Published online Sep 14, 2012. doi: 10.3748/wjg.v18.i34.4781
Revised: April 17, 2012
Accepted: April 22, 2012
Published online: September 14, 2012
AIM: To investigate the effect of hyperthermia on hypoxia-induced epithelial-mesenchymal transition (EMT) in HepG2 hepatocellular carcinoma (HCC) cells, and its mechanism.
METHODS: Cells were treated with hyperthermia at 43 °C for 0.5 h, followed by incubation under hypoxic or normoxic conditions for 72 h. Cell morphology was observed. Expressions of E-cadherin and vimentin were determined by immunofluorescence assay or Western blot. The protein and mRNA expressions of Snail were also determined by Western blot and reverse transcription-polymerase chain reaction. Cell migratory capacity was evaluated.
RESULTS: Hypoxia induced EMT in HepG2 cells, which was evidenced by morphological, molecular and functional changes, including the formation of a spindle shape and the loss of cell contact. The expression of E-cadherin was decreased but the expression of vimentin was increased; also, the migratory capability was increased by 2.2 ± 0.20-fold as compared with normoxia. However, those effects were inhibited by hyperthermia pretreatment. Furthermore, protein synthesis and mRNA expression of Snail in the cells were enhanced by hypoxia as compared with normoxia, and also significantly inhibited by hyperthermia pretreatment.
CONCLUSION: Hyperthermia may inhibit hypoxia-induced EMT in HepG2 HCC cells, and the mechanism may involve inhibition of induced expression of Snail.
- Citation: Yuan GJ, Li QW, Shan SL, Wang WM, Jiang S, Xu XM. Hyperthermia inhibits hypoxia-induced epithelial-mesenchymal transition in HepG2 hepatocellular carcinoma cells. World J Gastroenterol 2012; 18(34): 4781-4786
- URL: https://www.wjgnet.com/1007-9327/full/v18/i34/4781.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i34.4781
Hepatocellular carcinoma (HCC) is a major health problem worldwide, and ranks third in the leading causes of cancer mortality[1]. There are approximately 560 000 new cases diagnosed each year, and around 550 000 deaths occur mostly in developing countries[2]. However, the choices for its treatment, especially for advanced HCC, is limited, and the prognosis is poor.
Hyperthermia is a physical treatment with fewer side effects than chemotherapy and radiotherapy. Numerous phase II/III studies have shown that hyperthermia is feasible and effective especially if combined with chemotherapy or radiotherapy in a variety of solid tumors (e.g., melanoma, breast cancer, HCC)[3-5]. It is reported that local hyperthermia can inhibit metastasis in an animal model[6]; however, the exact mechanisms remain unclear. Epithelial-mesenchymal transition (EMT) is a key step during cancer invasion and metastasis[7], and has been associated with cancer stem cells and resistance to chemotherapy and radiotherapy[8-10]. In the present study, we investigated the effect of hyperthermia on hypoxia-induced EMT in HepG2 HCC cells, and its underlying mechanism.
The human HCC cell line HepG2 (Wuhan University Cell Center) was cultured in RPMI1640 medium supplemented with 10% fetal bovine serum, 100 U/mL streptomycin, and 100 U/mL penicillin in a humidified 5% CO2 atmosphere at 37 °C.
Water-bath warming was performed and the temperature was controlled at 43 °C. The cell culture bottles were packaged and put into the water at 43 °C for 0.5 h. Then the culture bottles were returned to the cell-incubator immediately and incubated at 37 °C for 24 h. Thereafter, the cells were incubated under hypoxic (1% O2, 5% CO2, 37 °C) or normoxic (21% O2, 5% CO2, 37 °C) conditions for 72 h.
After hyperthermia, cells were plated on sterile coverslips. After 24 h, the cells were treated with hypoxia as mentioned above. Then, the cells were fixed in 4% formaldehyde, permeabilized with 0.2% Triton X-100, and blocked with 10% goat serum for 60 min at room temperature. Thereafter, cells were incubated with antibodies specific for goat E-cadherin (SC-31020, diluted 1:50) at 4 °C overnight. After washes with phosphate-buffered saline (PBS) 3 times, the cells were incubated with Alexa Fluor® 488 mouse anti-goat IgG (H+L) (A11078, diluted 1:200) for 60 min at room temperature. The expression of E-cadherin was detected under the fluorescence microscope.
Protein lysates were prepared using total protein extraction kit (ProMab, SJ-200501), and stored at -20 °C until assay. The protein concentrations were determined with the Bradford method. The equivalent aliquots of protein were separated by 10% sodium dodecylsulfate polyacrylamide gel electrophoresis, then transferred onto a nitrocellulose filter. The filters were blocked with 5% nonfat dry milk in PBS for 2 h at 37 °C. After washing with PBST (PBS with Tween 20), they were incubated at 4 °C overnight with goat E-cadherin (SANTA, SC-31020, diluted 1:400), mouse vimentin (ProMab, 20301, diluted 1:1000), mouse Snail (CST, 3895, diluted 1:500) or mouse beta-actin (ProMab, 20270, diluted 1:1000) antibodies. Following 4-time PBST washing, the filters were incubated with secondary antibody of horseradish peroxidase-conjugated rabbit anti-goat IgG (diluted 1:4000, for E-cadherin) or goat anti-mouse IgG (diluted 1:5000, for vimentin, Snail and β-actin) for 1 h at room temperature. The immunoreactive proteins were detected using an enhanced chemiluminescent detection system.
Total RNA was isolated using TRIzol reagent (Invitrogen) as suggested by the supplier. One micrograms of total RNA were reverse-transcribed. The sequences for forward and reverse primers were 5’-TCAACTGCAAATACTGCAAC-3’ and 5’-CTTGACATCTGAGTGGGTCT-3’ (Snail), and 5’-ACCACAGTCCATGCCATCAC-3’ and 5’-TCCACCACCCTGTTGCTGTA-3’ (glyceraldehyde-3-phosphate dehydrogenase, GAPDH). The sizes of their amplified fragments were 242 and 450 bps, respectively. Amplification was performed with 40 cycles with initial incubation at 95 °C for 5 min and final extension at 72 °C for 5 min. Each cycle consisted of denaturation for 30 s at 94 °C, annealing for 30 s at 54 °C and extension for 30 s at 72 °C. Following PCR, 10 μL samples of amplified products were resolved by electrophoresis in 1% agarose gel, then stained with ethidium bromide. The levels of Snail expression were semiquantitatively evaluated using a Kodak ultraviolet imaging system and an image analysis system (Vilber Lourmat, France), and normalized to GAPDH.
Cell migration was assessed using 6.5-mm Costar Transwell chambers with 8 μm pores (Corning). Cells were trypsinized and resuspended in serum-free medium containing 0.5% bovine serum albumin. Cells (5 × 104) were plated in the upper chamber in the above serum-free medium, and 1 mL serum-containing standard medium was placed in the lower chamber as a chemoattractant. The system was incubated in 1.0% or 21% O2 for 24 h. After incubation, the nonmigrating cells were removed from the upper surface of the Transwell membrane by a cotton swab, and the membrane was fixed with 95% ethanol for 15-20 min before staining with hematoxylin and eosin. Cells that migrated through the membrane to the lower surface were counted under light microscopy.
Data were pooled from at least three independent experiments, and presented as mean ± SD unless otherwise indicated. Differences between groups were analyzed using one-way analysis of variance. All the statistical analyses were performed with SPSS13.0. P < 0.05 was considered statistically significant.
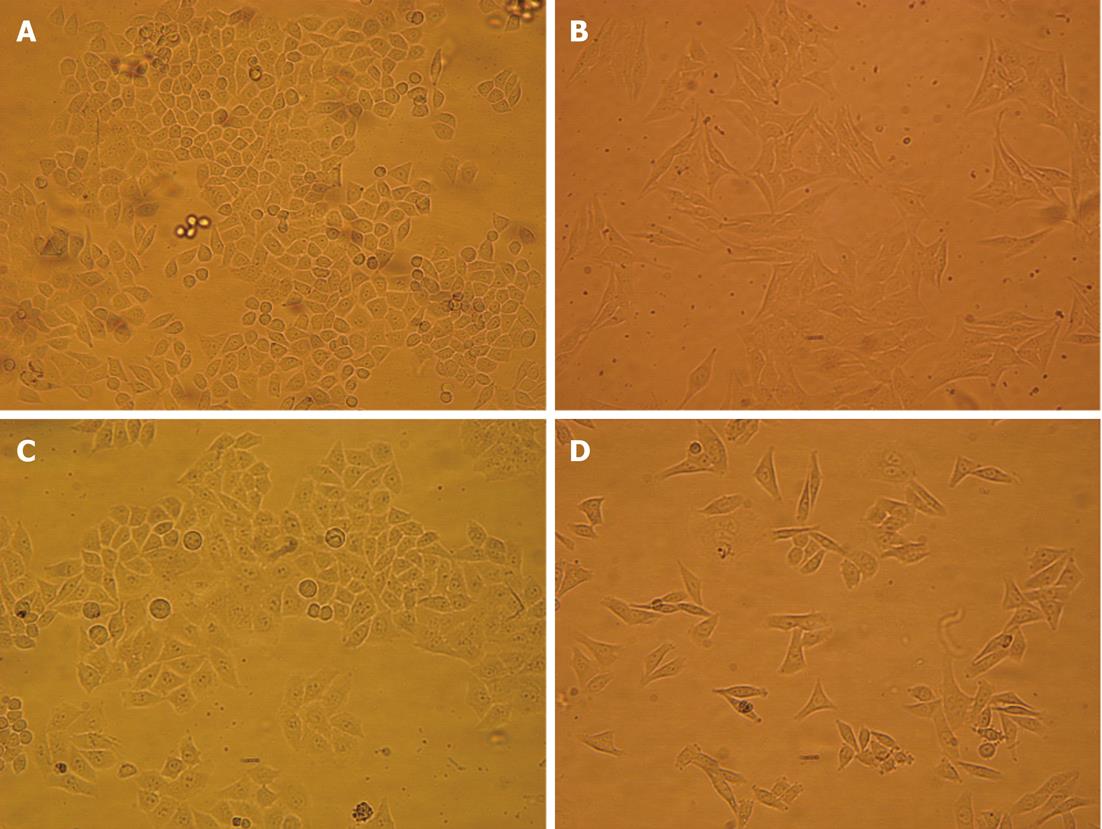
Folding and corrugation of the cell membrane, and a number of vacuoles in the cytoplasm were observed after hyperthermia at 43 °C for 0.5 h. The cells cultured in 1.0% O2 for 72 h showed a spindle shape and lost cell contact. However, these features were partially inhibited by pretreatment with hyperthermia (Figure 1).
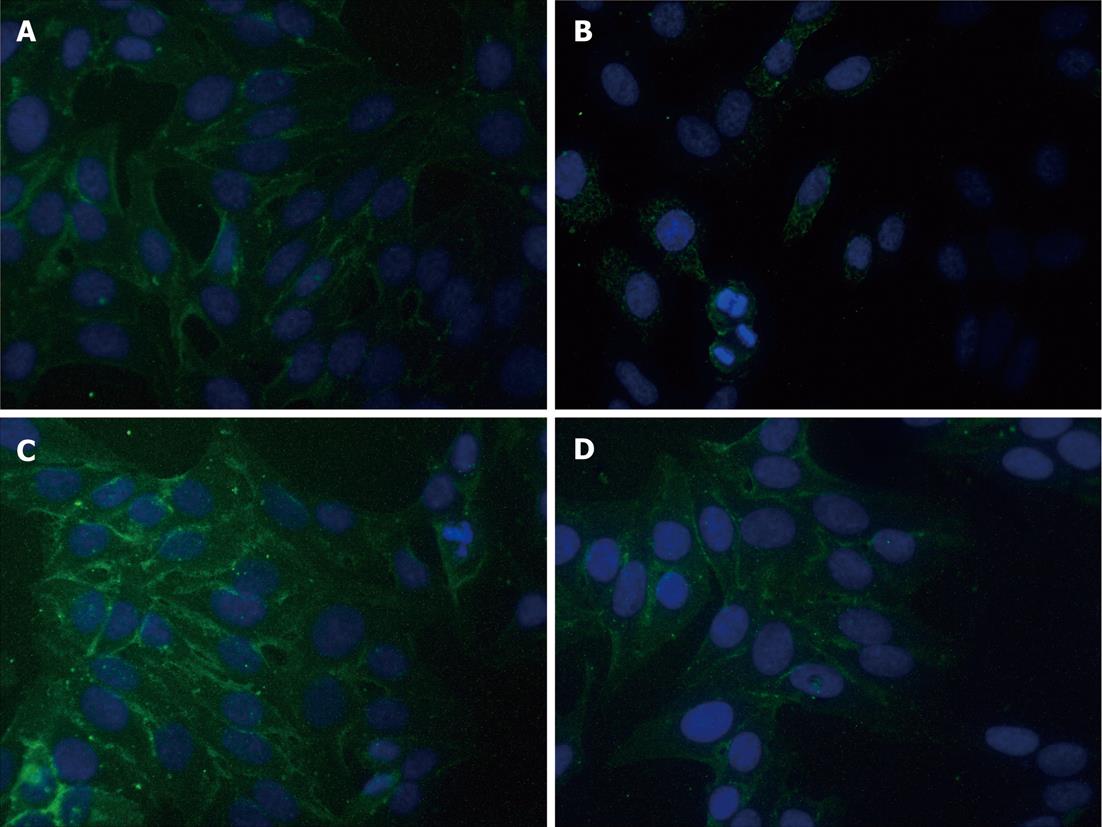
Expressions of E-cadherin were determined by immunofluorescence assay and Western blot. As shown in Figure 2, E-cadherin immunofluorescence staining intensity was significantly decreased in cells after incubation in 1.0% O2 for 72 h as compared with cells cultured in normoxic conditions. However, hyperthermia pretreatment significantly blunted the above effects caused by hypoxia. As compared with untreated cells, hyperthermia alone moderately increased the immunofluorescence intensity of E-cadherin staining.
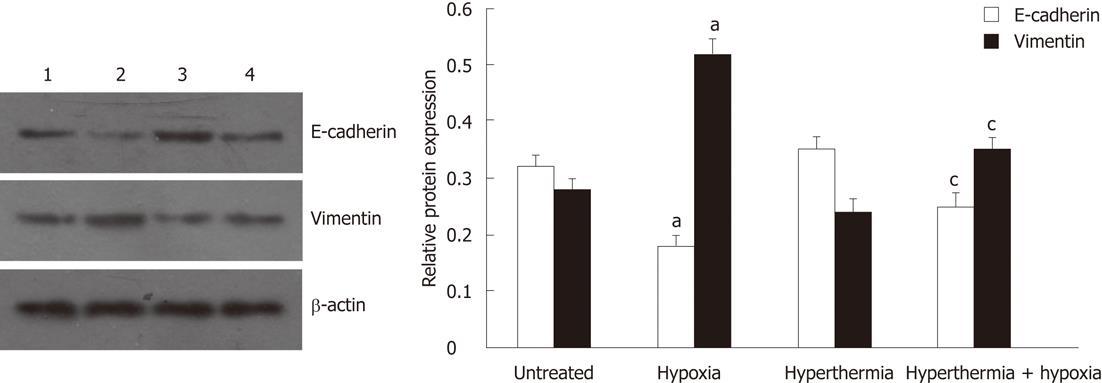
Consistent with the results obtained in immunofluorescence assays, Western blot analysis also showed that, under hypoxia, the expression of E-cadherin was significantly reduced, but the expression of vimentin was substantially increased when compared with normoxia. These effects were blunted after hyperthermia pretreatment (Figure 3). Hyperthermia alone also moderately increased the expression of E-cadherin but decreased the expression of vimentin compared with untreated cells.
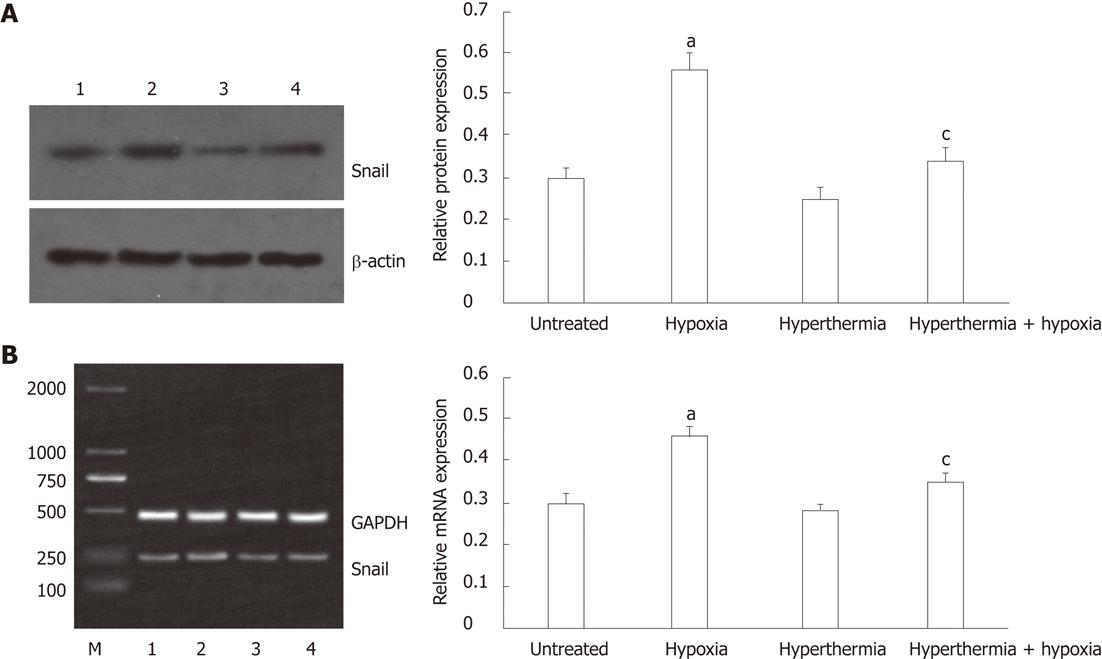
The protein and mRNA expressions of Snail were determined by Western blot and reverse transcription-polymerase chain reaction analysis, respectively. Similar to the expression of vimentin, the protein expression of Snail was significantly increased under hypoxia as compared with normoxia, and hyperthermia pretreatment inhibited the effect induced by hypoxia (Figure 4A). Hyperthermia alone moderately decreased the protein expression of Snail compared with untreated cells.
Consistent with protein expression, Snail mRNA expression was induced under hypoxia, but its induction was inhibited by hyperthermia pretreatment (Figure 4B). Hyperthermia alone also moderately decreased the mRNA expression of Snail compared with untreated cells.
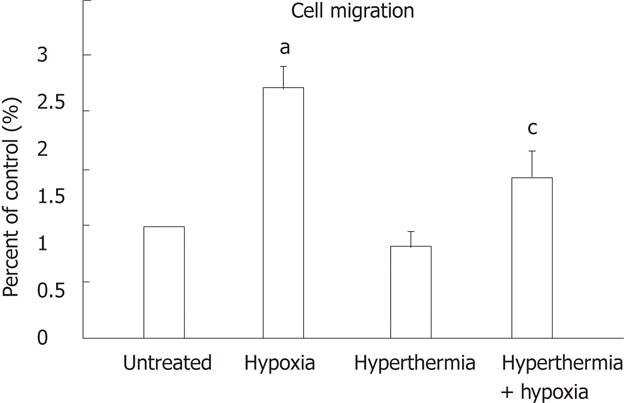
Transwell chamber assays were performed to investigate the migratory capability of HepG2 cells treated with hyperthermia and hypoxia. Under hypoxia, cell migration was increased by 2.2 ± 0.20-fold as compared with normoxia (P < 0.01), which was attenuated to 1.4 ± 0.25-fold by hyperthermia pretreatment (Figure 5). Hyperthermia alone moderately decreased the cell migration compared with untreated cells.
Hypoxia is a common hallmark of solid cancers, including liver cancer, and is associated with treatment failure and poor prognosis[11,12]. Previous studies have shown that it can induce both chemo- and radio-resistance, and also promotes cell migration, invasion and metastasis[11-13]. EMT, originally identified as a process vital for morphogenesis during embryonic development, is now recognized as a key step during cancer invasion and metastasis[7], and has been associated with cancer stem cells and resistance to chemotherapy and radiotherapy[8-10]. EMT is characterized by the loss of epithelial cell markers such as adherens protein E-cadherin, the acquisition of mesenchymal markers such as vimentin and fibronectin, and an increase in cell motility[9]. Several studies have shown that hypoxic conditions can induce EMT in cancer cells, the mechanisms of which may involve reactive oxygen species generated during hypoxia, hypoxia-inducible factor-1α and phosphoinositide-3-kinase/Akt signaling[14-16]. Consistent with other studies, the present study showed that hypoxia induced morphological (spindle shape and loss of cell contact), molecular (decreased E-cadherin but increased vimentin expressions) and functional (increased cell migration) changes in HepG2 HCC cells, all of which are in line with EMT.
In recent years, hyperthermia has emerged as an effective approach for cancers, with fewer side effects than chemotherapy and radiotherapy. Clinical studies have shown that hyperthermia, usually classified as local, regional and whole body, is a tolerable and clinical practical adjunctive therapy for the patients with advanced malignant tumors[3-5]. Previous reports have demonstrated that local hyperthermia can inhibit tumor metastasis in animal model[6]. Furthermore, the expressions of some genes associated with metastasis such as vascular endothelial growth factor, membrane type 1-matrix metalloproteinase, were shown to be suppressed by heating[17-19]. In the present study, we found that hyperthermia can inhibit hypoxia-induced EMT, as hyperthermia before hypoxia exposure significantly blunted the inhibition of E-cadherin, the induction of vimentin expressions, and the increase of cell migration triggered by hypoxia in HepG2 HCC cells. These findings suggested that hyperthermia may alter the metastatic potential of cancer cells and inhibit tumor metastasis.
A number of transcription factors have been found to play an important role in EMT, including zinc finger homologs (e.g., Snail), basic helix-loop-helix transcription factors (e.g., Twist) and ZEB family members (ZEB1, ZEB2/SIP1)[9]. Snail has been found to be expressed in numerous human tumors, including gastric and hepatic carcinoma[20-22], and has been described as a direct repressor of E-cadherin both in vitro and in vivo through binding to E-box elements in the E-cadherin promoter, repressing its expression and producing changes in cell phenotype consistent with EMT[23,24]. Our experiments showed that hypoxia induced mRNA and protein expressions of Snail in HepG2 HCC cells, which may be involved in the mechanism of hypoxia-induced EMT. However, the induction of Snail expressions by hypoxia was inhibited by hyperthermia pretreatment. Therefore, the mechanism by which hyperthermia inhibits hypoxia-induced EMT may involve the reduction in Snail expression.
Taken together, the present study demonstrated that hyperthermia inhibits hypoxia-induced EMT in HepG2 HCC cells, and the mechanism may be associated with the reduction of mRNA and protein expressions of Snail. This study provides further evidence that hyperthermia can inhibit tumor metastasis.
Epithelial-mesenchymal transition (EMT) has been recognized as a key step during cancer invasion and metastasis, and may be induced by environmental stresses such as hypoxia, reactive oxygen species, and growth factors such as transforming growth factor β1. Hypoxia is a common phenomenon in solid tumors, and hypoxic conditions are known to induce EMT in cancer cells. Hyperthermia is a potent approach for cancers with fewer side effects compared with chemotherapy and radiotherapy. Recent reports have shown that local hyperthermia can inhibit tumor metastasis in an animal model, and the mechanisms are associated with vascular endothelial growth factor, membrane type 1-matrix metalloproteinase. However, the effect of hyperthermia on EMT of cancer cells is unknown.
EMT plays an essential role in tumor progression and metastasis, and has been associated with cancer stem cells and resistance to chemotherapy and radiotherapy. To reverse EMT or to target EMT related pathways is a promising novel approach to overcome the chemoresistance and radioresistance for cancer treatment, and to help prevent tumor metastasis.
Hyperthermia can inhibit EMT induction in cancer cells, suggesting that hyperthermia may alter the properties of metastatic potential of cancer cells and inhibit tumor metastasis. Furthermore, the mechanism of inhibition is associated with inhibition of expression of the transcription factor Snail.
The results of the study provide a novel theoretical basis for hyperthermia in the treatment of malignant tumors, especially combined with chemotherapy or radiotherapy.
EMT is a physiological and pathological process in which epithelial cells acquire the motile and invasive characteristics of mesenchymal cells. EMT is characterized by the loss of epithelial cell markers such as E-cadherin, the acquisition of mesenchymal markers such as vimentin and fibronectin, and an increase of cell motility. EMT has been recognized as a key step during embryonic morphogenesis, heart development, chronic degenerative fibrosis, and cancer metastasis.
The paper described the effect of hyperthermia on EMT in HepG2 hepatocellular carcinoma cells, and its associated mechanism. The authors concluded that hyperthermia may inhibit EMT induction in cancer cells and the mechanism is associated with inhibition of expression of the transcription factor Snail. The results are interesting, but the mechanism needs to be further studied, especially employing Snail knockdown technique.
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13561] [Article Influence: 645.8] [Reference Citation Analysis (3)] |
| 2. | McGlynn KA, London WT. Epidemiology and natural history of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2005;19:3-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 250] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 3. | Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia. 2001;17:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 340] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1439] [Cited by in RCA: 1176] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 5. | Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol). 2007;19:418-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 315] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 6. | Nagashima K, Takagi R, Hoshina H. Effect of local hyperthermia on metastases in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2002;31:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796:75-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 354] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 8. | Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704-715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6972] [Cited by in RCA: 6910] [Article Influence: 383.9] [Reference Citation Analysis (0)] |
| 9. | Sabbah M, Emami S, Redeuilh G, Julien S, Prévost G, Zimber A, Ouelaa R, Bracke M, De Wever O, Gespach C. Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist Updat. 2008;11:123-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 254] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 10. | Theys J, Jutten B, Habets R, Paesmans K, Groot AJ, Lambin P, Wouters BG, Lammering G, Vooijs M. E-Cadherin loss associated with EMT promotes radioresistance in human tumor cells. Radiother Oncol. 2011;99:392-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 11. | Weinmann M, Belka C, Plasswilm L. Tumour hypoxia: impact on biology, prognosis and treatment of solid malignant tumours. Onkologie. 2004;27:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. 2003;29:297-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 416] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 13. | Rofstad EK, Gaustad JV, Egeland TA, Mathiesen B, Galappathi K. Tumors exposed to acute cyclic hypoxic stress show enhanced angiogenesis, perfusion and metastatic dissemination. Int J Cancer. 2010;127:1535-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Cannito S, Novo E, Compagnone A, Valfrè di Bonzo L, Busletta C, Zamara E, Paternostro C, Povero D, Bandino A, Bozzo F. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis. 2008;29:2267-2278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 15. | Krishnamachary B, Zagzag D, Nagasawa H, Rainey K, Okuyama H, Baek JH, Semenza GL. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;66:2725-2731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 339] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 16. | Yan W, Fu Y, Tian D, Liao J, Liu M, Wang B, Xia L, Zhu Q, Luo M. PI3 kinase/Akt signaling mediates epithelial-mesenchymal transition in hypoxic hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2009;382:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Sawaji Y, Sato T, Takeuchi A, Hirata M, Ito A. Anti-angiogenic action of hyperthermia by suppressing gene expression and production of tumour-derived vascular endothelial growth factor in vivo and in vitro. Br J Cancer. 2002;86:1597-1603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Sawaji Y, Sato T, Seiki M, Ito A. Heat shock-mediated transient increase in intracellular 3',5'-cyclic AMP results in tumor specific suppression of membrane type 1-matrix metalloproteinase production and progelatinase A activation. Clin Exp Metastasis. 2000;18:131-138. [PubMed] |
| 19. | Liang X, Zhou H, Liu X, He Y, Tang Y, Zhu G, Zheng M, Yang J. Effect of local hyperthermia on lymphangiogenic factors VEGF-C and -D in a nude mouse xenograft model of tongue squamous cell carcinoma. Oral Oncol. 2010;46:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Natalwala A, Spychal R, Tselepis C. Epithelial-mesenchymal transition mediated tumourigenesis in the gastrointestinal tract. World J Gastroenterol. 2008;14:3792-3797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Castro Alves C, Rosivatz E, Schott C, Hollweck R, Becker I, Sarbia M, Carneiro F, Becker KF. Slug is overexpressed in gastric carcinomas and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin. J Pathol. 2007;211:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Woo HY, Min AL, Choi JY, Bae SH, Yoon SK, Jung CK. Clinicopathologic significance of the expression of Snail in hepatocellular carcinoma. Korean J Hepatol. 2011;17:12-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1272] [Cited by in RCA: 1340] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 24. | Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol. 2004;48:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 436] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
Peer reviewer: Dr. Xian-Ming Chen, Department of Medical Microbiology and Immunology, Creighton University Medical Center, 2500 California Plaza, Omaha, NE 68178, United States
S- Editor Gou SX L- Editor Cant MR E- Editor Xiong L
.