's esophagus. Lab Invest. 2004;84:753-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Powell WS. Reversed-phase high-pressure liquid chromatography of arachidonic acid metabolites formed by cyclooxygenase and lipoxygenases. Anal Biochem. 1985;148:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Grippa E, Santini L, Castellano G, Gatto MT, Leone MG, Saso L. Simultaneous determination of hydrocortisone, dexamethasone, indomethacin, phenylbutazone and oxyphenbutazone in equine serum by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 2000;738:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Cheng P, Li JS, Gong J, Zhang LF, Chen RZ. Effects of refluxate pH values on duodenogastroesophageal reflux-induced esophageal adenocarcinoma. World J Gastroenterol. 2011;17:3060-3065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Hindmarsh A, Belshaw N, Mehta S, Johnson IT, Rhodes M. Can the rat be used as a valid model of human esophageal adenocarcinoma? Dis Esophagus. 2012;25:159-165. [PubMed] |
| 19. | Heath EI, Canto MI, Piantadosi S, Montgomery E, Weinstein WM, Herman JG, Dannenberg AJ, Yang VW, Shar AO, Hawk E. Secondary chemoprevention of Barrett's esophagus with celecoxib: results of a randomized trial. J Natl Cancer Inst. 2007;99:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Lanas A, Jiménez P, Ferrández A, Escartín A, Arenas J, Esteva F, Ortego J. Selective COX-2 inhibition is associated with decreased mucosal damage induced by acid and pepsin in rabbit esophagitis. Inflammation. 2003;27:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 63] [Reference Citation Analysis (1)] |
| 21. | Oyama K, Fujimura T, Ninomiya I, Miyashita T, Kinami S, Fushida S, Ohta T, Koichi M. A COX-2 inhibitor prevents the esophageal inflammation-metaplasia-adenocarcinoma sequence in rats. Carcinogenesis. 2005;26:565-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Chen X, Wang S, Wu N, Sood S, Wang P, Jin Z, Beer DG, Giordano TJ, Lin Y, Shih WC. Overexpression of 5-lipoxygenase in rat and human esophageal adenocarcinoma and inhibitory effects of zileuton and celecoxib on carcinogenesis. Clin Cancer Res. 2004;10:6703-6709. [PubMed] |
| 23. | Triadafilopoulos G, Kaur B, Sood S, Traxler B, Levine D, Weston A. The effects of esomeprazole combined with aspirin or rofecoxib on prostaglandin E2 production in patients with Barrett's oesophagus. Aliment Pharmacol Ther. 2006;23:997-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Orido T, Fujino H, Hasegawa Y, Toyomura K, Kawashima T, Murayama T. Indomethacin decreases arachidonic acid uptake in HCA-7 human colon cancer cells. J Pharmacol Sci. 2008;108:389-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Kim JH, Chang JH, Rhee KH, Yoon JH, Kwon SH, Song K, Lee KW, Cho CI, Jeon JH, Kim KS. Cyclooxygenase inhibitors induce apoptosis in sinonasal cancer cells by increased expression of nonsteroidal anti-inflammatory drug-activated gene. Int J Cancer. 2008;122:1765-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 1358] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 27. | Castro P, Nasser H, Abrahão A, Dos Reis LC, Riça I, Valença SS, Rezende DC, Quintas LE, Cavalcante MC, Porto LC. Aspirin and indomethacin reduce lung inflammation of mice exposed to cigarette smoke. Biochem Pharmacol. 2009;77:1029-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Hanif R, Pittas A, Feng Y, Koutsos MI, Qiao L, Staiano-Coico L, Shiff SI, Rigas B. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol. 1996;52:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 461] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 29. | Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001;15:2057-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 561] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
Peer reviewers: Jose Liberato Ferreira Caboclo, Professor, Department of Surgery, Medical School of Sao Jose do Rio Preto, Pizza Torre 2 Apt 91, Av Bady Bassit, SJ do Rio Preto 15025-900, Brazil; Andrew Ukleja, MD, Assistant Professor, Department of Gastroenterology, Cleveland Clinic Florida, 2950 Cleveland Clinic Blvd., Weston, FL 33331, United States
S- Editor Gou SX L- Editor Logan S E- Editor Zhang DN