Published online Sep 14, 2012. doi: 10.3748/wjg.v18.i34.4729
Revised: May 31, 2012
Accepted: June 15, 2012
Published online: September 14, 2012
AIM: To investigate the effects of titanium dioxide (TiO2) nanoparticles (NPTiO2) and microparticles (MPTiO2) on the inflammatory response in the small intestine of mice.
METHODS: Bl 57/6 male mice received distilled water suspensions containing TiO2 (100 mg/kg body weight) as NPTiO2 (66 nm), or MPTiO2 (260 nm) by gavage for 10 d, once a day; the control group received only distilled water. At the end of the treatment the duodenum, jejunum and ileum were extracted for assessment of cytokines, inflammatory cells and titanium content. The cytokines interleukin (IL)-1b, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, IL-23, tumor necrosis factor-α (TNF-α), intracellular interferon-γ (IFN-γ) and transforming growth factor-β (TGF-β) were evaluated by enzyme-linked immunosorbent assay in segments of jejunum and ileum (mucosa and underlying muscular tissue). CD4+ and CD8+ T cells, natural killer cells, and dendritic cells were evaluated in duodenum, jejunum and ileum samples fixed in 10% formalin by immunohistochemistry. The titanium content was determined by inductively coupled plasma atomic emission spectrometry.
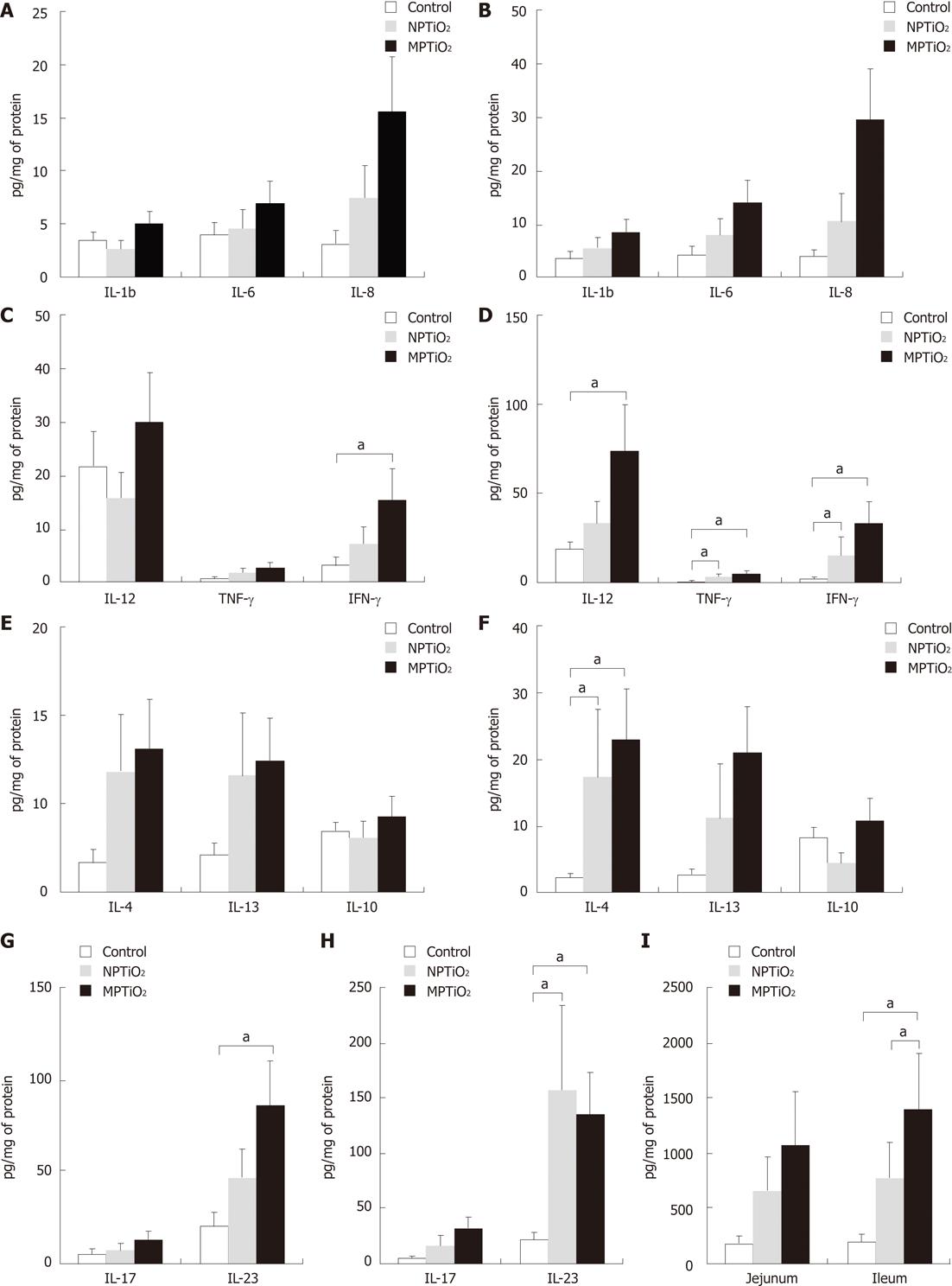
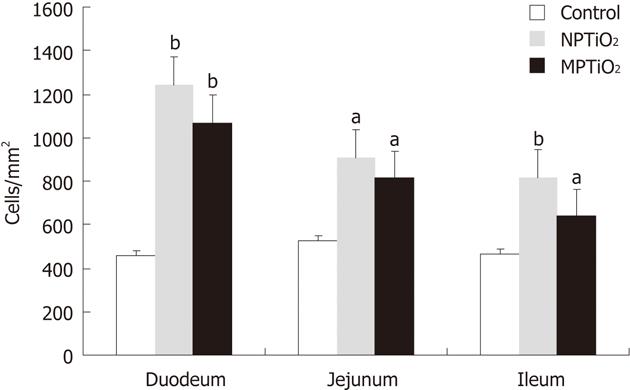
RESULTS: We found increased levels of T CD4+ cells (cells/mm2) in duodenum: NP 1240 ± 139.4, MP 1070 ± 154.7 vs 458 ± 50.39 (P < 0.01); jejunum: NP 908.4 ± 130.3, MP 813.8 ± 103.8 vs 526.6 ± 61.43 (P < 0.05); and ileum: NP 818.60 ± 123.0, MP 640.1 ± 32.75 vs 466.9 ± 22.4 (P < 0.05). In comparison to the control group, the groups receiving TiO2 showed a statistically significant increase in the levels of the inflammatory cytokines IL-12, IL-4, IL-23, TNF-α, IFN-γ and TGF-β. The cytokine production was more pronounced in the ileum (mean ± SE): IL-12: NP 33.98 ± 11.76, MP 74.11 ± 25.65 vs 19.06 ± 3.92 (P < 0.05); IL-4: NP 17.36 ± 9.96, MP 22.94 ± 7.47 vs 2.19 ± 0.65 (P < 0.05); IL-23: NP 157.20 ± 75.80, MP 134.50 ± 38.31 vs 22.34 ± 5.81 (P < 0.05); TNFα: NP 3.71 ± 1.33, MP 5.44 ± 1.67 vs 0.99 ± 019 (P < 0.05); IFNγ: NP 15.85 ± 9.99, MP 34.08 ± 11.44 vs 2.81 ± 0.69 (P < 0.05); and TGF-α: NP 780.70 ± 318.50, MP 1409.00 ± 502.20 vs 205.50 ± 63.93 (P < 0.05).
CONCLUSION: Our findings indicate that TiO2 particles induce a Th1-mediated inflammatory response in the small bowel in mice.
- Citation: Nogueira CM, Azevedo WM, Dagli MLZ, Toma SH, Leite AZA, Lordello ML, Nishitokukado I, Ortiz-Agostinho CL, Duarte MIS, Ferreira MA, Sipahi AM. Titanium dioxide induced inflammation in the small intestine. World J Gastroenterol 2012; 18(34): 4729-4735
- URL: https://www.wjgnet.com/1007-9327/full/v18/i34/4729.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i34.4729
We are exposed daily through inhalation, ingestion or contact to many environmental and engineered particles. The gastrointestinal tract is continuously exposed to particles in the diet, such as titanium dioxide (TiO2), which is an insoluble white powder that is used as an anti-caking and whitening agent in many commercial products, including paint, cosmetics, plastics, paper, pharmaceuticals, and food colorants[1]. The ingestion of exogenous particles is substantial, as demonstrated by Lomer et al[2], who reported that about 40 mg of exogenous particles are ingested per person per day in the United Kingdom. Besides the amount of exogenous particles that are ingested, nanoparticulates can be inhaled and trapped on the mucus of the respiratory tract and then swallowed, thus reaching the gastrointestinal tract[3]. Clearance studies in animals involving radiolabeled nanoparticles (NP) showed that 30%-50% of inhaled NP are translocated to the gastrointestinal tract[4].
TiO2 can be found as microparticles (MP) (diameter of 0.1 to 1.0 μm) and NP (diameter less than 100 nm). The increasing utilization of nanomaterials in industrial as well as consumer products has increased the possibility of human exposure but their influence on human health remains uncertain. Although studies reporting TiO2 toxicity are still limited, some suggest that smaller particles produce a greater inflammatory response in comparison with the larger fine-sized particles of similar chemistry at the same mass concentrations[5-7].
Many studies have revealed that exposure to TiO2 can cause adverse effects such as the generation of reactive oxygen species[8-10], inflammatory responses[5,6,11-13], tumors[14], cytotoxicity[15] and apoptosis[16]. In vivo studies showed that NP can be accumulated in many organs such as the liver, kidney, spleen, lung, heart and brain[17,18], thus generating a number of adverse effects. Previous investigations have found that TiO2 accumulates in the intestine in rats[19] and fish[20] and migrates to other organs. Accumulation of TiO2 inside the intestinal cells, especially in lymphoid-rich areas (Peyer’s patch), might lead to damaging outcomes such as inflammation and could be involved in the pathogenesis of inflammatory bowel disease[21,22]. However, little is known about the influence of either micro- or NP on the gut, which is potentially exposed to particles in the diet, such as TiO2. To date, most of the studies regarding the adverse effects of TiO2 particles on human health have involved the pulmonary tract. No available in vivo work has evaluated the impacts of TiO2 particles in terms of their inflammatory potential within the gastrointestinal tract.
Therefore, the present study was designed to investigate the effects of TiO2 as MP and as NP on the inflammatory response in the small intestine of mice. We aimed to evaluate cytokine production and inflammatory cell proliferation in the small intestine of mice after oral exposure to TiO2.
Uncoated anatase TiO2 microparticles (MPTiO2) (260 nm) that are commercially available for use in food, pharmaceuticals, and cosmetics were obtained from Evonik Degussa (Kronos® 1171). Uncoated TiO2 nanoparticles (NPTiO2) (mean diameter of 66 nm), consisting mostly of anatase, were synthesized by Professor de Azevedo WM from the Department of Fundamental Chemistry of the Federal University of Pernambuco (Recife, Brazil) at pH = 2.0, followed by centrifugation. Particle size was determined by dynamic light scattering Nanotrac® (Microtrac Inc., United States) by Professor Toma SH from the Laboratory of Supramolecular Chemistry and Nanotechnology of the Chemistry Institute of the University of São Paulo (São Paulo, Brazil). Particle phase was characterized using an X-ray diffractometer Rigaku Miniflex® (Rigaku Corporation, Japan) under monochromatic radiation, Cu Kα (1.541 Å, 30 kV, 15 mA, 0.02°, 2° to 61° range), also by Professor Toma SH.
Bl 57/6 male mice (20 to 25 g) were obtained from the Center of Bioterism of the School of Medicine, University of São Paulo (São Paulo, Brazil). Animals were housed in cages in a ventilated room in a 12-h light/dark cycle. Food and water were available ad libitum. They were acclimated to this environment for 1 wk before treatment. All animal experimental procedures were in compliance with the School of Medicine, University of São Paulo Ethics Committee. Mice were randomly divided into three groups of 12 animals, and received either distilled water suspensions containing TiO2 (100 mg/kg body weight) as MP, or as NP, or distilled water as a control. The suspension was given by gavage for 10 d, once a day. TiO2 particles were suspended in 500 μL of distilled water. The suspension was mixed and sonicated immediately before being administered to animals to minimize particle aggregation. At the end of the treatment the animals were weighed and killed in a CO2 chamber, and had their duodenum, jejunum and ileum extracted for assessment of cytokines, inflammatory cells and titanium content.
Segments of jejunum and ileum - mucosa and underlying muscular tissue - were extracted from animals, stored at -80 °C and subsequently homogenized with Tris-buffer (10 mmol)-ethylenediamine tetraacetic acid (1 mmol)-Triton (1%) containing protease, aprotinin, chymostatin and leupeptin inhibitors (1 μg/mL of solution) and phenylmethylsulfonyl fluoride (1 μL/mL of solution). After homogenization, the sample was centrifuged at 14 000 g for 10 min at 4 °C and the supernatant was stored at -80 °C until the cytokines were analyzed using an enzyme-linked immunosorbent assay (ELISA). Interleukin-1β (IL-1β) IL-4, IL-6, IL-8 (Keratinocyte Chemoattractant), IL-10, IL-12, IL-13, IL-17, IL-23, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ) and transforming growth factor-β (TGF-β) were evaluated using ELISA kits (IL-1β, IL-4, IL-6, IL-12, IL-13, IL-17, IL-23, TNF-α, IFN-γ, and TGF-β from eBioscience; and IL-10 and IL-8 from R and D systems) according to the manufacturer’s recommendations.
Duodenum, jejunum and ileum samples were fixed in 10% formalin and were embedded in paraffin. Five-mm thick sections were cut for evaluation of CD4+ and CD8+ T cells, natural killer cells (CD57), and dendritic cells (S100+) by immunohistochemistry using specific antibodies: anti-CD4 [Dako M0834 (OPD4 clone)], anti-CD8 [Dako® M7103 (clone C8/144B)], anti-S100+ [Dako® M7103 (clone C8/144B)], and anti-CD57 [Dako® M7103 (clone C8/144B)]. For cell quantification, the observers randomly selected ten areas and counted positive cells under the 40 × objective.
Duodenum, jejunum and ileum samples were fixed in 10% formalin and embedded in paraffin. Five-mm thick sections were cut, placed onto glass slides and then stained with HE. The slides were examined by optical microscopy (E800, Nikon, United States) by Professor Dagli MLZ from the Department of Pathology of the School of Veterinary Medicine, University of São Paulo (São Paulo, Brazil).
The titanium content in intestinal samples from each group was analyzed with the purpose of verifying the efficacy of the treatment, and to make sure that the animals of the control group did not contain titanium (Ti) in their tissues. To determine the presence of Ti in the small intestine of the animals receiving TiO2 particles, the experiment was repeated including two animals in each group. At the end of the treatment, the small intestine was extracted for analysis of the titanium content by inductively coupled plasma atomic emission spectrometry (ICP-AES). The small intestine was removed in its entirety (from the pylorus to the ileocecal valve), preserving the mucosa and muscle tissue, stored at -80 °C and taken to the Basic Analysis Laboratory of the Analytical Center, Chemistry Institute, University of São Paulo (São Paulo, Brazil) where it was homogenized and processed by the local team following their standard protocol.
Data for each parameter evaluated are shown as the mean ± SE. Data were analyzed assuming a gamma distribution (identity link) and using generalized linear models. Pairwise comparisons and Fisher’s least significant difference post-hoc test were applied to evaluate the differences between the groups. P < 0.05 was considered significant. Commercially available software was used for analysis (PASW Statistics Version 18, United States).
The titanium content in the small intestine from each group was determined by ICP-AES. The control group showed no detectable levels of titanium in the intestine. The titanium content (mg/kg of tissue) in the intestine was (animal 1/animal 2): 1.43/11.68 in the NP group, and 0.39/0.22 in the MP group.
In comparison to the control group, MPTiO2 and NPTiO2 generated a statistically significant increase in inflammatory cytokines. The group receiving MPTiO2 showed an enhanced concentration of IFN-γ and IL-23 in the jejunum, and IL-12, TNF-α, IFN-γ, IL-4, IL-23, and TGF-β in the ileum (Figure 1). The group receiving NPTiO2 showed increased TNF-α, IFN-γ, IL-4, IL-23, and TGF-β, but only in the ileum. There was no significant difference between the groups receiving MPTiO2 and NPTiO2, as both showed statistically similar levels of cytokine production. A more important cytokine production was found in the ileum compared to the jejunum.
We found a statistically significant increase in T CD4+ cells in the duodenum, jejunum and ileum of mice treated with MPTiO2 and NPTiO2, compared to the control group. There was no significant difference between the MPTiO2 and NPTiO2 groups. The results are illustrated as the mean and SE in Figure 2. Mice treated with MPTiO2 or NPTiO2 showed no increase in T CD8+, natural killers, or dendritic cells in the small intestine (data not shown).
No major histological changes were found in small intestine samples of experimental animals, except for hypertrophy and hyperplasia of the mucosal epithelium of mice receiving TiO2 particles, which were not seen in the control group. These findings were observed in all three regions of the small intestine (duodenum, jejunum and ileum) of mice treated with NPTiO2, while animals treated with MPTiO2 showed these effects only in the ileum.
To date, there have been few data regarding the inflammatory potential of TiO2 particles in the intestine. Here, we sought to evaluate inflammatory responses induced by TiO2 in the small intestine in mice. We found that TiO2 as micro- and nano-sized particles produced a pro-inflammatory response in the small intestine by generating increased inflammatory cytokine production and T CD4+ cell proliferation. The generation of pro-inflammatory cytokines and T CD4+ cells induced by NPTiO2 was also described by Schanen et al[23] in an in vivo immune construct study.
The main cytokines enhanced in our study were IL-12, IFN-γ, TNF-α, IL-4, IL-23 and TGF-β. IL-12, TNF-α and IFN-γ are Th1-type cytokines, whereas IL-23 and TGF-β are both involved in the Th17 pathway. In murine models, TGF-β together with IL-6 promotes differentiation of naïve T cells to Th17 cells[24]. Here we found no significant increase in IL-6 or IL-17, which is produced by Th17 cells. Taken together, these data suggest that TiO2 particles provoked a pro-inflammatory response mainly through the Th1-mediated pathway in the small bowel in mice. Other authors found a Th-2 mediated immune response in the lungs, induced by nano-sized TiO2 exposure in mice[13,25]. However, inflammatory responses differ depending on the organ, and thus the immune response caused by exposure to TiO2 may diverge between the respiratory tract and the gut.
Cytokine production was more pronounced in the ileum. These findings might be related to differences concerning particle uptake throughout the gut. It is known that the ileum presents the greatest concentration of M cells (Peyer’s patch) in the intestine, which are believed to represent the main pathway of particle uptake across the gastrointestinal tract[26,27]. Li et al[28] observed greater absorption of lipid NP in the ileum and colon of rats when compared to other segments of the intestine, reinforcing the importance of M cells as a pathway of particle uptake. Given that the ileum represents the major site of particle uptake, we would expect to find a more substantial inflammatory response in this area.
We also evaluated histopathological changes in the small intestine of mice after exposure to TiO2. We observed hypertrophy and hyperplasia of the mucosal epithelium in both groups receiving TiO2 particles. These findings were also described by other authors in TiO2-related studies. Alveolar epithelium hypertrophy was observed in the lungs of rats exposed to nano-sized TiO2[29]. Mice, rats and hamsters showed histopathological changes consistent with alveolar epithelial cell hypertrophy and hyperplasia after long-term inhalation of fine TiO2[30].
Taken together our data provide evidence that micro- and nano-sized TiO2 particles induce a pro-inflammatory response in the small intestine in mice, after a short period of oral exposure.
The titanium content of the small intestine samples was determined by ICP-AES at the end of the experiments to guarantee the efficacy of the treatment with TiO2 and to ensure that the control group had no detectable levels of titanium in their tissues. Our results demonstrated that TiO2 particles were absorbed by the small intestine in mice after a short period of oral exposure, as the animals that received TiO2 particles had titanium in their tissues at the end of the experiment. The control group showed no detectable levels of titanium in any sample. We found greater amounts of titanium in the small intestine of the animals receiving NPTiO2 in comparison to those receiving MPTiO2. These findings indicate that smaller particles may be absorbed to a greater extent than larger ones in the gut.
Previous investigations of TiO2 particles found that TiO2 as nano-sized particles is more toxic than similarly composed, but larger sized particles[5-7,11,29,31]. Thus, we aimed to compare the effects of micro- and nano-sized TiO2 on the small intestine. However, we found no statistically significant difference in cytokine secretion or T CD4+ cell proliferation between the groups who received MPTiO2 and NPTiO2. Other authors have already reported the lack of significant differences in the pulmonary effects in rats exposed to TiO2 particles of different sizes[14,32-35].
In conclusion, We demonstrated that over a short period TiO2 as micro- and nano-particles induced a Th1-mediated inflammatory response in the small intestine of mice, especially in the ileum. These findings provide evidence of the inflammatory potential of TiO2 particles in the gastrointestinal tract. Since we are exposed to TiO2 particles on a daily basis, as well as to many other engineered particles, these data should be taken into consideration when evaluating the safety of biomaterials.
Titanium dioxide (TiO2) is a white pigment that is widely found as nano- and micro-sized particles added to commercial products such as food, drugs, cosmetics, etc. Many studies involving the respiratory tract warn of adverse effects resulting from exposure to TiO2. Although the gastrointestinal tract is exposed to TiO2 particles on a daily basis there is little information regarding their adverse effects on the intestine. Adverse effects resulting from exposure to TiO2, such as cytotoxicity, generations of reactive oxygen species, tumors, inflammation, allergic reactions, lung emphysema, among others, have been reported in studies involving the respiratory tract. Although the lung is the most widely studied organ as far as exposure to particles such as TiO2 is concerned, the gastrointestinal tract is considerably more exposed to environmental particles.
The present study shows that nano- and micro-sized particles may lead to an inflammatory process in the bowel. As a result, small particles of TiO2, which are present in the diet, may be involved in the development of inflammatory bowel disease.
Most researches related to TiO2 particles studied their effects on the respiratory tract or were performed in vitro. This study was conducted in vivo to investigate the effects of TiO2 as micro- and nanoparticles on the inflammatory response in the intestine of mice. To do so, mice received high doses of TiO2 by gavage for 10 d and subsequently the small intestine was extracted for assessment of cytokines, infiltration of inflammatory cells and morphological alterations of tissue. Authors found the highest concentration of cytokines [interleukin (IL)-12, IL-4, IL-23, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ) and transforming growth factor-β (TGF-β) and of CD4+ T cell in the intestine of animals that received TiO2, suggesting the pro-inflammatory effect of these particles on the small intestine of mice.
Based on their results, authors can infer that TiO2 elicited a pro-inflammatory response in the small intestine of mice, which seems to be predominantly a T-helper 1-type response and more pronounced in the ileum. These data are in vivo evidence of the inflammatory potential of TiO2 particles on the gastrointestinal tract. Their findings emphasize the need to investigate the adverse effects of TiO2 particles on health since they are exposed to products containing this substance on a daily basis.
Microparticles are particles whose size ranges from 0.1 and 100 μm; Nanoparticles are particles smaller than 100 nm. Nanoscale materials are developed to display some specific features based on their size, shape, surface, etc. TiO2 is a white powder that is used as an anti-caking and whitening agent in many commercial products, including paint, cosmetics, plastics, paper, pharmaceuticals and food colorants
This study reports on the mice intestinal inflammatory responses to TiO2 nano- and micro-particles administration. The presented results show that daily administration of 100 mg/kg of TiO2 nano- as well as micro-particles elicited substantial inflammatory responses in the intestinal tissue and jejunum, as evidenced by the increase in IL-12, TNF-α, IFN-γ, IL-4, IL-23 and TGF-β. These effects were observed with both types of particles. Considering the increasing use of various form of titanium in the food industry and pharmaceuticals and implants, this adds an important evidence to a growing list of potential adverse effects of Ti on human health.
Peer reviewers: Dr. Bronislaw L Slomiany, Professor, Dental School, Research Center, University of Medicine and Dentistry of New Jersey, 110 Bergen Street, PO Box 1709, Room C875, Newark, NJ 07103-2400, United States; Dr. Vibeke Andersen, Department of Medical, Viborg Regional Hospital, Heibergs Alle 2-4, 8800 Viborg, Denmark
S- Editor Gou SX L- Editor A E- Editor Xiong L
.
| 1. | Available from: http://www.cdc.gov/niosh/review/public/TiO2/pdfs/TiO2Draft.pdf. |
| 2. | Lomer MC, Hutchinson C, Volkert S, Greenfield SM, Catterall A, Thompson RP, Powell JJ. Dietary sources of inorganic microparticles and their intake in healthy subjects and patients with Crohn's disease. Br J Nutr. 2004;92:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Wang L, Nagesha DK, Selvarasah S, Dokmeci MR, Carrier RL. Toxicity of CdSe Nanoparticles in Caco-2 Cell Cultures. J Nanobiotechnology. 2008;6:11. [PubMed] |
| 4. | Madl AK, Pinkerton KE. Health effects of inhaled engineered and incidental nanoparticles. Crit Rev Toxicol. 2009;39:629-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Oberdörster G, Ferin J, Gelein R, Soderholm SC, Finkelstein J. Role of the alveolar macrophage in lung injury: studies with ultrafine particles. Environ Health Perspect. 1992;97:193-199. [PubMed] [DOI] [Full Text] |
| 6. | Oberdörster G, Ferin J, Lehnert BE. Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Perspect. 1994;102 Suppl 5:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 321] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Renwick LC, Brown D, Clouter A, Donaldson K. Increased inflammation and altered macrophage chemotactic responses caused by two ultrafine particle types. Occup Environ Med. 2004;61:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 222] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Jin CY, Zhu BS, Wang XF, Lu QH. Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells. Chem Res Toxicol. 2008;21:1871-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 249] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Kim KT, Klaine SJ, Cho J, Kim SH, Kim SD. Oxidative stress responses of Daphnia magna exposed to TiO(2) nanoparticles according to size fraction. Sci Total Environ. 2010;408:2268-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Wang J, Li N, Zheng L, Wang S, Wang Y, Zhao X, Duan Y, Cui Y, Zhou M, Cai J. P38-Nrf-2 signaling pathway of oxidative stress in mice caused by nanoparticulate TiO2. Biol Trace Elem Res. 2011;140:186-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Ferin J, Oberdörster G, Penney DP. Pulmonary retention of ultrafine and fine particles in rats. Am J Respir Cell Mol Biol. 1992;6:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 368] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Bermudez E, Mangum JB, Wong BA, Asgharian B, Hext PM, Warheit DB, Everitt JI. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol Sci. 2004;77:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 382] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 13. | Park EJ, Yoon J, Choi K, Yi J, Park K. Induction of chronic inflammation in mice treated with titanium dioxide nanoparticles by intratracheal instillation. Toxicology. 2009;260:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Elder AC, Finkelstein J, Johnston C, Gelein R, Oberdörster G. Induction of adaptation to inhaled lipopolysaccharide in young and old rats and mice. Inhal Toxicol. 2000;12:225-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Wang JJ, Sanderson BJ, Wang H. Cyto- and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutat Res. 2007;628:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 359] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 16. | Rahman Q, Lohani M, Dopp E, Pemsel H, Jonas L, Weiss DG, Schiffmann D. Evidence that ultrafine titanium dioxide induces micronuclei and apoptosis in Syrian hamster embryo fibroblasts. Environ Health Perspect. 2002;110:797-800. [PubMed] |
| 17. | Borm PJ, Robbins D, Haubold S, Kuhlbusch T, Fissan H, Donaldson K, Schins R, Stone V, Kreyling W, Lademann J. The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol. 2006;3:11. [PubMed] |
| 18. | Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, Jia G, Gao Y, Li B, Sun J. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett. 2007;168:176-185. [PubMed] |
| 19. | Jani PU, McCarthy DE, Florence AT. Titanium dioxide (rutile) particle uptake from the rat GI tract and translocation to systemic organs after oral administration. Int J Pharm. 1994;105:157–168. [RCA] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 110] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Zhang X, Sun H, Zhang Z, Niu Q, Chen Y, Crittenden JC. Enhanced bioaccumulation of cadmium in carp in the presence of titanium dioxide nanoparticles. Chemosphere. 2007;67:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 225] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Lomer MC, Thompson RP, Powell JJ. Fine and ultrafine particles of the diet: influence on the mucosal immune response and association with Crohn's disease. Proc Nutr Soc. 2002;61:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 239] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Evans SM, Ashwood P, Warley A, Berisha F, Thompson RP, Powell JJ. The role of dietary microparticles and calcium in apoptosis and interleukin-1beta release of intestinal macrophages. Gastroenterology. 2002;123:1543-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Schanen BC, Karakoti AS, Seal S, Drake DR, Warren WL, Self WT. Exposure to titanium dioxide nanomaterials provokes inflammation of an in vitro human immune construct. ACS Nano. 2009;3:2523-2532. [PubMed] |
| 24. | Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5541] [Article Influence: 277.1] [Reference Citation Analysis (0)] |
| 25. | Larsen ST, Roursgaard M, Jensen KA, Nielsen GD. Nano titanium dioxide particles promote allergic sensitization and lung inflammation in mice. Basic Clin Pharmacol Toxicol. 2010;106:114-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Kreuter J. Peroral administration of nanoparticles. Adv Drug Deliv Rev. 1991;7:71–86. [RCA] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 114] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Florence AT. The oral absorption of micro- and nanoparticulates: neither exceptional nor unusual. Pharm Res. 1997;14:259-266. [PubMed] |
| 28. | Li H, Zhao X, Ma Y, Zhai G, Li L, Lou H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Control Release. 2009;133:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 444] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 29. | Kobayashi N, Naya M, Endoh S, Maru J, Yamamoto K, Nakanishi J. Comparative pulmonary toxicity study of nano-TiO(2) particles of different sizes and agglomerations in rats: different short- and long-term post-instillation results. Toxicology. 2009;264:110-118. [PubMed] |
| 30. | Everitt JI, Mangum JB, Bermudez E, Wong BA, Asgharian B, Reverdy EE, Warheit DB. Comparison of selected pulmonary responses of rats, mice, and syrian golden hamsters to inhaled pigmentary titanium dioxide. Inhal Toxicol. 2000;12 Suppl 3:275–282. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Ferin J, Oberdorster G, Penney DP, Soderholm C, Gelein R, Piper HC. Increased pulmonary toxicity of ultrafine particles? I. Particle clearance, translocation, morphology. J Aerosol Sci. 1990;21:381-384. [DOI] [Full Text] |
| 32. | Höhr D, Steinfartz Y, Schins RP, Knaapen AM, Martra G, Fubini B, Borm PJ. The surface area rather than the surface coating determines the acute inflammatory response after instillation of fine and ultrafine TiO2 in the rat. Int J Hyg Environ Health. 2002;205:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Rehn B, Seiler F, Rehn S, Bruch J, Maier M. Investigations on the inflammatory and genotoxic lung effects of two types of titanium dioxide: untreated and surface treated. Toxicol Appl Pharmacol. 2003;189:84-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Sayes CM, Wahi R, Kurian PA, Liu Y, West JL, Ausman KD, Warheit DB, Colvin VL. Correlating nanoscale titania structure with toxicity: a cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol Sci. 2006;92:174-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 512] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 35. | Warheit DB, Webb TR, Sayes CM, Colvin VL, Reed KL. Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: toxicity is not dependent upon particle size and surface area. Toxicol Sci. 2006;91:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 300] [Article Influence: 15.0] [Reference Citation Analysis (0)] |