Published online Sep 14, 2012. doi: 10.3748/wjg.v18.i34.4659
Revised: July 6, 2012
Accepted: July 18, 2012
Published online: September 14, 2012
Endoscopic ultrasonography (EUS) has gained wide acceptance as an important, minimally invasive diagnostic tool in gastroenterology, pulmonology, visceral surgery and oncology. This review focuses on data regarding risks and complications of non-interventional diagnostic EUS and EUS-guided fine-needle biopsy (EUS-FNB). Measures to improve the safety of EUS und EUS-FNB will be discussed. Due to the specific mechanical properties of echoendoscopes in EUS, there is a low but noteworthy risk of perforation. To minimize this risk, endoscopists should be familiar with the specific features of their equipment and their patients’ specific anatomical situations (e.g., tumor stenosis, diverticula). Most diagnostic EUS complications occur during EUS-FNB. Pain, acute pancreatitis, infection and bleeding are the primary adverse effects, occurring in 1% to 2% of patients. Only a few cases of needle tract seeding and peritoneal dissemination have been reported. The mortality associated with EUS and EUS-FNB is 0.02%. The risks associated with EUS-FNB are affected by endoscopist experience and target lesion. EUS-FNB of cystic lesions is associated with an increased risk of infection and hemorrhage. Peri-interventional antibiotics are recommended to prevent cyst infection. Adequate education and training, as well consideration of contraindications, are essential to minimize the risks of EUS and EUS-FNB. Restricting EUS-FNB only to patients in whom the cytopathological results may be expected to change the course of management is the best way of reducing the number of complications.
- Citation: Jenssen C, Alvarez-Sánchez MV, Napoléon B, Faiss S. Diagnostic endoscopic ultrasonography: Assessment of safety and prevention of complications. World J Gastroenterol 2012; 18(34): 4659-4676
- URL: https://www.wjgnet.com/1007-9327/full/v18/i34/4659.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i34.4659
Endoscopic ultrasonography (EUS) was first introduced in 1980, when teams from the Wolfgang von Goethe University in Frankfurt, Germany[1] and the Mayo Clinic in Rochester, United States[2,3] incorporated a rotating mechanical ultrasound scanner or an electronic linear ultrasound array into the tip of side-viewing gastroscopes (Olympus GF-B3; ACMI FX-5), respectively. The clinical use of these early echoendoscopes was limited by the length (80 mm) and diameter (13 mm) of their rigid tips. Due to the devices’ limited flexibility, endoscopists often were unable to pass the pyloric channel. Despite these disadvantageous mechanical properties, early reports showed no complications[1,3-5]. The first experience with needle biopsy under direct endosonographic guidance was published in 1992[6]. Over the last three decades, technological improvements have allowed EUS and EUS-guided fine-needle biopsy (EUS-FNB) to become established tools for the diagnosis, staging, and treatment of gastrointestinal diseases and benign and malignant pulmonary disorders[7-12]. In the United States, 60% of gastroenterologists use EUS, and approximately 43% of gastroenterologists and visceral surgeons in four European countries have access to EUS[13,14]. In Germany, EUS systems are used in universities and tertiary referral centers, as well as in 40% of the approximately 2000 hospitals[8]. Data from the prospective EUS registry of the German Society of Ultrasound in Medicine show that EUS-FNB is performed during one out of seven endosonographic examinations. The two basic EUS-FNB techniques are: EUS-guided fine-needle aspiration (EUS-FNA) using hollow-bore 19 Gauge (G) to 25 G needles, and EUS-guided trucut-biopsy (EUS-TCB) using side-notch 19 G core needles[8]. After 30 years of clinical experience with EUS and 20 years’ experience with EUS-FNB, reliable data on the incidence of complications and specific risk factors are available and may be used as the background information when obtaining informed consent.
Endoscopic ultrasound systems differ from traditional forward-viewing and oblique-viewing endoscopes in the rigidity and stiffness of the scope tip that carries the ultrasound transducer. Moreover, echoendoscopes have a somewhat larger diameter (12.4 mm to 14.6 mm) compared to gastroscopes (9.0 mm to 9.8 mm) and duodenoscopes (11.0 mm to 13.1 mm), and a less flexible bending section[15]. Additionally, most echoendoscopes have a longer non-flexible segment just proximal to the transducer (Figure 1). The mean EUS examination time (20 min in a German multicenter survey[16] or 34 ± 20 min in one US-center with a high case load[17]) is significantly longer than that needed for standard esophagogastroduodenoscopy. With the exception of the electronic radial scanning echoendoscopes of Pentax/Hitachi and Fujinon, all other currently available echoendoscopes are oblique-viewing instruments[15]. Therefore, intubation and advancement of the echoendoscopes, especially through the esophagus, are semi-blind maneuvers. Moreover, in oblique-viewing instruments, the rigid segment containing the transducer extends beyond the optical lens.
EUS-FNA and EUS-TCB differ from forceps biopsies in that the needle penetrates from the non-sterile gastrointestinal tract into sterile extra-intestinal anatomical structures to access the target organ or structure. The target lesions are often near to vascular structures[18] and may themselves have pathological vascularization. For these reasons, it is not possible to apply safety data directly from upper gastrointestinal endoscopy to upper gastrointestinal EUS.
Due to its mechanical and optical properties, echoendoscope advancement may perforate the gastrointestinal wall, particularly at: (1) areas of angulation (e.g., hypopharynx, hiatal hernia, tip of the duodenal bulb, and rectosigmoidal junction); (2) in the presence of unexpected anatomical alterations (e.g., esophageal or duodenal diverticula); and (3) in luminal obstruction (e.g., gastrointestinal cancer). Several investigations of EUS staging have documented non-transversable malignant stenosis in 15% to 42% of patients with esophageal cancer[19-25], and in 7% to 23% (mean 12%) of patients with rectal cancer[26].
Esophageal perforation: In a survey of members of the American Endosonography Club published in 2002, 86 members reported 16 cervical esophageal perforations among 43 852 upper gastrointestinal EUS procedures performed using radial echoendoscopes (0.03%)[27]. Almost all of these patients were elderly; seven of the 16 (44%) had a history of difficult intubation during previous endoscopy. Large cervical osteophytes were identified in three cases. In nine of 16 cases (56%), the endoscopist had less than one year of experience in EUS. Two patients required surgery. For 13 patients (81%), the complication was managed successfully with conservative treatment. However, one patient died[27].
Another early multicenter survey including 34 centers from Europe, Japan and the United States[28] reported 13 esophageal perforations in 37 915 radial EUS examinations (0.03%); esophageal perforations accounted for 68% of all complications. Most of these patients (77%) had undergone pre-EUS dilatation of esophageal strictures to allow complete esophageal cancer staging, including celiac lymph-node station. Nine of these patients underwent surgery, four were managed conservatively, and one patient died. Further major complications in this survey were two pharyngeal and one duodenal perforation and two cases of bleeding[28].
Only two studies have investigated the frequency of esophageal perforations prospectively. A single-center study in the United States enrolled all patients who underwent upper gastrointestinal EUS by the same experienced endosonographer over a 7-year period. Cervical esophageal perforations occurred in three of 4844 patients (0.03%). Longitudinal echoendoscopes had been used in all three patients[29]. The second prospective study, performed in a department of surgical endoscopy in Denmark, evaluated EUS complications of 3324 consecutive patients. Esophageal perforation occurred in 5 of 10 patients with complications (0.15% of all EUS examinations; 0.94% of all patients with esophageal cancer). Balloon dilation had been performed prior to EUS in two cases. All five patients recovered fully with conservative (n = 4) or surgical treatment (n = 1)[30].
From 2004 to 2006, we conducted a survey in German centers performing EUS[31]. Of 67 centers responding to the questionnaire, 32 registered EUS complications prospectively. Esophageal perforation occurred in only eight of 85 084 reported diagnostic EUS procedures (0.009%). None of the perforations were associated with previous dilation of esophageal strictures. Stenosing esophageal cancer was present in five of eight cases[31].
Duodenal perforations: In contrast to the international multicenter survey conducted from 1982 to 1992[28], the German retrospective survey showed that duodenal perforations occurred significantly more often (19 additional cases) compared to esophageal perforations (0.022%)[31]. In 10 of 19 cases (47.4%), duodenal diverticula (n = 4), duodenal stenosis (n = 3), duodenal ulcer (n = 1), duodenal scarring (n = 1), or acute pancreatitis (n = 1) were reported as potentially contributing factors. Twenty-seven of 28 gastrointestinal perforations were managed surgically, and all the patients survived[31] (Table 1). In a prospective EUS online registry of the German Society of Ultrasound in Medicine, participants reported 10 cases of gastrointestinal perforation in 13 988 diagnostic EUS procedures (0.07%). Again, duodenal perforation was the most common type of perforation, accounting for six out of ten cases[32]. The increasing proportion of duodenal perforations in recent multicenter studies compared to the older surveys may partially reflect changing trends in indications for EUS[7,33].
In a large series of 233 EUS-FNA biopsies in patients with presumed pancreatic cancer, Raut and colleagues reported two cases of duodenal perforation requiring surgical intervention (0.86%)[34]. There was no luminal narrowing of the duodenum in either case[34]. One published case report describes iatrogenic duodenal perforation during EUS, which was managed successfully by endoscopic closure using hemoclips, followed by conservative treatment[35]. In a series of 224 EUS-FNAs, one duodenal perforation accounted for one of five severe complications[36]. A large single-center series of 1034 pancreatic EUS-FNAs found one case of fatal duodenal perforation in a 63-year-old woman with an advanced neuroendocrine tumor of the duodenal wall; the perforation likely resulted from mechanical injury of the duodenal wall by the echoendoscope, rather than from biopsy[37].
A national survey in Israel which investigated mortality associated with diagnostic EUS[38] showed that 13 of 18 reported fatal complications (seven in Israel and six from outside the country) resulted from duodenal tears which led to retroperitoneal perforations. Two of the fatalities were secondary to esophageal perforation. At least four of six cases of duodenal perforation reported from Israel involved patients with duodenal diverticula. Five of eight fatal complications in Israel occurred during examinations by endoscopists who had performed fewer than 300 EUS procedures[38].
Other gastrointestinal perforations: EUS-related gastric and rectal perforation seems very rare. There is only one case of rectal perforation reported in the prospective German EUS registry[32]. One study of 2490 endorectal ultrasound examinations reported no procedure-related perforations[39]. One case of gastric perforation occurred in each of the German retrospective study[31] and the German prospective registry[32]. In all three cases of rectal or gastric perforations the indication for EUS was staging of a stenosing tumor[31,32]. A few pharyngeal and hypopharyngeal perforations have been reported as well[28,32]. Possible risk factors for EUS-related gastrointestinal perforations are summarized in Table 2.
| Risk factor | References |
| Operator inexperience | Das et al[27], Jenssen et al[31], Lachter[38] |
| Esophagus | |
| Stenosing cancer of the esophagus and esophagogastric junction | Das et al[27], Mortensen et al[30], Rösch et al[28] |
| Dilatation of esophageal stricture prior to EUS | Das et al[27], Mortensen et al[30], Rösch et al[28] |
| Advanced patient age | Das et al[27] |
| Difficult previous endoscopic esophageal intubation | Das et al[27] |
| Large cervical osteophytes | Das et al[27] |
| Duodenum | |
| Diverticula | Jenssen et al[31], Lachter[38] |
| Stenosis, pancreatic head tumor | Raut et al[34] |
| Longitudinal echoendoscope (long, rigid tip) | Lachter[38] |
For appropriate acoustic coupling of the scanner to the gastrointestinal wall, EUS textbooks suggest instillation of water into the gastric lumen for some indications (e.g., gastrointestinal cancer staging, examination of subepithelial tumors). In a Danish prospective study, tracheal suction was needed in 15 of 293 patients (5.1%), and one patient aspirated during the procedure (0.3%). However, the "water-in-stomach" method was only used in nine patients (3.1%)[30]. Only five cases of aspiration pneumonia have been reported following EUS[32,38,41].
Few data are available concerning the frequency of bacteremia following diagnostic EUS without EUS-FNB. Janssen et al[42] investigated the incidence of bacteremia in 100 consecutive patients undergoing longitudinal diagnostic EUS using paired blood cultures: immediately before and again 5 min after EUS. Significant bacteremia (Streptococcus viridans and Propionibacterium species; Streptococcus mitis) occurred in only two patients (2%), both of whom underwent endosonographic staging of esophageal cancer (seven of nine patients with esophageal cancer did not experience bacteremia)[42]. Levy et al[43] noted transient bacteremia in one of 52 patients who underwent diagnostic EUS with a radial-scanning echoendoscope (1.9%). None of these three patients with bacteremia following diagnostic non-interventional EUS in the two studies developed symptoms of infection[42,43].
Hematogenous tumor cell dissemination has been documented in 11 of 45 patients (24%) following transrectal ultrasound (TRUS)-staging of rectal cancer. In 17 other patients (38%), circulating tumor cells were detected in peripheral blood samples before and after TRUS[44]. The clinical and prognostic significance of this observation is presently unclear, although data from the same group suggest that the detection of circulating tumor cells in blood samples of patients with stage II colorectal cancer correlates with poor outcome[44]. However, a recent retrospective analysis of metastatic rates of locally advanced esophageal cancer showed no difference between those who required and those who did not require esophageal dilation to complete EUS staging[25]. Prospective data are needed to determine whether passage of an endoscope beyond a stenosing gastrointestinal tumor causes clinically relevant hematogenous tumor cell dissemination.
EUS is reported to be safe in pediatric and elderly populations. No complications were reported in five case series of EUS and EUS-guided procedures performed in a total of 166 children[45-49] plus several case reports. A retrospective analysis of EUS and EUS-FNB in 265 consecutive patients aged 80 years or older showed that diagnostic EUS is feasible and safe in geriatric patients[50]. Another study compared complications of endoscopic retrograde cholangiopancreatography (ERCP) and EUS in elderly (≥ 75 years, n = 184) and non-elderly (< 75 years, n = 816) patient cohorts. The EUS complication rate of 4.8% among elderly patients did not statistically differ from the 3.1% complication rate among non-elderly patients[51].
Patients who undergo EUS-FNB are approximately ten times more likely to suffer complications or report symptoms following EUS compared to patients undergoing diagnostic non-interventional EUS (Tables 3 and 4)[30-32,36,59,60].
| Complications | Non-interventional diagnostic EUS (n = 85 084) | EUS-FNB (n = 13 223) | Total (n = 98 307) |
| Total | 29 (0.034) | 38 (0.29) | 104 (0.10) |
| Major | 29 (0.034) | 23 (0.17) | 52 (0.053) |
| Conservative treatment | 2 | 15 | 17 |
| Interventional treatment | 0 | 4 | 4 |
| Surgical treatment | 27 | 4 | 31 |
| Mortality | 4 (0.005) | 0 (0.000) | 4 (0.004) |
| Minor (no treatment necessary) | 0 | 15 (0.11) | 16 (0.016) |
| First author (yr) | Study features | Frequency of complications (%) | ||
| EUS1 | EUS-FNB | EUS-I | ||
| Prospective, single-center | ||||
| Williams et al[52] | 333 EUS-FNA | - | 0.3 | - |
| Mortensen et al[30] | 2518 EUS; 670 EUS-FNA; 136 EUS-I | 0.22 | 0.3 | 0.74 |
| Bournet et al[36] | 3207 EUS; 224 EUS-FNA | 0.093 | 2.2 | - |
| Eloubeidi et al[53] | 355 pancreatic EUS-FNA | - | 2.542 | - |
| Al-Haddad et al[54] | 483 EUS-FNA | - | 1.4 | - |
| Eloubeidi et al[55] | 656 EUS-FNA | - | 1.12 | - |
| Thomas et al[56] | 247 EUS-TCB | - | 2.43 | - |
| Gerke et al[57] | 44 EUS-TCB; 36 EUS-FNA4 | - | 2.3 (EUS-TCB);2.8 (EUS-FNA) | - |
| Prospective, multicenter (registry) | ||||
| Gottschalk et al[32] | 11 889 EUS; 2099 EUS-FNB; 438 EUS-I | 0.14 | 2.1 | 4.1 |
| Retrospective, single-center | ||||
| O'Toole et al[41] | 322 EUS-FNA | - | 1.6 | - |
| Carrara et al[37] | 1034 pancreatic EUS-FNA | - | 1.25 | - |
| Retrospective, multicenter | ||||
| Rösch et al[28] | 42 105 EUS (34 EUS centers) | 0.05 | - | - |
| Wiersema et al[58] | 457 EUS-FNA (4 EUS centers) | - | 1.1 | - |
| Buscarini et al[59] | 11 539 EUS; 787 EUS-FNA; 21 EUS-I (6 EUS centers) | 0.046 | 0.88 | 9.5 |
| Jenssen et al[31] | 85 084 EUS; 13 223 EUS-FNB; 2297 EUS-I (67 EUS centers) | 0.034 | 0.29 | 1.61 |
According to a recent systematic review of 51 EUS-FNB trials, the most common complications are post-procedural pain (34%), acute pancreatitis (34%), fever and infectious complications (16%), and bleeding (13%). Perforations and bile leaks are uncommon (3%). The overall complication rate of EUS-FNB in this systematic analysis of 10 941 patients was 0.98% (1.72% in 31 prospective studies). The procedure-related mortality was estimated at 0.02%[61].
Bacteremia following upper gastrointestinal tract EUS-FNB appears to be rare. Its frequency has been investigated in three prospective studies of 202 patients[42,43,62]. In one study of 100 patients, all blood cultures obtained 30 min and 60 min after the procedure were negative, except in six patients in whom the culture was positive for coagulase-negative Staphylococcus, which the investigators regarded as contaminating and non-pathogenic[62]. Among 102 patients studied by Janssen et al[42] and Levy et al[43], five had bacteremia following EUS-FNB [three with Streptococcus viridans, one with Streptococcus group F (1), and one with Gram-negative bacilli]. No signs or symptoms of infection developed in these five patients. Large case series of EUS-FNB for solid pancreatic mass showed that 0.4% to 1.0% of patients experienced febrile episodes following the procedure[63,64]. In one patient undergoing EUS-FNA of a large pancreatic head mass, pyrexia and abdominal pain emerged followed by extensive acute portal vein thrombosis[65]. In addition to two cases of fever after lymph node EUS-FNA which resolved with antibiotics[54,66], there have been 12 published reports of serious infectious complications following EUS-FNA or EUS-TCB of mediastinal lymph nodes. In one case, a mediastinal abscess developed after EUS-guided aspiration biopsy of a mediastinal lymph-node metastasis from hepatocellular carcinoma (HCC). The iatrogenic abscess was treated successfully by EUS-guided drainage and subsequent endoscopic closure of the resulting esophagomediastinal fistula[67]. Another mediastinal abscess developed following EUS-FNA of a mediastinal metastasis of malignant teratoma; the lesion required computed tomography (CT)-assisted drainage and subsequent thoracotomy[68]. Infectious mediastinitis has also occurred as a result of transesophageal EUS-FNA of an enlarged necrotic lymph node[69] and of a small benign lymph node in the aortopulmonary window[70]. Another case of mediastinitis after EUS-FNB for mediastinal lymphadenopathy developed as a consequence of perforation due to technical problems with the needle[71]. In one patient, EUS-FNA of a tuberculous subcarinal lymph node led to multiple symptomatic mediastinal-esophageal fistulae, which resolved in the course of tuberculostatic treatment[72]. Only one case has been published describing infectious endocarditis (Streptococcus salivarius) secondary to EUS-FNA of a mediastinal lymph node in an oncology patient[73]. Recently, five cases of mediastinal abscess have been reported following EUS-FNA in patients with sarcoidosis; four required surgical treatment[74].
There are six published reports of infection after EUS-FNA or EUS-TCB of subepithelial tumors. In one case, EUS-FNA of an esophageal leiomyoma caused severe intramural infection which resulted in esophagectomy[75]. In another case, fever developed 2 h after EUS-FNA of a large mesenchymal esophageal tumor, presumably due to left lobar pneumonia[41]. In the third case a large duodenal gastrointestinal stromal tumor (GIST) became infected with Enterobacter cloacae following EUS-FNA. The resulting abscess resolved following endoscopic transduodenal drainage and antibiotic treatment[76]. In a series of 52 consecutive EUS-TCBs of gastric subepithelial tumors, two cases of severe septic complications (Streptococcus sepsis and gastric wall abscess) occurred (3.9%)[77]. In our own series of 46 EUS-guided biopsies of hypoechoic subepithelial gastric tumors using a 19 G aspiration-needle, one extramural abscess and septic infection (Morganella morganii) developed in a patient with a large gastric GIST who was receiving 15 mg of prednisolone per day[78]. Despite the high colonization rate of the lower digestive tract lumen with pathogenic bacteria, EUS-FNB of (peri-)rectal and (peri-)colonic solid masses appears relatively safe. Only two cases of infectious complication following transrectal or transcolonic EUS-FNB of solid mass lesions has been published[79,80]. In a prospective risk assessment of bacteremia in 100 patients undergoing EUS-FNB of rectal and perirectal lesions, six patients developed positive blood cultures[81]. However, four of these cases were considered to be contaminants (coagulase-negative Staphylococcus, Moraxella, Peptostreptococcus stomatis). Two patients had true-positive blood cultures (Bacteroides fragilis and Gemella morbillorum). No immediate or delayed infectious complications occurred in any patient[81]. Five other studies (476 patients) showed no complications in patients who underwent transrectal or transcolonic EUS-FNB of solid masses[82-85].
In contrast, EUS-FNB of cystic lesions appears to carry a significant risk of severe infectious complications. In a large multicenter study of EUS-FNA, two of 18 patients undergoing EUS-FNA for cystic pancreatic lesions without peri-procedural antibiotics developed cyst infection[58]. Despite the large number of EUS-guided diagnostic procedures performed for cystic pancreatic lesions, there have been only four reports of infection after EUS-FNB performed with prophylactic antibiotics[52,86,87]. Of the 909 patients who underwent EUS-FNB of pancreatic cystic lesions included in the systematic review of Wang et al[61], 93.7% received prophylactic antibiotics (as recommended by the American Society of Gastrointestinal Endoscopy[88]), and the rate of cyst infection was estimated at 0.55%. However, there is no randomized prospective study supporting the utility of peri-procedural antimicrobial therapy. One recent retrospective study comparing the incidence of infectious complications after 263 EUS-FNBs of cystic pancreatic lesions for 88 cases with and 178 cases without antibiotic prophylaxis showed no significant difference[86]. The authors of one experimental study proposed to implement the combination of systemic antibiotics and local 5% povidone iodine during EUS-FNB of cystic lesions[89].
EUS-FNB of bronchogenic and other mediastinal cysts may cause cyst infection, mediastinitis and severe sepsis. Foregut duplication cysts account for 6% to 15% of primary mediastinal masses[90]. The differentiation between esophageal bronchogenic cysts and subepithelial tumors or paraesophageal masses may be difficult because of the hypoechoic and inhomogeneous mucoid content[91,92]. One study of 19 patients with bronchogenic cysts diagnosed by EUS demonstrated that 13 were anechoic, but the other six were hypoechoic, which suggested, erroneously in these cases, a solid mass lesion. CT or magnetic resonance imaging were diagnostic for a cyst in only four cases[93]. In another study, 9 of 27 benign mediastinal cysts were misdiagnosed as solid masses by CT[94]. Eight cases have been reported of mediastinitis following EUS-FNA or EUS-TCB of mediastinal mass lesions that were hypoechoic on EUS and had high CT attenuation values, but were later shown to be bronchogenic cysts. Because solid lesions were suspected, 5 of these patients did not receive antibiotics[36,92,93,95,96]. In three other cases, severe infection requiring surgical intervention occurred despite pre- and post-interventional intravenous antibiotics[97]. Moreover, one case of asymptomatic contamination of a mediastinal foregut duplication cyst with Candida albicans developed following pre-procedural antibiotic prophylaxis[98]. However, no infectious complications were seen among 22 patients who underwent transesophageal EUS-FNA of suspected mediastinal cysts using peri-interventional antibiotic prophylaxis[91].
In one recently published study on the utility of EUS-guided biopsy of extramural pelvic masses, two patients with cystic pelvic masses developed abscesses (7%)[99]. Also EUS-FNA of ascites and pleural fluid carries a risk of infection. Despite levofloxacin prophylaxis, one of 25 patients undergoing transgastric or transduodenal EUS-FNA of suspected malignant ascites developed bacterial peritonitis. Treatment with intravenous antibiotics was successful[100]. In another study, two of 60 patients (3%) developed self-limited fever following EUS-guided paracentesis[101]. Three other studies (47 patients) did not report any complications following EUS-FNA of ascites or peritoneal nodules in ascites in patients who did not receive prophylactic antibiotics[102-104].
Biliary peritonitis is a severe complication which may develop secondary to penetration or perforation of the common bile duct during EUS-FNB of a pancreatic mass in patients with biliary obstruction[105]. During a study to identify patients with microlithiasis as a cause of idiopathic pancreatitis, EUS-guided gallbladder bile duct aspiration resulted in biliary peritonitis in two of the three patients enrolled; these complications prompted the investigators to discontinue the study[106]. However, a recent systematic review of nine studies (284 patients) showed EUS-FNB of gallbladder masses or bile duct strictures to be safe, with no reported complications[107]. During an international survey of the indications, yield, and safety of EUS-FNB of liver lesions, one death due to septic cholangitis associated with EUS-FNB of a liver metastasis was reported. The patient who died had an occluded biliary stent, with biliary obstruction secondary to pancreatic cancer[108]. Mechanical irritation caused by the tip of the scope or by a water-filled balloon during pancreatic EUS may cause proximal or distal biliary stent migration, possibly followed by cholangitis[109].
Hyperamylasemia was common following pancreatic EUS-FNB in one recent prospective study, occurring in 11 of 100 patients. However, only two patients developed mild acute pancreatitis[110]. Nine large studies have reported iatrogenic acute pancreatitis following EUS-FNB of pancreatic lesions, with rates ranging from 0.19% to 2.0%[37,41,52,53,60,111-113]. A pooled analysis of 4909 solid pancreatic mass EUS-FNBs from 19 US centers identified 14 cases of iatrogenic post-procedural acute pancreatitis (0.29%)[114]. Among them, 71% were mild, 21% moderate, and 7% severe. One patient with significant co-morbidity died secondary to severe procedure-related pancreatitis. The frequency of acute pancreatitis at individual centers ranged from 0% to 2.35%. Only two centers (413 cases of pancreatic EUS-FNB, two cases of acute pancreatitis) assessed EUS-FNB complications prospectively. Some centers with retrospective complication assessment reported no cases of iatrogenic acute pancreatitis, suggesting that acute pancreatitis may have been underreported for the retrospective cohort. Interestingly, the survey showed that half of patients who developed acute pancreatitis after EUS-FNB had benign disease, suggesting that these patients may carry a higher risk. Another possible risk factor for iatrogenic acute pancreatitis following EUS-FNB is a history of acute recurrent pancreatitis[114]. In a German retrospective survey, eight of nine patients (86%) with reported acute pancreatitis after pancreatic mass EUS-FNB had benign disease[31]. In a large retrospective series of EUS-FNB for pancreatic cystic lesions, acute pancreatitis occurred in six of 603 cases (1%)[52]. A retrospective analysis of pancreatic EUS-FNB detected pancreatitis in three of 114 (2.6%) patients with cystic lesions, but none in the 134 patients with solid lesions[41].
Two studies have investigated the value of EUS-FNA and EUS-TCB in the diagnosis of chronic pancreatitis. Mild acute pancreatitis developed in two of 27 patients (7.4%) following EUS-FNA[115] and in one of 16 patients (6%) following EUS-TCB[116].
This rare complication was reported only recently in two cases following EUS-FNA of pancreatic mass lesions. Both cases resolved after antibiotic treatment, transpapillary duct drainage and percutaneous drainage[117,118].
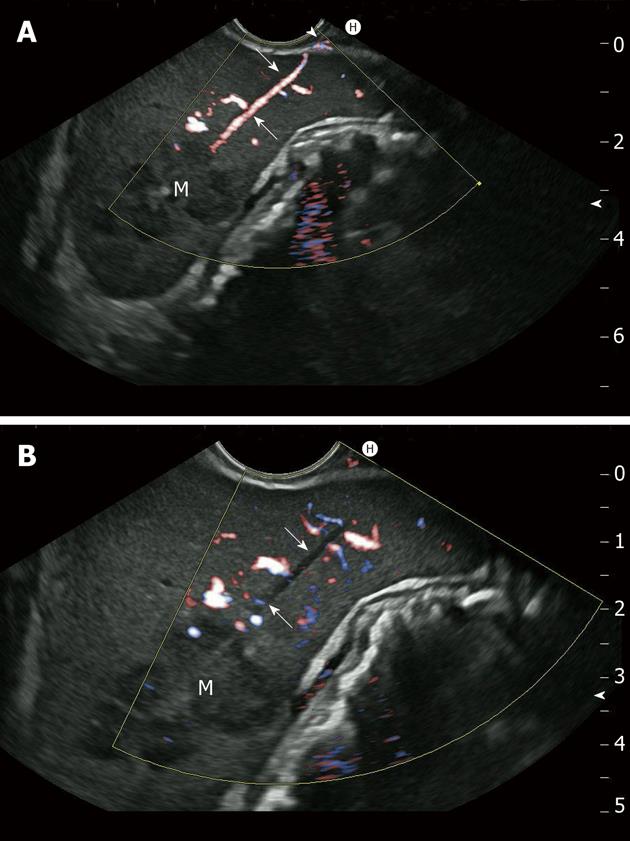
Self-limiting mild intraluminal bleeding due to EUS-FNB has been reported in up to 4% of cases[64] (Figure 2). A prospective study evaluating the risk of extraluminal hemorrhage caused by EUS-FNA found only three cases among 227 patients (1.3%)[119]. Extraluminal bleeding following EUS-FNB can be visualized clearly using EUS. Bleeding appears as an expanding hyperechoic or hypoechoic region adjacent to the sampled lesion[119]. Occasionally, color-coded Doppler may demonstrate blood flow within a “patent needle track” (Figure 3). In a retrospective survey of German EUS centers (13 223 EUS-FNB; Table 3), bleeding was the most common complication of EUS-FNB (0.15% of cases)[31]. Mild and self-limited bleeding was reported in 15 patients, whereas five patients suffered from severe hemorrhages requiring transfusion, and two of those patients required surgical intervention. One patient was treated with low-molecular-weight heparin (LMWH) immediately after the procedure and developed a large mediastinal hematoma and hemothorax; thoracoscopy and transfusion of seven units of packed red cells were required. Another patient required transfusion of four units of packed red cells following EUS-FNB of pancreatic head carcinoma with associated portal hypertension[31]. The prospective German EUS registry also showed that bleeding was the most frequent complication, reported in 1.4% of cases of EUS-FNB. However, 29 of 30 cases were judged to be mild, requiring no endoscopic intervention, surgery, or transfusion[32]. Patients with highly vascularized lesions (e.g., subepithelial mesenchymal tumors, neuroendocrine tumors, and some metastases), as well as those with cystic lesions, may be at greater risk. An Italian multicenter retrospective study of EUS complications showed that three out of seven EUS-FNA complications were intracystic bleeding[59]. Three of 50 patients (6%) in a retrospective study developed acute intracystic hemorrhage following EUS-FNB of pancreatic cystic lesions[120]. Two patients experienced transient abdominal pain, and none of the three patients required blood transfusion. Intracystic bleeding episodes were readily recognized during the investigation as expanding hyperechoic areas surrounding needle puncture sites within the target cysts. Contributing risk factors could not be identified for the bleeding events[120]. In a large Italian single-center experience, bleeding was the most frequent pancreatic EUS-FNA complication (0.96%; n = 1034), with seven of ten cases occurring in cystic lesions[37].
A prospective analysis compared the risk of bleeding after EUS-FNA or EUS-TCB in patients taking non-steroidal anti-inflammatory drugs (NSAIDs), aspirin (ASA) or prophylactic LMWH to those who did not receive those medications[121]. Two of six patients (33.3%) receiving prophylactic LMWH developed extraluminal bleeding, compared to 0 of 26 patients receiving platelet inhibitors, and 7 of 190 patients (3.7%) not receiving those medications. There appears to be no increased bleeding risk during EUS-FNB for patients taking ASA or NSAIDs, but a possible risk for patients receiving prophylactic LMWH[121]. There are no published data regarding the risk of EUS-FNB in patients treated with clopidogrel and other thienopyridines. However, one study of patients undergoing transbronchial lung biopsy showed that clopidogrel, especially when combined with ASA, greatly increased bleeding risk[122].
There have been several case reports of severe bleeding associated with EUS-FNB, including hemosuccus pancreaticus in cystic pancreatic lesions[123], left adrenal gland hemorrhage[124], retroperitoneal bleeding following EUS-FNA of solid and cystic pancreatic lesions[59,125,126], mesenteric bleeding[127], and intramural bleeding following EUS-TCB of subepithelial tumors[78,96,128]. Three fatal cases of extraluminal bleeding have been reported following pancreatic mass EUS-FNA. One patient developed uncontrolled bleeding from a pseudoaneurysm and died following EUS-FNB performed using a radial-scanning echoendoscope[129]. Another patient died in the consequence of massive gastrointestinal bleeding 6 h after EUS-FNA of a pancreatic cancer[31]. The third fatal outcome was observed after a 19 G EUS-FNA and intracystic brushing of a cystic pancreatic lesion in a patient receiving anticoagulation therapy. The patient died one month later due to a subacute retroperitoneal bleed related to the EUS-guided procedure and further complications[130].
Several studies have described abdominal pain (following EUS-FNB of pancreatic lesions and abdominal lymph nodes) or thoracic pain (following EUS-FNB of mediastinal lymph nodes and mediastinal mass lesions) without clinical, laboratory, or radiological findings suggestive of any specific complication[30,53,60]. One case of massive pneumoperitoneum due to transduodenal EUS-FNB of a solid pancreatic lesion resolved without any intervention other than antibiotics[131]. In the absence of clinical and radiological evidence of bowel perforation, the authors explained that the pneumoperitoneum resulted from insufflated air tracking into the peritoneal cavity through the EUS-FNB site. They postulate that some patients with abdominal pain following EUS-FNB have unrecognized pneumoperitoneum[131].
Pneumothorax following EUS-FNB has been reported as one of three complications in a series of 159 EUS-FNBs for transesophageal biopsy of mediastinal masses[132].
Needle-tract seeding is a rare complication following FNB of intra-abdominal tumors. Based on large retrospective surveys, its frequency is estimated at 0.003% to 0.009%[133]. However, one prospective comparative study reported the incidence of needle-tract implantation of HCC and pancreatic carcinoma after ultrasound-guided percutaneous puncture to be 1.5%[134]. A systematic review and a meta-analysis of percutaneous biopsy of HCC reported tumor seeding frequencies of 2.29%[135] and 2.7%[136], respectively. After percutaneous biopsy of colorectal cancer liver metastases, the risk of needle-track metastases seems even higher, occurring in 10% to 19% of cases[133].
The actual frequency of EUS-FNB-related needle-tract seeding is unknown, but six published case reports have raised concern that the substantial rise of EUS-guided biopsies used to diagnose pancreatic masses and lymphadenopathy may increase the incidence of this complication. In one case, a large gastric wall implantation metastasis with retroperitoneal invasion (50 mm × 30 mm) was diagnosed 16 mo after potentially curative resection of a small (8 mm) T1N0M0 pancreatic tail carcinoma. The tumor had previously been sampled using transgastric EUS-FNA (with five needle passes)[137]. Two similar cases have been reported recently. In both cases, gastric wall metastases occurred at 26 mo and nearly 4 years after transgastric EUS-FNB and pancreatic surgery with curative intent for solid-cystic pancreatic cancer[138,139]. In the fourth case, transgastric EUS-FNB (one needle pass) of a melanoma metastasis to a perigastric lymph node was complicated by a large (30 mm) gastric implantation metastasis along the needle tract. It was detected and resected 6 mo later during surgical resection of lymph node metastasis following neoadjuvant chemotherapy[140]. A recent case report describes needle tract implantation metastasis of the esophageal wall following 19 G EUS-FNA of a large metastatic subcarinal lymph node metastasis of gastric cancer. The esophageal implantation metastasis developed despite perioperative systemic chemotherapy and disappearance of the subcarinal metastatic lymph node; fortunately, it resolved after 2 mo of radiotherapy[141]. The potential risk of peritoneal carcinomatosis due to transgastric EUS-FNA of pancreatic malignancies was highlighted by the case of peritoneal dissemination of an intraductal papillary mucinous neoplasm of the pancreas following EUS-FNA of the tumor[142]. Nevertheless, the risk of peritoneal carcinomatosis appears to be significantly lower with EUS-FNB compared to percutaneous needle biopsy under computed-tomographic or ultrasonographic guidance[143].
Recent data demonstrate vital tumor cell presence within gastrointestinal luminal fluid in 48% of patients with luminal cancers (resulting from tumor cell sloughing), as well as in 10% of patients who underwent EUS-FNB of pancreatic cancer lesions[144]. The importance of this finding with regard to potential tumor cell dissemination by EUS-guided transmural biopsy is speculative at this time. Whereas pancreatic head cancer EUS-FNBs are performed typically through the duodenal wall (which is later resected with the pancreatic head in cases of resectable cancers), pancreatic body and tail tumor EUS-FNBs are performed transgastrically, crossing the peritoneal structures of the Bursa omentalis. Due to the risk of tumor cell seeding along the needle tract, which is proven in principle by the above described case reports, the indication for preoperative EUS-FNB of suspected distal pancreatic cancer is controversial[145-147]. To date, prospective comparative studies of the long-term impact of pre-operative EUS-FNB of suspected pancreatic malignancies are lacking. A recent retrospective study investigated the long-term outcomes of patients undergoing distal pancreatectomy for primary pancreatic neoplasias, comparing patients who received (n = 179) and who did not receive pre-operative EUS-FNB (n = 51)[112]. After a 16-mo median follow-up, no local recurrences were detected within the gastric wall among 91 patients with adenocarcinoma or neuroendocrine tumors who underwent pre-operative EUS-FNB. Median disease-specific survival and recurrence-free survival in patients with pancreatic adenocarcinoma were not statistically worse in 57 patients with pre-operative EUS-FNB as compared with six patients without pre-operative transgastric biopsy. However, partial gastrectomy due to suspected infiltration of the gastric wall by pancreatic adenocarcinoma was performed only in patients with pre-operative EUS-FNB. The study authors expressed concern that surgical pathology showed direct transmural gastric invasion from the pancreatic primary site in six of these seven patients[112].
Scarce information exists regarding the potential impact of pre-operative EUS-FNB on complications of subsequent surgery. One study showed no correlation with overall morbidity, specific complications, or technical difficulties during distal pancreatectomy, when analyzed according to specific pancreatic pathology sub-classifications. However, subgroup analysis revealed a higher incidence of postoperative complications in patients with cystic neoplasms who underwent pre-operative EUS-FNB[112].
A false-positive diagnosis obtained from EUS-FNB may result in inappropriate treatment decisions, which could negatively impact the patient’s clinical outcome. Therefore, we regard false-positive results as a major EUS-FNB complication in this review. The overwhelming majority of studies report EUS-FNB specificity and positive predictive value for detecting cancer at 100%. A thorough review of EUS-FNB studies conducted in 2008 identified only ten studies (1750 cases) plus one case study that altogether reported 27 false-positive diagnoses using EUS-FNB[8]. In most of these cases, benign pancreatic diseases (chronic pancreatitis, intrapancreatic splenic heterotopia, mucinous cystic adenoma) were misdiagnosed as ductal adenocarcinoma or neuroendocrine pancreatic tumor[64,66,145,148-154]. However, few studies have correlated EUS-FNB findings with surgical pathology[149,150,155-158]. Therefore, the “true false-positive rate” of EUS-FNB was unknown until a remarkable 2010 study presented a more realistic picture[159]. A cohort of 337 consecutive patients with positive or suspicious EUS-FNB results underwent surgery in the absence of neoadjuvant therapy; there were 19 false-positive cases (5.6%) (based on “positive” cytopathological interpretations) and 25 false-positive cases (7.3%) (based on “positive” plus “suspicious” cytopathological findings), respectively. For pancreatic lesions, the authors reported a 2.2% rate of discordance between pre-operative cytological diagnosis and subsequent surgical pathology. In this study, most discordant cases resulted from malignant cell contamination by luminal cancers (primarily esophageal) when performing EUS-FNB of nearby lymph nodes. Retrospectively, only 11 cases were identified as resulting from cytopathological interpretive error[159]. These results are supported by a retrospective cohort study of 367 patients who underwent surgical resection without previous neoadjuvant treatment[160]. This study showed a 1.1% false-positive rate of EUS-FNB for solid pancreatic lesions. When suspicious cytological findings were considered, the false-positive rate was estimated at 3.8%. On review of discordant cases, almost all were caused by pathological misinterpretation in the setting of chronic pancreatitis[160]. Another possible explanation for false-positive diagnosis by EUS-FNB may be inadvertent aspiration of contaminated luminal fluid or traversal of mucosal high-grade dysplasia or malignant infiltration of the gastrointestinal wall[144,161]. Malignant cells are identified within gastrointestinal luminal fluid not only in 48% of cases of luminal cancer, but also in 10% of cases of extraluminal (pancreatic) cancer undergoing EUS-FNB[144].
Due to its high accuracy and lower complication rate compared to ERCP for suspected pancreaticobiliary disease (especially for obstructive jaundice), EUS and EUS-FNB should be performed first when possible. Moreover, biliary stent placement prior to EUS is believed to negatively affect the accuracy of endosonographic staging of pancreatic head malignancies[162]. To perform EUS, EUS-FNB and therapeutic ERCP in one session has advantages with regard to cost, total procedure time and workflow. Several studies have shown that single-session EUS and ERCP does not result in higher complication rates[163-168]. However, ERCP is more widely available than EUS and some cases (e.g., obstructive cholangitis) require biliary decompression more urgently than cytological diagnosis. Therefore, biliary stenting often precedes EUS-FNB. A recent retrospective comparative study showed that pre-EUS stenting of biliary obstructions due to pancreatic cancer did not increase the EUS-FNB complication rate. Moreover, the accuracy of tissue diagnosis was not impaired if ERCP and endoscopic stenting were performed more than one day prior to EUS-FNB[169].
Due to the relatively low number of complications associated with EUS-FNB, available studies are statistically underpowered to adequately address the question of risk factors. Endosonographer experience seems to be important. Of five nonfatal complications in a large multicenter EUS-FNA study (n = 457), none occurred during the last 12 mo of the study[58]. Similarly, the largest EUS-TCB study (n = 247) showed that all complications were observed among the first 100 patients[56].
There is no evidence that needle size affects complication rate. Only one retrospective multicenter study reported a complication rate of 2% in 540 patients undergoing 22 G EUS-FNA of solid pancreatic masses, versus 0% in 302 patients undergoing 25 G EUS-FNA[113]. Small prospective studies comparing needle sizes reported no complications in any group[170,171]. EUS-FNB using a 19 G needle, when combined with cytobrushing of pancreatic cystic lesions, conveys a significant complication risk associated primarily with cytobrush trauma to the cyst wall. In a prospective controlled study of 37 patients with 39 cystic pancreatic lesions, seven experienced complications (19%): one experienced severe bleeding and one experienced severe pancreatitis[172]. In another study using the same technique (n = 30), three patients experienced complications, including one case of fatal bleeding[130]. This procedure-related morbidity is considerably higher than the 2.2% to 5.2% complication rate reported in several EUS-FNB studies of cystic pancreatic lesions[54,86,87,173]. Nevertheless, the overall EUS-FNB complication rate seems higher for cystic than solid pancreatic masses[54,58,61]. In a systematic review which included 7337 EUS-FNBs of solid pancreatic masses and 909 EUS-FNBs of pancreatic cystic lesions, complications were reported in 60 patients with solid masses (0.82%; only prospective studies: 2.44%) and 25 patients (2.75%; only prospective studies: 5.07%) with pancreatic cystic lesions[61]. Data from the same systematic review confirmed that EUS-FNB is exceptionally safe for mediastinal lesions (n = 1310; complication rate: 0.38%), abdominal masses (n = 381; complication rate: 0.26%) and adrenal gland biopsies (n = 81; complication rate: 0%). EUS-FNB had an associated overall morbidity of 1.03% for pancreatic lesions (n = 8246; only prospective studies: 2.64%), 2.33% for liver lesions (n = 344), 2.07% for perirectal lesions (n = 193), and 3.53% for ascites (n = 85)[61].
Safe and effective EUS and EUS-FNB performance require adequate sedation. Only a few studies report on the frequency of sedation-related complications in EUS and EUS-FNB. In the prospective German EUS registry, oxygen desaturation (SpO2 < 80%) was reported in 0.39% of patients, but most patients (94.4%) did not require intubation or mechanical ventilation[32]. A retrospective single-center study on the safety of nurse-administered propofol sedation for upper gastrointestinal EUS showed that 6 of 806 patients (0.7%) experienced declines in oxygen saturation (SpO2 < 90%), and four patients (0.5%) required assisted positive pressure ventilation[17]. Comparable results have been reported by other authors[174]. Several studies show that EUS with deep sedation using propofol (administered by an anesthesiologist, by a second gastroenterologist, or by a trained nurse) is as safe as conscious sedation using benzodiazepines (midazolam) and pethidine[174-177].
Strategies aimed to minimize potential risks of EUS and EUS-FNB include: (1) adequate education and examiner training; (2) familiarity of the endosonographer with the results of previous investigations and of possible therapeutic implications of EUS and EUS-FNB results; (3) appropriate consideration of the patient’s clinical condition and of indications and contraindications for EUS and EUS-FNB; (4) careful consideration of a meaningful clinical strategy for investigating and treating each patient; and (5) optimization of prerequisites and conditions for the planned investigation or procedure[40].
We suggest the following measures, which seem appropriate to minimize the risks of EUS and EUS-FNB: (1) participation in supervised training programs in EUS and EUS-guided interventions. EUS and EUS-FNB are complex, technically demanding procedures in which the findings are difficult to interpret and which require advanced endoscopic skills, outstanding experience in diagnostic ultrasonography, and a fundamental understanding of the anatomy of the gastrointestinal tract and surrounding structures. The guidelines for credentialing and granting privileges for endoscopic ultrasound published by the American Society for Gastrointestinal Endoscopy recommend a minimum of 150 supervised EUS investigations, including 75 pancreatobiliary EUS investigations and 50 EUS-FNA procedures, to establish comprehensive competence in all aspects of EUS[178]; (2) specific guidelines for EUS training should be implemented in national gastroenterological societies’ training programs[7,179,180]. Curricula of formal training programs should include sessions on complications and contraindications of EUS and EUS-FNB; (3) EUS and EUS-FNB should only be carried out in cooperative and adequately sedated patients. Oxygen saturation, heart rate, blood pressure and (whenever possible and necessary) electrocardiography should be monitored[181-183]; (4) water filling in the upper gastrointestinal tract should be restricted to the minimum amount necessary and the endoscopy unit and team should be prepared to perform nasopharyngeal suction; (5) in EUS examinations of the pancreas, biliary duct, ampulla, and duodenum in patients with biliary stents, the correct position of the stent should be checked prior to examination; (6) in the case of stenosing tumors and for specific anatomical locations (e.g., the tip of the duodenal bulb), care should be taken when maneuvering the echoendoscope. Dilation to achieve echoendoscope passage through malignant strictures should be carried out only in selected cases; (7) EUS and EUS-FNB are contraindicated in all patients and in all conditions in which the risks of the procedure outweigh the expected benefits of its diagnostic information (Table 5)[8,180]. According to recent guidelines, EUS-FNB of solid masses and lymph nodes may be performed in patients taking ASA or NSAIDs, but not in patients receiving: thienopyridines (clopidogrel, prasugrel, ticagrelor), oral anticoagulation, standard heparin treatment, LMWH or fondaparinux in therapeutic doses. EUS-FNB of cystic lesions should be avoided in patients taking any antiplatelet agent (e.g., ASA, thienopyridines). If EUS-guided sampling is indicated in a patient on LWMH, the biopsy should be performed no earlier than 8 h following the last administration of the drug[180,189,190]; (8) whenever possible, color-coded duplexsonography (CCDS) should be used before puncture to avoid vessels and highly vascularized structures in the needle trajectory; (9) before attempting EUS-FNB of large, solid-appearing mediastinal mass lesions, all appropriate methods (e.g., CCDS, contrast-enhanced endosonography (CE-EUS) and real-time elastography) should be used to exclude cystic lesions; (10) transgastric EUS-FNB of pancreatic masses should be restricted to those patients in whom definitive cytopathological diagnosis is likely to alter management significantly. In general, there is no indication for EUS-FNB of a pancreatic mass lesion that is typical of adenocarcinoma in patients who have no criteria of non-resectability[8,9,147,180]. Advanced EUS techniques (e.g., CCDS, CE-EUS, real-time elastography) may help to select resectable pancreatic masses that are atypical of ductal pancreatic adenocarcinoma[191-197]; (11) trucut needles should be used with caution in selected cases and indications. Although a prospective randomized study found no significant difference in the complication rates between EUS-TCB and EUS-FNA[57], the use of EUS-TCB is limited by technical drawbacks, especially for pancreatic head lesions[198]. Subepithelial tumors, unexplained gastrointestinal wall thickening, suspected malignant lymphoma, and suspected autoimmune pancreatitis are now emerging as special indications for EUS-TCB[8] or, alternatively, for a new type of aspiration needle that is suited to obtain core particles for histological evaluation[199]; (12) antibiotic prophylaxis should be administered in patients undergoing EUS-FNB of cystic lesions and fluid collections[88,100,180]. Some experts also recommend peri-procedural antibiotic prophylaxis for EUS-FNB of the perirectal space[200] and for EUS-TCB of subepithelial tumors[76]. However, there are no valid data supporting routine use of antibiotics for these indications; and (13) complications should be assessed in a prospective manner, and all complications should be analyzed. Prospective risk assessment is prerequisite to the prevention and analysis of complications. Careful prospective studies show higher EUS-FNB complication rates compared to retrospective studies (2.44% vs 0.35%)[61] (Table 4). The American Society for Gastrointestinal Endoscopy recommends that specific quality indicators (e.g., indications, sedation, immediate and delayed complications, procedure success) should be tracked routinely in all patients undergoing EUS[201].
| Contraindications to EUS and EUS-FNB |
| Lack of informed consent1 |
| Lack of cooperation or insufficient sedation1 |
| Results of examination are unlikely to have a significant clinical impact1 |
| Anticoagulation treatment or non-substituted coagulopathy (international normalized ratio > 1.5; platelet count < 50 000/mL; heparin administration at therapeutic doses)2 |
| Inhibition of platelet aggregation by clopidogrel and other thienopyridines2 |
| Failure of ultrasonographic needle control2 |
| EUS-FNB of lesions upstream of a bile duct stricture which is insufficiently drained2 |
| Cystic mediastinal and pelvic lesions23 |
| Interposition of vessels24 |
In conclusion, diagnostic EUD is a safe procedure. The EUS complication rate of 0.03% to 0.15% is comparable to that of upper gastrointestinal tract diagnostic endoscopy (Table 6)[40,88,180]. Due to specific mechanical and optical properties of echoendoscopes, the risk of esophageal or duodenal perforation seems somewhat higher compared to esophagogastroduodenoscopy. The major risks of EUS-FNB are pain, acute pancreatitis, infection and hemorrhage[61]. Tumor cell seeding is exceedingly rare. The complication rate of EUS-FNB is 0.3% to 6.3%, which is comparable to that of colonoscopy with polypectomy (Table 6). Very few fatal complications of diagnostic EUS have been reported (overall mortality 0.02%)[31,38,61]. Adherence to guidelines on indications and contraindications, adequate education and training, prospective registration, and careful assessment of all complications are essential preconditions for enhancing safety and efficacy of EUS and EUS-FNB. A constructive process of continuous quality and safety assessment should be implemented in all centers performing endoscopic endosonography.
| Complications | Total | Bleeding | Perforation |
| Diagnostic esophagogastroduodenoscopy | 0.009-0.2 | 0.002-0.06 | 0.0009-0.04 |
| Diagnostic colonoscopy | 0.02-0.25 | 0-0.03 | 0.005-0.2 |
| Colonoscopy + polypectomy | 0.36-9.7 | 0.26-8.6 | 0.06-1.1 |
| Diagnostic ERCP | 1.38 | 0 | 0.11 |
| Therapeutic ERCP | 5.0-9.8 | 0.49-2.0 | 0.3-0.8 |
| EUS | 0.03-0.15 | 0-0.03 | 0.03-0.15 |
| EUS-FNB | 0.3-6.3 | 0.15-3.7 | 0-0.86 |
| 1. | Strohm WD, Phillip J, Hagenmüller F, Classen M. Ultrasonic tomography by means of an ultrasonic fiberendoscope. Endoscopy. 1980;12:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 96] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | DiMagno EP, Buxton JL, Regan PT, Hattery RR, Wilson DA, Suarez JR, Green PS. Ultrasonic endoscope. Lancet. 1980;1:629-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 210] [Article Influence: 4.6] [Reference Citation Analysis (11)] |
| 3. | Dimagno EP, Regan PT, Clain JE, James EM, Buxton JL. Human endoscopic ultrasonography. Gastroenterology. 1982;83:824-829. [PubMed] |
| 4. | Lux G, Heyder N, Lutz H, Demling L. Endoscopic ultrasonography--technique, orientation and diagnostic possibilities. Endoscopy. 1982;14:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 418] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 6. | Dietrich CF, Jenssen C. [Evidence based endoscopic ultrasound]. Z Gastroenterol. 2011;49:599-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Jenssen C. [Diagnostic endosonography - state of the art 2009]. Endo heute. 2009;22:89-104. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Jenssen C, Dietrich CF. Endoscopic ultrasound-guided fine-needle aspiration biopsy and trucut biopsy in gastroenterology - An overview. Best Pract Res Clin Gastroenterol. 2009;23:743-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 9. | Dumonceau JM, Polkowski M, Larghi A, Vilmann P, Giovannini M, Frossard JL, Heresbach D, Pujol B, Fernández-Esparrach G, Vazquez-Sequeiros E. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 10. | Jue TL, Sharaf RN, Appalaneni V, Anderson MA, Ben-Menachem T, Decker GA, Fanelli RD, Fukami N, Ikenberry SO, Jain R. Role of EUS for the evaluation of mediastinal adenopathy. Gastrointest Endosc. 2011;74:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Jenssen C, Dietrich CF, Burmester E. [Malignant neoplasias of the gastrointestinal tract--endosonographic staging revisited]. Z Gastroenterol. 2011;49:357-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Gan SI, Rajan E, Adler DG, Baron TH, Anderson MA, Cash BD, Davila RE, Dominitz JA, Harrison ME, Ikenberry SO. Role of EUS. Gastrointest Endosc. 2007;66:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Ahmad NA, Kochman ML, Ginsberg GG. Practice patterns and attitudes toward the role of endoscopic ultrasound in staging of gastrointestinal malignancies: a survey of physicians and surgeons. Am J Gastroenterol. 2005;100:2662-2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Kalaitzakis E, Panos M, Sadik R, Aabakken L, Koumi A, Meenan J. Clinicians' attitudes towards endoscopic ultrasound: a survey of four European countries. Scand J Gastroenterol. 2009;44:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Tierney WM, Adler DG, Chand B, Conway JD, Croffie JM, DiSario JA, Mishkin DS, Shah RJ, Somogyi L, Wong Kee Song LM. Echoendoscopes. Gastrointest Endosc. 2007;66:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Parusel M, Krakamp B, Janssen J, Rünzi M. [Endoscopic ultrasonography (EUS) of the upper gastrointestinal tract - prospective multicenter study to evaluate time and staff requirements]. Z Gastroenterol. 2003;41:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Fatima H, DeWitt J, LeBlanc J, Sherman S, McGreevy K, Imperiale TF. Nurse-administered propofol sedation for upper endoscopic ultrasonography. Am J Gastroenterol. 2008;103:1649-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 18. | Eloubeidi MA, Iseman DT, Chen VK, Vickers SM, Wilcox CM. Prevalence and significance of periduodenal venous collaterals in patients evaluated for pancreaticobiliary disorders by endosonography. Endoscopy. 2003;35:1015-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Mallery S, Van Dam J. Increased rate of complete EUS staging of patients with esophageal cancer using the nonoptical, wire-guided echoendoscope. Gastrointest Endosc. 1999;50:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Pfau PR, Kochman ML. Pretreatment staging by endoscopic ultrasonography does not predict complete response to neoadjuvant chemoradiation in patients with esophageal carcinoma. Gastrointest Endosc. 2000;52:583-586. [PubMed] |
| 21. | Wallace MB, Hawes RH, Sahai AV, Van Velse A, Hoffman BJ. Dilation of malignant esophageal stenosis to allow EUS guided fine-needle aspiration: safety and effect on patient management. Gastrointest Endosc. 2000;51:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Eloubeidi MA, Wallace MB, Hoffman BJ, Leveen MB, Van Velse A, Hawes RH, Reed CE. Predictors of survival for esophageal cancer patients with and without celiac axis lymphadenopathy: impact of staging endosonography. Ann Thorac Surg. 2001;72:212-29; discussion 212-29;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Kallimanis GE, Gupta PK, al-Kawas FH, Tio LT, Benjamin SB, Bertagnolli ME, Nguyen CC, Gomes MN, Fleischer DE. Endoscopic ultrasound for staging esophageal cancer, with or without dilation, is clinically important and safe. Gastrointest Endosc. 1995;41:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Jacobson BC, Shami VM, Faigel DO, Larghi A, Kahaleh M, Dye C, Pedrosa M, Waxman I. Through-the-scope balloon dilation for endoscopic ultrasound staging of stenosing esophageal cancer. Dig Dis Sci. 2007;52:817-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Hancock SM, Gopal DV, Frick TJ, Pfau PR. Dilation of malignant strictures in endoscopic ultrasound staging of esophageal cancer and metastatic spread of disease. Diagn Ther Endosc. 2011;2011:356538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Marusch F, Ptok H, Sahm M, Schmidt U, Ridwelski K, Gastinger I, Lippert H. Endorectal ultrasound in rectal carcinoma--do the literature results really correspond to the realities of routine clinical care? Endoscopy. 2011;43:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Das A, Sivak MV, Chak A. Cervical esophageal perforation during EUS: a national survey. Gastrointest Endosc. 2001;53:599-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Rosch T, Dittler HJ, Fockens P, Yasuda K, Lightdale C. Major complications of endoscopic ultrasonography: Results of a survey of 42105 cases[abstract]. Gastrointest Endosc. 1993;39:370. |
| 29. | Eloubeidi MA, Tamhane A, Lopes TL, Morgan DE, Cerfolio RJ. Cervical esophageal perforations at the time of endoscopic ultrasound: a prospective evaluation of frequency, outcomes, and patient management. Am J Gastroenterol. 2009;104:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Mortensen MB, Fristrup C, Holm FS, Pless T, Durup J, Ainsworth AP, Nielsen HO, Hovendal C. Prospective evaluation of patient tolerability, satisfaction with patient information, and complications in endoscopic ultrasonography. Endoscopy. 2005;37:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Jenssen C, Faiss S, Nürnberg D. [Complications of endoscopic ultrasound and endoscopic ultrasound-guided interventions - results of a survey among German centers]. Z Gastroenterol. 2008;46:1177-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Gottschalk U, Dffelmeyer M, Jenssen C. [omplikationserfassung der diagnostischen und therapeutischen Endosonografie]. Z Gastroenterol. 2011;49:V118. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Noh KW, Woodward TA, Raimondo M, Savoy AD, Pungpapong S, Hardee JD, Wallace MB. Changing trends in endosonography: linear imaging and tissue are increasingly the issue. Dig Dis Sci. 2007;52:1014-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Raut CP, Grau AM, Staerkel GA, Kaw M, Tamm EP, Wolff RA, Vauthey JN, Lee JE, Pisters PW, Evans DB. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration in patients with presumed pancreatic cancer. J Gastrointest Surg. 2003;7:118-126; discussion 127-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 195] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Sebastian S, Byrne AT, Torreggiani WC, Buckley M. Endoscopic closure of iatrogenic duodenal perforation during endoscopic ultrasound. Endoscopy. 2004;36:245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Bournet B, Migueres I, Delacroix M, Vigouroux D, Bornet JL, Escourrou J, Buscail L. Early morbidity of endoscopic ultrasound: 13 years' experience at a referral center. Endoscopy. 2006;38:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Carrara S, Arcidiacono PG, Mezzi G, Petrone MC, Boemo C, Testoni PA. Pancreatic endoscopic ultrasound-guided fine needle aspiration: complication rate and clinical course in a single centre. Dig Liver Dis. 2010;42:520-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Lachter J. Fatal complications of endoscopic ultrasonography: a look at 18 cases. Endoscopy. 2007;39:747-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Morken JJ, Baxter NN, Madoff RD, Finne CO. Endorectal ultrasound-directed biopsy: a useful technique to detect local recurrence of rectal cancer. Int J Colorectal Dis. 2006;21:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Jenssen C, Mayr M, Nuernberg D, Faiss S. Complications of Endoscopic Ultrasound: Risk Assessment and Prevention. Endoscopic Ultrasound: An Introductory Manual and Atlas. Stuttgart, New York: Thieme 2011; 176-196. |
| 41. | O'Toole D, Palazzo L, Arotcarena R, Dancour A, Aubert A, Hammel P, Amaris J, Ruszniewski P. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:470-474. |
| 42. | Janssen J, König K, Knop-Hammad V, Johanns W, Greiner L. Frequency of bacteremia after linear EUS of the upper GI tract with and without FNA. Gastrointest Endosc. 2004;59:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Levy MJ, Norton ID, Wiersema MJ, Schwartz DA, Clain JE, Vazquez-Sequeiros E, Wilson WR, Zinsmeister AR, Jondal ML. Prospective risk assessment of bacteremia and other infectious complications in patients undergoing EUS-guided FNA. Gastrointest Endosc. 2003;57:672-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Koch M, Antolovic D, Kienle P, Horstmann J, Herfarth C, von Knebel Doeberitz M, Weitz J. Increased detection rate and potential prognostic impact of disseminated tumor cells in patients undergoing endorectal ultrasound for rectal cancer. Int J Colorectal Dis. 2007;22:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Al-Rashdan A, LeBlanc J, Sherman S, McHenry L, DeWitt J, Al-Haddad M. Role of endoscopic ultrasound for evaluating gastrointestinal tract disorders in pediatrics: a tertiary care center experience. J Pediatr Gastroenterol Nutr. 2010;51:718-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Cohen S, Kalinin M, Yaron A, Givony S, Reif S, Santo E. Endoscopic ultrasonography in pediatric patients with gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2008;46:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Attila T, Adler DG, Hilden K, Faigel DO. EUS in pediatric patients. Gastrointest Endosc. 2009;70:892-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Varadarajulu S, Wilcox CM, Eloubeidi MA. Impact of EUS in the evaluation of pancreaticobiliary disorders in children. Gastrointest Endosc. 2005;62:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Roseau G, Palazzo L, Dumontier I, Mougenot JF, Chaussade S, Navarro J, Couturier D. Endoscopic ultrasonography in the evaluation of pediatric digestive diseases: preliminary results. Endoscopy. 1998;30:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Attila T, Faigel DO. Endoscopic ultrasound in patients over 80 years old. Dig Dis Sci. 2011;56:3065-3071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Benson ME, Byrne S, Brust DJ, Manning B, Pfau PR, Frick TJ, Reichelderfer M, Gopal DV. EUS and ERCP complication rates are not increased in elderly patients. Dig Dis Sci. 2010;55:3278-3283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Williams DB, Sahai AV, Aabakken L, Penman ID, van Velse A, Webb J, Wilson M, Hoffman BJ, Hawes RH. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999;44:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 438] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 53. | Eloubeidi MA, Tamhane A, Varadarajulu S, Wilcox CM. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc. 2006;63:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 54. | Al-Haddad M, Wallace MB, Woodward TA, Gross SA, Hodgens CM, Toton RD, Raimondo M. The safety of fine-needle aspiration guided by endoscopic ultrasound: a prospective study. Endoscopy. 2008;40:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 55. | Eloubeidi MA, Tamhane A. Prospective assessment of diagnostic utility and complications of endoscopic ultrasound-guided fine needle aspiration. Results from a newly developed academic endoscopic ultrasound program. Dig Dis. 2008;26:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Thomas T, Kaye PV, Ragunath K, Aithal G. Efficacy, safety, and predictive factors for a positive yield of EUS-guided Trucut biopsy: a large tertiary referral center experience. Am J Gastroenterol. 2009;104:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 57. | Gerke H, Rizk MK, Vanderheyden AD, Jensen CS. Randomized study comparing endoscopic ultrasound-guided Trucut biopsy and fine needle aspiration with high suction. Cytopathology. 2010;21:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 739] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 59. | Buscarini E, De Angelis C, Arcidiacono PG, Rocca R, Lupinacci G, Manta R, Carucci P, Repici A, Carrara S, Vallisa D. Multicentre retrospective study on endoscopic ultrasound complications. Dig Liver Dis. 2006;38:762-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Eloubeidi MA, Chen VK, Eltoum IA, Jhala D, Chhieng DC, Jhala N, Vickers SM, Wilcox CM. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663-2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 284] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 61. | Wang KX, Ben QW, Jin ZD, Du YQ, Zou DW, Liao Z, Li ZS. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011;73:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 304] [Article Influence: 20.3] [Reference Citation Analysis (1)] |
| 62. | Barawi M, Gottlieb K, Cunha B, Portis M, Gress F. A prospective evaluation of the incidence of bacteremia associated with EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:189-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Chang KJ, Nguyen P, Erickson RA, Durbin TE, Katz KD. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 405] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 64. | Voss M, Hammel P, Molas G, Palazzo L, Dancour A, O'Toole D, Terris B, Degott C, Bernades P, Ruszniewski P. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 249] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 65. | Matsumoto K, Yamao K, Ohashi K, Watanabe Y, Sawaki A, Nakamura T, Matsuura A, Suzuki T, Fukutomi A, Baba T. Acute portal vein thrombosis after EUS-guided FNA of pancreatic cancer: case report. Gastrointest Endosc. 2003;57:269-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Eloubeidi MA, Wallace MB, Reed CE, Hadzijahic N, Lewin DN, Van Velse A, Leveen MB, Etemad B, Matsuda K, Patel RS. The utility of EUS and EUS-guided fine needle aspiration in detecting celiac lymph node metastasis in patients with esophageal cancer: a single-center experience. Gastrointest Endosc. 2001;54:714-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 67. | Will U, Meyer F, Bosseckert H. Successful endoscopic management of iatrogenic mediastinal infection and subsequent esophagomediastinal fistula, following endosonographically guided fine-needle aspiration biopsy. Endoscopy. 2005;37:88-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 68. | Pai KR, Page RD. Mediastinitis after EUS-guided FNA biopsy of a posterior mediastinal metastatic teratoma. Gastrointest Endosc. 2005;62:980-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 69. | Aerts JG, Kloover J, Los J, van der Heijden O, Janssens A, Tournoy KG. EUS-FNA of enlarged necrotic lymph nodes may cause infectious mediastinitis. J Thorac Oncol. 2008;3:1191-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 70. | Savides TJ, Margolis D, Richman KM, Singh V. Gemella morbillorum mediastinitis and osteomyelitis following transesophageal endoscopic ultrasound-guided fine-needle aspiration of a posterior mediastinal lymph node. Endoscopy. 2007;39 Suppl 1:E123-E124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 71. | Hirdes MM, Schwartz MP, Tytgat KM, Schlösser NJ, Sie-Go DM, Brink MA, Oldenburg B, Siersema PD, Vleggaar FP. Performance of EUS-FNA for mediastinal lymphadenopathy: impact on patient management and costs in low-volume EUS centers. Surg Endosc. 2010;24:2260-2267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 72. | von Bartheld MB, van Kralingen KW, Veenendaal RA, Willems LN, Rabe KF, Annema JT. Mediastinal-esophageal fistulae after EUS-FNA of tuberculosis of the mediastinum. Gastrointest Endosc. 2010;71:210-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | van Fraeyenhove F, Lamot C, Vogelaers D, Van Belle S, Cesmeli E, Rottey S. A rare infectious complication after endoscopic ultrasound guided fine needle aspiration in an oncological patient and review of the literature. Acta Clin Belg. 2009;64:147-149. [PubMed] |
| 74. | von Bartheld M, van der Heijden E, Annema J. Mediastinal abscess formation after EUS-guided FNA: are patients with sarcoidosis at increased risk? Gastrointest Endosc. 2012;75:1104-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Grandval P, Picon M, Coste P, Giovannini M, Thomas P, Lafon J. [Infection of submucosal tumor after endosonography-guided needle biopsy]. Gastroenterol Clin Biol. 1999;23:566-568. [PubMed] |
| 76. | DeWitt J, Al-Haddad M, Fogel E, Sherman S, LeBlanc JK, McHenry L, Schmidt CM. Endoscopic transduodenal drainage of an abscess arising after EUS-FNA of a duodenal GI stromal tumor. Gastrointest Endosc. 2009;70:185-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 77. | Polkowski M, Gerke W, Jarosz D, Nasierowska-Guttmejer A, Rutkowski P, Nowecki ZI, Ruka W, Regula J, Butruk E. Diagnostic yield and safety of endoscopic ultrasound-guided trucut [corrected] biopsy in patients with gastric submucosal tumors: a prospective study. Endoscopy. 2009;41:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 78. | Eckardt AJ, Adler A, Gomes EM, Jenssen C, Siebert C, Gottschalk U, Koch M, Röcken C, Rösch T. Endosonographic large-bore biopsy of gastric subepithelial tumors: A prospective multicentre study. Eur J Gastroenterol Hepatol. 2012;Jul 12; Epub ahead of print. [PubMed] |
| 79. | Faias S, Kaplan R, Hawes RH, Romagnuolo J, Paquin SC, Chong AK, Hunter BS, Hoffman BJ. Role of endoscopic ultrasound with guided fine needle aspiration (EUS-FNA) in the diagnosis of pelvic masses. Gastrointest Endosc. 2005;61:AB276. [DOI] [Full Text] |
| 80. | Mezzi G, Arcidiacono PG, Carrara S, Freschi M, Boemo C, Testoni PA. Complication after endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) of rectal lesion. Endoscopy. 2007;39 Suppl 1:E137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 81. | Levy MJ, Norton ID, Clain JE, Enders FB, Gleeson F, Limburg PJ, Nelson H, Rajan E, Topazian MD, Wang KK. Prospective study of bacteremia and complications With EUS FNA of rectal and perirectal lesions. Clin Gastroenterol Hepatol. 2007;5:684-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Sailer M, Bussen D, Fein M, Freys S, Debus SE, Thiede A, Fuchs KH. Endoscopic ultrasound-guided transrectal biopsies of pelvic tumors. J Gastrointest Surg. 2002;6:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 83. | Sasaki Y, Niwa Y, Hirooka Y, Ohmiya N, Itoh A, Ando N, Miyahara R, Furuta S, Goto H. The use of endoscopic ultrasound-guided fine-needle aspiration for investigation of submucosal and extrinsic masses of the colon and rectum. Endoscopy. 2005;37:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 84. | Gleeson FC, Clain JE, Rajan E, Topazian MD, Wang KK, Levy MJ. EUS-FNA assessment of extramesenteric lymph node status in primary rectal cancer. Gastrointest Endosc. 2011;74:897-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Knight CS, Eloubeidi MA, Crowe R, Jhala NC, Jhala DN, Chhieng DC, Eltoum IA. Utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of colorectal carcinoma. Diagn Cytopathol. 2011;Sep 19; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 86. | Guarner-Argente C, Shah P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: a retrospective, comparative analysis. Gastrointest Endosc. 2011;74:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 87. | Lee LS, Saltzman JR, Bounds BC, Poneros JM, Brugge WR, Thompson CC. EUS-guided fine needle aspiration of pancreatic cysts: a retrospective analysis of complications and their predictors. Clin Gastroenterol Hepatol. 2005;3:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 88. | Adler DG, Jacobson BC, Davila RE, Hirota WK, Leighton JA, Qureshi WA, Rajan E, Zuckerman MJ, Fanelli RD, Baron TH. ASGE guideline: complications of EUS. Gastrointest Endosc. 2005;61:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 89. | Sing J, Erickson R, Fader R. An in vitro analysis of microbial transmission during EUS-guided FNA and the utility of sterilization agents. Gastrointest Endosc. 2006;64:774-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 90. | Ribet ME, Copin MC, Gosselin B. Bronchogenic cysts of the mediastinum. J Thorac Cardiovasc Surg. 1995;109:1003-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 114] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 91. | Fazel A, Moezardalan K, Varadarajulu S, Draganov P, Eloubeidi MA. The utility and the safety of EUS-guided FNA in the evaluation of duplication cysts. Gastrointest Endosc. 2005;62:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Westerterp M, van den Berg JG, van Lanschot JJ, Fockens P. Intramural bronchogenic cysts mimicking solid tumors. Endoscopy. 2004;36:1119-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 93. | Wildi SM, Hoda RS, Fickling W, Schmulewitz N, Varadarajulu S, Roberts SS, Ferguson B, Hoffman BJ, Hawes RH, Wallace MB. Diagnosis of benign cysts of the mediastinum: the role and risks of EUS and FNA. Gastrointest Endosc. 2003;58:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 94. | Eloubeidi MA, Cohn M, Cerfolio RJ, Chhieng DC, Jhala N, Jhala D, Eltoum IA. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of foregut duplication cysts: the value of demonstrating detached ciliary tufts in cyst fluid. Cancer. 2004;102:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 95. | Annema JT, Veseliç M, Versteegh MI, Rabe KF. Mediastinitis caused by EUS-FNA of a bronchogenic cyst. Endoscopy. 2003;35:791-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 96. | Varadarajulu S, Fraig M, Schmulewitz N, Roberts S, Wildi S, Hawes RH, Hoffman BJ, Wallace MB. Comparison of EUS-guided 19-gauge Trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004;36:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 97. | Diehl DL, Cheruvattath R, Facktor MA, Go BD. Infection after endoscopic ultrasound-guided aspiration of mediastinal cysts. Interact Cardiovasc Thorac Surg. 2010;10:338-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 98. | Ryan AG, Zamvar V, Roberts SA. Iatrogenic candidal infection of a mediastinal foregut cyst following endoscopic ultrasound-guided fine-needle aspiration. Endoscopy. 2002;34:838-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 99. | Mohamadnejad M, Al-Haddad MA, Sherman S, McHenry L, Leblanc JK, DeWitt J. Utility of EUS-guided biopsy of extramural pelvic masses. Gastrointest Endosc. 2012;75:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 100. | Kaushik N, Khalid A, Brody D, McGrath K. EUS-guided paracentesis for the diagnosis of malignant ascites. Gastrointest Endosc. 2006;64:908-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 101. | DeWitt J, LeBlanc J, McHenry L, McGreevy K, Sherman S. Endoscopic ultrasound-guided fine-needle aspiration of ascites. Clin Gastroenterol Hepatol. 2007;5:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 102. | Nguyen PT, Chang KJ. EUS in the detection of ascites and EUS-guided paracentesis. Gastrointest Endosc. 2001;54:336-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 103. | Peter S, Eltoum I, Eloubeidi MA. EUS-guided FNA of peritoneal carcinomatosis in patients with unknown primary malignancy. Gastrointest Endosc. 2009;70:1266-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 104. | Rana SS, Bhasin DK, Srinivasan R, Singh K. Endoscopic ultrasound-guided fine needle aspiration of peritoneal nodules in patients with ascites of unknown cause. Endoscopy. 2011;43:1010-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 105. | Chen HY, Lee CH, Hsieh CH. Bile peritonitis after EUS-guided fine-needle aspiration. Gastrointest Endosc. 2002;56:594-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 106. | Jacobson BC, Waxman I, Parmar K, Kauffman JM, Clarke GA, Van Dam J. Endoscopic ultrasound-guided gallbladder bile aspiration in idiopathic pancreatitis carries a significant risk of bile peritonitis. Pancreatology. 2002;2:26-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 107. | Wu LM, Jiang XX, Gu HY, Xu X, Zhang W, Lin LH, Deng X, Yin Y, Xu JR. Endoscopic ultrasound-guided fine-needle aspiration biopsy in the evaluation of bile duct strictures and gallbladder masses: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2011;23:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 108. | tenBerge J, Hoffman BJ, Hawes RH, Van Enckevort C, Giovannini M, Erickson RA, Catalano MF, Fogel R, Mallery S, Faigel DO. EUS-guided fine needle aspiration of the liver: indications, yield, and safety based on an international survey of 167 cases. Gastrointest Endosc. 2002;55:859-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 109. | Gaylord KM, Nawras A. Incidence of biliary stent migration as a direct complication of performing endoscopic ultrasound. Gastrointest Endosc. 2005;61:AB279. [DOI] [Full Text] |
| 110. | Fernández-Esparrach G, Ginès A, García P, Pellisé M, Solé M, Cortés P, Gimeno-García AZ, Sendino O, Navarro S, Llach J. Incidence and clinical significance of hyperamylasemia after endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) of pancreatic lesions: a prospective and controlled study. Endoscopy. 2007;39:720-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Gress F, Michael H, Gelrud D, Patel P, Gottlieb K, Singh F, Grendell J. EUS-guided fine-needle aspiration of the pancreas: evaluation of pancreatitis as a complication. Gastrointest Endosc. 2002;56:864-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 112. | Beane JD, House MG, Coté GA, DeWitt JM, Al-Haddad M, LeBlanc JK, McHenry L, Sherman S, Schmidt CM, Zyromski NJ. Outcomes after preoperative endoscopic ultrasonography and biopsy in patients undergoing distal pancreatectomy. Surgery. 2011;150:844-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 113. | Yusuf TE, Ho S, Pavey DA, Michael H, Gress FG. Retrospective analysis of the utility of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in pancreatic masses, using a 22-gauge or 25-gauge needle system: a multicenter experience. Endoscopy. 2009;41:445-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 114. | Eloubeidi MA, Gress FG, Savides TJ, Wiersema MJ, Kochman ML, Ahmad NA, Ginsberg GG, Erickson RA, Dewitt J, Van Dam J. Acute pancreatitis after EUS-guided FNA of solid pancreatic masses: a pooled analysis from EUS centers in the United States. Gastrointest Endosc. 2004;60:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 115. | Hollerbach S, Klamann A, Topalidis T, Schmiegel WH. Endoscopic ultrasonography (EUS) and fine-needle aspiration (FNA) cytology for diagnosis of chronic pancreatitis. Endoscopy. 2001;33:824-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 116. | DeWitt J, McGreevy K, LeBlanc J, McHenry L, Cummings O, Sherman S. EUS-guided Trucut biopsy of suspected nonfocal chronic pancreatitis. Gastrointest Endosc. 2005;62:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 117. | Babich JP, Bonasera RJ, Klein J, Friedel DM. Pancreatic ascites: complication after endoscopic ultrasound-guided fine needle aspiration of a pancreatic cyst. Endoscopy. 2009;41 Suppl 2:E211-E212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 118. | Reddymasu S, Oropeza-Vail MM, Williamson S, Jafri F, Olyaee M. Pancreatic leak after endoscopic ultrasound guided fine needle aspiration managed by transpapillary pancreatic duct stenting. JOP. 2011;12:489-490. [PubMed] |
| 119. | Affi A, Vazquez-Sequeiros E, Norton ID, Clain JE, Wiersema MJ. Acute extraluminal hemorrhage associated with EUS-guided fine needle aspiration: frequency and clinical significance. Gastrointest Endosc. 2001;53:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 120. | Varadarajulu S, Eloubeidi MA. Frequency and significance of acute intracystic hemorrhage during EUS-FNA of cystic lesions of the pancreas. Gastrointest Endosc. 2004;60:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 121. | Kien-Fong Vu C, Chang F, Doig L, Meenan J. A prospective control study of the safety and cellular yield of EUS-guided FNA or Trucut biopsy in patients taking aspirin, nonsteroidal anti-inflammatory drugs, or prophylactic low molecular weight heparin. Gastrointest Endosc. 2006;63:808-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 122. | Ernst A, Eberhardt R, Wahidi M, Becker HD, Herth FJ. Effect of routine clopidogrel use on bleeding complications after transbronchial biopsy in humans. Chest. 2006;129:734-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 123. | Singh P, Gelrud A, Schmulewitz N, Chauhan S. Hemosuccus pancreaticus after EUS-FNA of pancreatic cyst (with video). Gastrointest Endosc. 2008;67:543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 124. | Haseganu LE, Diehl DL. Left adrenal gland hemorrhage as a complication of EUS-FNA. Gastrointest Endosc. 2009;69:e51-e52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 125. | Carrara S, Arcidiacono PG, Giussani A, Testoni PA. Acute hemorrhage with retroperitoneal hematoma after endoscopic ultrasound-guided fine-needle aspiration of an intraductal papillary mucinous neoplasm of the pancreas. Am J Gastroenterol. 2009;104:1610-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 126. | Lim WC, Leblanc JK. Retroperitoneal bleeding after EUS-guided FNA of a pancreatic mass. Gastrointest Endosc. 2006;63:499-500; discussion 500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 127. | Siddiqui A, Burdick S, Yang K, Cryer B. Acute mesenteric hemorrhage associated with EUS-guided fine needle aspiration. J Clin Gastroenterol. 2007;41:722-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 128. | Inoue H, Mizuno N, Sawaki A, Takahashi K, Aoki M, Bhatia V, Matuura K, Tabata T, Yamao K. Life-threatening delayed-onset bleeding after endoscopic ultrasound-guided 19-gauge Trucut needle biopsy of a gastric stromal tumor. Endoscopy. 2006;38 Suppl 2:E38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 129. | Gress FG, Hawes RH, Savides TJ, Ikenberry SO, Lehman GA. Endoscopic ultrasound-guided fine-needle aspiration biopsy using linear array and radial scanning endosonography. Gastrointest Endosc. 1997;45:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 263] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 130. | Sendino O, Fernández-Esparrach G, Solé M, Colomo L, Pellisé M, Llach J, Navarro S, Bordas JM, Ginès A. Endoscopic ultrasonography-guided brushing increases cellular diagnosis of pancreatic cysts: A prospective study. Dig Liver Dis. 2010;42:877-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 131. | Andrews AH, Horwhat JD. Massive pneumoperitoneum after EUS-FNA aspiration of the pancreas. Gastrointest Endosc. 2006;63:876-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 132. | Larino-Noia J, Iglesias-Garcia J, Seijo-Rios S, Vilario-Insua M, Sobrino-Faya M, Dominguez-Muoz JE. Assessment of EUS and EUS-guided FNA complications in a large cohort of patients [abstract]. Endoscopy. 2006;39 Suppl 1:39-FR46. |
| 133. | Jenssen C, Dietrich CF. Kontraindikationen, Komplikationen, Komplikationsmanagement. Interventioneller Ultraschall: Lehrbuch und Atlas fr die Interventionelle Sonografie. Stuttgart, New York: Thieme 2011; . |
| 134. | Kosugi C, Furuse J, Ishii H, Maru Y, Yoshino M, Kinoshita T, Konishi M, Nakagohri T, Inoue K, Oda T. Needle tract implantation of hepatocellular carcinoma and pancreatic carcinoma after ultrasound-guided percutaneous puncture: clinical and pathologic characteristics and the treatment of needle tract implantation. World J Surg. 2004;28:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 135. | Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007;33:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 222] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 136. | Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 381] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 137. | Paquin SC, Gariépy G, Lepanto L, Bourdages R, Raymond G, Sahai AV. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005;61:610-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 138. | Chong A, Venugopal K, Segarajasingam D, Lisewski D. Tumor seeding after EUS-guided FNA of pancreatic tail neoplasia. Gastrointest Endosc. 2011;74:933-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 139. | Ahmed K, Sussman JJ, Wang J, Schmulewitz N. A case of EUS-guided FNA-related pancreatic cancer metastasis to the stomach. Gastrointest Endosc. 2011;74:231-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 140. | Shah JN, Fraker D, Guerry D, Feldman M, Kochman ML. Melanoma seeding of an EUS-guided fine needle track. Gastrointest Endosc. 2004;59:923-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 141. | Doi S, Yasuda I, Iwashita T, Ibuka T, Fukushima H, Araki H, Hirose Y, Moriwaki H. Needle tract implantation on the esophageal wall after EUS-guided FNA of metastatic mediastinal lymphadenopathy. Gastrointest Endosc. 2008;67:988-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 142. | Hirooka Y, Goto H, Itoh A, Hashimoto S, Niwa K, Ishikawa H, Okada N, Itoh T, Kawashima H. Case of intraductal papillary mucinous tumor in which endosonography-guided fine-needle aspiration biopsy caused dissemination. J Gastroenterol Hepatol. 2003;18:1323-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 143. | Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 279] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 144. | Levy MJ, Gleeson FC, Campion MB, Caudill JL, Clain JE, Halling K, Rajan E, Topazian MD, Wang KK, Wiersema MJ. Prospective cytological assessment of gastrointestinal luminal fluid acquired during EUS: a potential source of false-positive FNA and needle tract seeding. Am J Gastroenterol. 2010;105:1311-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 145. | Eloubeidi MA, Varadarajulu S, Desai S, Shirley R, Heslin MJ, Mehra M, Arnoletti JP, Eltoum I, Wilcox CM, Vickers SM. A prospective evaluation of an algorithm incorporating routine preoperative endoscopic ultrasound-guided fine needle aspiration in suspected pancreatic cancer. J Gastrointest Surg. 2007;11:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 146. | Lachter J, Rosenthal Y, Kluger Y. A multidisciplinary survey on controversies in the use of EUS-guided FNA: assessing perspectives of surgeons, oncologists and gastroenterologists. BMC Gastroenterol. 2011;11:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 147. | Mizuno N, Hara K, Hijioka S, Bhatia V, Shimizu Y, Yatabe Y, Yamao K. Current concept of endoscopic ultrasound-guided fine needle aspiration for pancreatic cancer. Pancreatology. 2011;11 Suppl 2:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 148. | Volmar KE, Vollmer RT, Jowell PS, Nelson RC, Xie HB. Pancreatic FNA in 1000 cases: a comparison of imaging modalities. Gastrointest Endosc. 2005;61:854-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 149. | Annema JT, Versteegh MI, Veseliç M, Welker L, Mauad T, Sont JK, Willems LN, Rabe KF. Endoscopic ultrasound added to mediastinoscopy for preoperative staging of patients with lung cancer. JAMA. 2005;294:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 150. | Ardengh JC, de Paulo GA, Ferrari AP. EUS-guided FNA in the diagnosis of pancreatic neuroendocrine tumors before surgery. Gastrointest Endosc. 2004;60:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 151. | Anand D, Barroeta JE, Gupta PK, Kochman M, Baloch ZW. Endoscopic ultrasound guided fine needle aspiration of non-pancreatic lesions: an institutional experience. J Clin Pathol. 2007;60:1254-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 152. | Vander Noot MR, Eloubeidi MA, Chen VK, Eltoum I, Jhala D, Jhala N, Syed S, Chhieng DC. Diagnosis of gastrointestinal tract lesions by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer. 2004;102:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 153. | Fritscher-Ravens A, Sriram PV, Schröder S, Topalidis T, Bohnacker S, Soehendra N. Stromal tumor as a pitfall in EUS-guided fine-needle aspiration cytology. Gastrointest Endosc. 2000;51:746-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 154. | Schwartz DA, Unni KK, Levy MJ, Clain JE, Wiersema MJ. The rate of false-positive results with EUS-guided fine-needle aspiration. Gastrointest Endosc. 2002;56:868-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 155. | Ando N, Goto H, Niwa Y, Hirooka Y, Ohmiya N, Nagasaka T, Hayakawa T. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 233] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 156. | Caddy G, Conron M, Wright G, Desmond P, Hart D, Chen RY. The accuracy of EUS-FNA in assessing mediastinal lymphadenopathy and staging patients with NSCLC. Eur Respir J. 2005;25:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 157. | Fritscher-Ravens A, Bohuslavizki KH, Brandt L, Bobrowski C, Lund C, Knöfel WT, Pforte A. Mediastinal lymph node involvement in potentially resectable lung cancer: comparison of CT, positron emission tomography, and endoscopic ultrasonography with and without fine-needle aspiration. Chest. 2003;123:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 158. | Okubo K, Yamao K, Nakamura T, Tajika M, Sawaki A, Hara K, Kawai H, Yamamura Y, Mochizuki Y, Koshikawa T. Endoscopic ultrasound-guided fine-needle aspiration biopsy for the diagnosis of gastrointestinal stromal tumors in the stomach. J Gastroenterol. 2004;39:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 159. | Gleeson FC, Kipp BR, Caudill JL, Clain JE, Clayton AC, Halling KC, Henry MR, Rajan E, Topazian MD, Wang KK. False positive endoscopic ultrasound fine needle aspiration cytology: incidence and risk factors. Gut. 2010;59:586-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 160. | Siddiqui AA, Kowalski TE, Shahid H, O'Donnell S, Tolin J, Loren DE, Infantolino A, Hong SK, Eloubeidi MA. False-positive EUS-guided FNA cytology for solid pancreatic lesions. Gastrointest Endosc. 2011;74:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 161. | van Hemel BM, Lamprou AA, Weersma R, Plukker JT, Suurmeijer AJ, van Dullemen HM. Procedure-related, false-positive cytology results during EUS-guided FNA in patients with esophageal cancer. Gastrointest Endosc. 2010;71:1130-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 162. | Fusaroli P, Manta R, Fedeli P, Maltoni S, Grillo A, Giovannini E, Bucchi L, Caletti G. The influence of endoscopic biliary stents on the accuracy of endoscopic ultrasound for pancreatic head cancer staging. Endoscopy. 2007;39:813-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 163. | Ascunce G, Ribeiro A, Rocha-Lima C, Larsen M, Sleeman D, Merchan J, Szabo D, Levi JU. Single-session endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography for evaluation of pancreaticobiliary disorders. Surg Endosc. 2010;24:1447-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 164. | Aslanian HR, Estrada JD, Rossi F, Dziura J, Jamidar PA, Siddiqui UD. Endoscopic ultrasound and endoscopic retrograde cholangiopancreatography for obstructing pancreas head masses: combined or separate procedures? J Clin Gastroenterol. 2011;45:711-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 165. | Che K, Muckova N, Olafsson S, Srikureja W. Safety of same-day endoscopic ultrasound and endoscopic retrograde cholangiopancreatography under conscious sedation. World J Gastroenterol. 2010;16:3287-3291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 166. | Ross WA, Wasan SM, Evans DB, Wolff RA, Trapani LV, Staerkel GA, Prindiville T, Lee JH. Combined EUS with FNA and ERCP for the evaluation of patients with obstructive jaundice from presumed pancreatic malignancy. Gastrointest Endosc. 2008;68:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 167. | Vila JJ, Kutz M, Goñi S, Ostiz M, Amorena E, Prieto C, Rodriguez C, Fernández-Urien I, Jiménez FJ. Endoscopic and anesthetic feasibility of EUS and ERCP combined in a single session versus two different sessions. World J Gastrointest Endosc. 2011;3:57-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 168. | Tarantino I, Barresi L, Di Pisa M, Traina M. Simultaneous endoscopic ultrasound fine needle aspiration and endoscopic retrograde cholangio-pancreatography: Evaluation of safety. World J Gastroenterol. 2007;13:3861-3863. [PubMed] |
| 169. | Fisher JM, Gordon SR, Gardner TB. The impact of prior biliary stenting on the accuracy and complication rate of endoscopic ultrasound fine-needle aspiration for diagnosing pancreatic adenocarcinoma. Pancreas. 2011;40:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 170. | Siddiqui UD, Rossi F, Rosenthal LS, Padda MS, Murali-Dharan V, Aslanian HR. EUS-guided FNA of solid pancreatic masses: a prospective, randomized trial comparing 22-gauge and 25-gauge needles. Gastrointest Endosc. 2009;70:1093-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 171. | Sakamoto H, Kitano M, Komaki T, Noda K, Chikugo T, Dote K, Takeyama Y, Das K, Yamao K, Kudo M. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 172. | Al-Haddad M, Gill KR, Raimondo M, Woodward TA, Krishna M, Crook JE, Skarvinko LN, Jamil LH, Hasan M, Wallace MB. Safety and efficacy of cytology brushings versus standard fine-needle aspiration in evaluating cystic pancreatic lesions: a controlled study. Endoscopy. 2010;42:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 173. | de Jong K, Poley JW, van Hooft JE, Visser M, Bruno MJ, Fockens P. Endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions provides inadequate material for cytology and laboratory analysis: initial results from a prospective study. Endoscopy. 2011;43:585-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 174. | Dewitt J, McGreevy K, Sherman S, Imperiale TF. Nurse-administered propofol sedation compared with midazolam and meperidine for EUS: a prospective, randomized trial. Gastrointest Endosc. 2008;68:499-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 175. | Nayar DS, Guthrie WG, Goodman A, Lee Y, Feuerman M, Scheinberg L, Gress FG. Comparison of propofol deep sedation versus moderate sedation during endosonography. Dig Dis Sci. 2010;55:2537-2544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 176. | Pagano N, Arosio M, Romeo F, Rando G, Del Conte G, Carlino A, Strangio G, Vitetta E, Malesci A, Repici A. Balanced Propofol Sedation in Patients Undergoing EUS-FNA: A Pilot Study to Assess Feasibility and Safety. Diagn Ther Endosc. 2011;2011:542159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 177. | Trummel JM, Surgenor SD, Cravero JP, Gordon SR, Blike GT. Comparison of differing sedation practice for upper endoscopic ultrasound using expert observational analysis of the procedural sedation. J Patient Saf. 2009;5:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 178. | Eisen GM, Dominitz JA, Faigel DO, Goldstein JA, Petersen BT, Raddawi HM, Ryan ME, Vargo JJ, Young HS, Wheeler-Harbaugh J. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastrointest Endosc. 2001;54:811-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 153] [Article Influence: 6.1] [Reference Citation Analysis (3)] |
| 179. | Van Dam J, Brady PG, Freeman M, Gress F, Gross GW, Hassall E, Hawes R, Jacobsen NA, Liddle RA, Ligresti RJ. Guidelines for training in electronic ultrasound: guidelines for clinical application. From the ASGE. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc. 1999;49:829-833. [PubMed] |
| 180. | Polkowski M, Larghi A, Weynand B, Boustière C, Giovannini M, Pujol B, Dumonceau JM. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 181. | Wehrmann T, Riphaus A. Sedation with propofol for interventional endoscopic procedures: a risk factor analysis. Scand J Gastroenterol. 2008;43:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 182. | Riphaus A, Wehrmann T, Weber B, Arnold J, Beilenhoff U, Bitter H, von Delius S, Domagk D, Ehlers AF, Faiss S. [S3-guidelines--sedation in gastrointestinal endoscopy]. Z Gastroenterol. 2008;46:1298-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 183. | Dumonceau JM, Riphaus A, Aparicio JR, Beilenhoff U, Knape JT, Ortmann M, Paspatis G, Ponsioen CY, Racz I, Schreiber F. European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Guideline: Non-anesthesiologist administration of propofol for GI endoscopy. Endoscopy. 2010;42:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 184. | von Bartheld MB, Rabe KF, Annema JT. Transaortic EUS-guided FNA in the diagnosis of lung tumors and lymph nodes. Gastrointest Endosc. 2009;69:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 185. | Wallace MB, Woodward TA, Raimondo M, Al-Haddad M, Odell JA. Transaortic fine-needle aspiration of centrally located lung cancer under endoscopic ultrasound guidance: the final frontier. Ann Thorac Surg. 2007;84:1019-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 186. | Fritscher-Ravens A, Ganbari A, Mosse CA, Swain P, Koehler P, Patel K. Transesophageal endoscopic ultrasound-guided access to the heart. Endoscopy. 2007;39:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 187. | Jenssen C, Siebert C, Bartho S. [Leiomyosarcoma of the inferior vena cava. Diagnosis using endoscopic ultrasound-guided fine-needle aspiration biopsy]. Dtsch Med Wochenschr. 2008;133:769-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 188. | Lai R, Stephens V, Bardales R. Diagnosis and staging of hepatocellular carcinoma by EUS-FNA of a portal vein thrombus. Gastrointest Endosc. 2004;59:574-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 189. | Boustière C, Veitch A, Vanbiervliet G, Bulois P, Deprez P, Laquiere A, Laugier R, Lesur G, Mosler P, Nalet B. Endoscopy and antiplatelet agents. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2011;43:445-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 190. | Veitch AM, Baglin TP, Gershlick AH, Harnden SM, Tighe R, Cairns S. Guidelines for the management of anticoagulant and antiplatelet therapy in patients undergoing endoscopic procedures. Gut. 2008;57:1322-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 191. | Dietrich CF, Ignee A, Braden B, Barreiros AP, Ott M, Hocke M. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin Gastroenterol Hepatol. 2008;6:590-597.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 192. | Hocke M, Schmidt C, Zimmer B, Topalidis T, Dietrich CF, Stallmach A. [Contrast enhanced endosonography for improving differential diagnosis between chronic pancreatitis and pancreatic cancer]. Dtsch Med Wochenschr. 2008;133:1888-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 193. | Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, Kamata K, Imai H, Chiba Y, Okada M. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 194. | Matsubara H, Itoh A, Kawashima H, Kasugai T, Ohno E, Ishikawa T, Itoh Y, Nakamura Y, Hiramatsu T, Nakamura M. Dynamic quantitative evaluation of contrast-enhanced endoscopic ultrasonography in the diagnosis of pancreatic diseases. Pancreas. 2011;40:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 195. | Napoleon B, Alvarez-Sanchez MV, Gincoul R, Pujol B, Lefort C, Lepilliez V, Labadie M, Souquet JC, Queneau PE, Scoazec JY. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: results of a pilot study. Endoscopy. 2010;42:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 196. | Săftoiu A, Iordache SA, Gheonea DI, Popescu C, Maloş A, Gorunescu F, Ciurea T, Iordache A, Popescu GL, Manea CT. Combined contrast-enhanced power Doppler and real-time sonoelastography performed during EUS, used in the differential diagnosis of focal pancreatic masses (with videos). Gastrointest Endosc. 2010;72:739-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 197. | Săftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, Iglesias-Garcia J, Arcidiacono P, Will U, Giovannini M. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: a multicenter study. Endoscopy. 2011;43:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 198. | Itoi T, Itokawa F, Sofuni A, Nakamura K, Tsuchida A, Yamao K, Kawai T, Moriyasu F. Puncture of solid pancreatic tumors guided by endoscopic ultrasonography: a pilot study series comparing Trucut and 19-gauge and 22-gauge aspiration needles. Endoscopy. 2005;37:362-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 199. | Iglesias-Garcia J, Poley JW, Larghi A, Giovannini M, Petrone MC, Abdulkader I, Monges G, Costamagna G, Arcidiacono P, Biermann K. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 200. | Schwartz DA, Harewood GC, Wiersema MJ. EUS for rectal disease. Gastrointest Endosc. 2002;56:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 201. | Jacobson BC, Chak A, Hoffman B, Baron TH, Cohen J, Deal SE, Mergener K, Petersen BT, Petrini JL, Safdi MA. Quality indicators for endoscopic ultrasonography. Am J Gastroenterol. 2006;101:898-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Peer reviewers: Adrian Saftoiu, MD, PhD, Professor, Research Center of Gastroenterology and Hepatology Craiova, University of Medicine and Pharmacy Craiova, 2 Petru Rares str., 200349 Craiova, Romania; Dr. Massimo Raimondo, Division of Gastroenterology and Hepatology, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224, United States; Dr. Hyoung-Chul Oh, Assistant Professor, Department of Internal Medicine, College of Medicine, Chung-Ang University, No. 65-207 Hanganro-3ga Yongsan-gu, Seoul 140757, South Korea
S- Editor Lv S L- Editor A E- Editor Xiong L
.