Published online Sep 7, 2012. doi: 10.3748/wjg.v18.i33.4618
Revised: March 27, 2012
Accepted: May 26, 2012
Published online: September 7, 2012
AIM: To investigate whether the 7-difluoromethoxyl-5, 4’-di-n-octylgenistein (DFOG), a novel synthetic genistein analogue, affects the growth of gastric cancer cells and its mechanisms.
METHODS: A series of genistein analogues were prepared by difluoromethylation and alkylation, and human gastric cancer cell lines AGS and SGC-7901 cultured in vitro were treated with various concentrations of genistein and genistein analogues. The cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The cells were incubated by DFOG at different concentrations. The growth inhibitory effects were evaluated using MTT and clonogenic assay. The distribution of the phase in cell cycle was analyzed using flow cytometric analysis with propidium iodide staining. The expression of the transcription factor forkhead box M1 (FOXM1) was analyzed by reverse transcription-polymerase chain reaction and Western blotting. The expression levels of CDK1, Cdc25B, cyclin B and p27KIP1 protein were detected using Western blotting.
RESULTS: Nine of the genistein analogues had more effective antitumor activity than genistein. Among the tested analogues, DFOG possessed the strongest activity against AGS and SGC-7901 cells in vitro. DFOG significantly inhibited the cell viability and colony formation of AGS and SGC-7901 cells. Moreover, DFOG efficaciously arrested the cell cycle in G2/M phase. DFOG decreased the expression of FOXM1 and its downstream genes, such as CDK1, Cdc25B, cyclin B, and increased p27KIP1 at protein levels. Knockdown of FOXM1 by small interfering RNA before DFOG treatment resulted in enhanced cell growth inhibition in AGS cells. Up-regulation of FOXM1 by cDNA transfection attenuated DFOG-induced cell growth inhibition in AGS cells.
CONCLUSION: DFOG inhibits the growth of human gastric cancer cells by down-regulating the FOXM1 expression.
- Citation: Xiang HL, Liu F, Quan MF, Cao JG, Lv Y. 7-difluoromethoxyl-5,4’-di-n-octylgenistein inhibits growth of gastric cancer cells through downregulating forkhead box M1. World J Gastroenterol 2012; 18(33): 4618-4626
- URL: https://www.wjgnet.com/1007-9327/full/v18/i33/4618.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i33.4618
Genistein, 5,7,4’-trihydroxylisoflavone, as one of the active constituents of soybean products, has been reported to possess anti-cancer activities[1,2]. But probably because of its low solubility in both water and organic solvent, genistein has a very low bioavailability. The introduction of the fluorine or fluoride-bearing alkyl such as CF3 or HCF2 into organic molecules dramatically changes their physiological, physical and chemical properties[3]. To our best knowledge, the introduction of fluorine moiety into the aryl part of the flavonoid molecule can enhance their biological activities, including anti-bacterial, anti-fungal and anti-viral activities[3]. Fluorinated 3, 4-dihydroxychalcones have illustrated interesting biological activities, including anti-peroxidation and antitumor activity in vitro[4]. We have previously reported that introduction of CF3 or HCF2 group into chrysin (5,7-dihydroxylflavone) molecule can improve their anticancer activities[5-7]. Recently, we synthesized a series of difluoromethoxylated genistein analogues and determined their protective effects against vascular endothelial cells[8]. However, there are few studies which reported the anti-cancer effect of fluorinated genistein analogues.
Gastric cancer is one of the most common malignancies in the world and its incidence and mortality rank first in China[9]. Recent data indicate that the mortality of gastric cancer in China tends to increase and it severely threatens the health and life of people[10]. At present, surgery and chemotherapy remain as the main modalities in the management of gastric cancer, but the curative effect of the existing chemotherapeutic drugs is not satisfactory, which cause numerous side effects. Therefore, it has been a focus to search for new drugs capable of preventing and treating gastric cancer and other malignancies. Gastric cancer has been shown to have activated forkhead box protein M1 (FOXM1) signaling pathway[11]. The FOXM1 belongs to a family of evolutionary conserved transcriptional regulators that were characterized by the presence of a DNA-binding domain called the forkhead box or winged helix domain[12]. It has been shown that FOXM1 signaling plays an important role in cellular developmental pathways, and activation of FOXM1 signaling is associated with carcinogenesis[13]. FOXM1 signaling is frequently up-regulated in cancers, including lung, breast, pancreatic and gastric cancer[11,14-16]. Moreover, FOXM1 has been shown to regulate transcription of cell cycle genes, including Cdc25B, CDK1, cyclin B and p27KIP1[13,17]. Recently, it has been reported that FOXM1 expression could serve as an independent predictor of a poor survival of gastric cancer patients[11]. Studies by Wang et al[18] have shown that genistein may inhibit FOXM1 activation in pancreatic cancer cells, leading to apoptotic cell death. Therefore, it is believed that the targeted inactivation of FOXM1 could represent a promising strategy for the development of novel selective anti-cancer therapies.
In the present study, we investigated whether the growth inhibitory effects of genistein and the novel synthetic genistein analogue 7-difluoromethoxyl-5,4’-di-n-octylgenistein (DFOG) on gastric cancer cells could be attributed to modulation of FOXM1 activity. We found that DFOG and genistein down-regulated the FOXM1 expression and its downstream genes, including cdc25B, CDK1, cyclin B and up-regulated p27KIP1, resulting in the growth inhibition of gastric cancer cells. These results provide strong evidences to support that FOXM1 is a rational target in gastric cancer, and the targeted inactivation of FOXM1, especially by genistein and its analogue DFOG, may provide new insight into the strategy development for better prevention of tumor progression and/or treatment of gastric cancer.
Human gastric cancer cell lines AGS and SGC-7901 were purchased from China Center for Type Culture Collection (CCTCC, Wuhan, China). Cells were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA, United States) supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, 100 mg/L penicillin, and 100 mg/L streptomycin, and incubated in a humidified atmosphere of 5% CO2 at 37 °C. The difluoromethoxylated genistein analogues 2,4a-4h were prepared by the method described elsewhere[8] (Table 1). Primary antibodies for FOXM1, CDK1, cyclin B, p27KIP1 and Cdc25B were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, United States). Anti-β-actin antibody and Horseradish peroxidase-conjugated rabbit anti-mouse secondary antibody were purchased from Santa Cruz Biotechnology. Lipofectamine 2000 was purchased from Invitrogen. Protease inhibitor cocktail, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and all other chemicals were obtained from Sigma (St. Louis, MO, United States). Genistein (Sigma) and the difluoromethoxylated genistein analogues were dissolved in dimethyl sulfoxide (DMSO) to make a 10 mmol stock solution and were added directly to the media at different concentrations before use.
| Compound | R1 | R2 | |
| 1 | Genistein (5,7,4’-trihydroxylisoflavone) | H | H |
| 2 | 7-difluoromethyl genistein | CHF2 | H |
| 4a | 7-difluoromethyl-5,4’-dimethyl genistein | CHF2 | CH3 |
| 4b | 7-difluoromethyl-5,4’-diethyl genistein | CHF2 | CH3CH2 |
| 4c | 7-difluoromethyl-5,4’-di-n-propyl genistein | CHF2 | n-C3H7 |
| 4d | 7-difluoromethyl-5,4’-di-benzyl genistein | CHF2 | C6H5CH2 |
| 4e | 7-difluoromethyl-5,4’-diheptyl genistein | CHF2 | n-C7H15 |
| 4f | 7-difluoromethyl-5,4’-di-n-octyl genistein | CHF2 | n-C8H17 |
| 4g | 7-difluoromethyl-5,4’-didecyl genistein | CHF2 | n-C10H21 |
| 4h | 7-difluoromethyl-5,4’-diisobutyl genistein | CHF2 | iso-C4H9 |
Cells were seeded in a 96-well plate at a density of 5000 cells/well as described previously[19]. After incubation for 24 h to allow cell attachment, different concentrations of genistein and genistein analogues (0.1, 0.3, 1.0, 3.0, 10.0 and 30.0 μmol/L) were added to each well and cultured for 24 h. Medium was removed and then incubated with 5.0 mg/mL MTT for 4 h. Then supernatant was removed after centrifugation. Finally, 100 μL of DMSO was added and absorbance at 570 nm wavelength (A570) was measured by means of an Enzyme-labeling instrument (ELX-800 type; Bio-Tek, Shanghai, China). Relative cell proliferation inhibition rate = (1 - average A570 of the experimental group/average A570 of the control group) × 100%. The IC50 (defined as the drug concentration which 50% cell viability was inhibited) was assessed from the dose-response curves using GraphPad Prism program (Version 4, GraphPad Software).
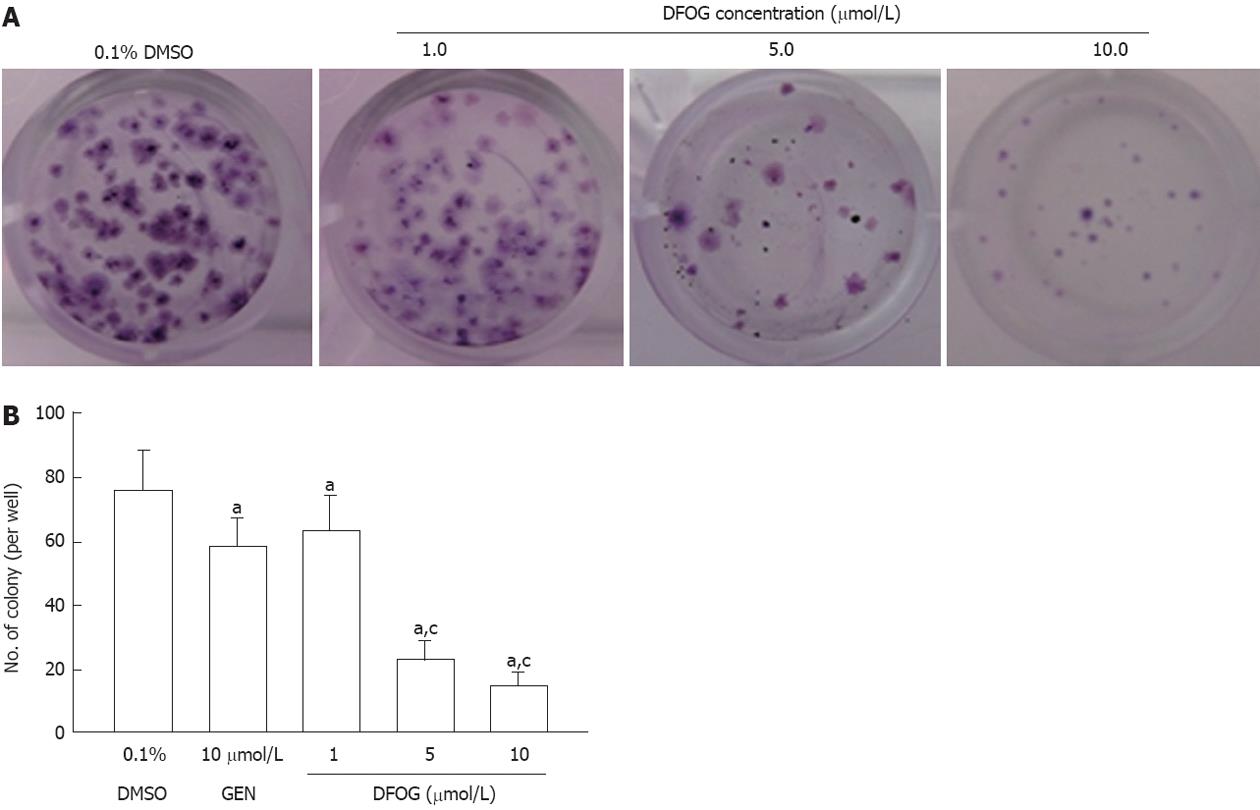
Cells were plated in 24-well plates at a density of 300 cells/well for 24 h, prior to the addition of various concentrations of DFOG (1, 5 and 10 μmol/L) and 10 μmol/L genistein. After 48 h of treatment, the drug-containing medium was removed and replaced with complete growth medium. Medium was changed every 3 d for 8-10 d until visible colonies formed. Colonies were simultaneously fixed and stained with 0.5% crystal violet in methanol, and manually counted. Individually stained colonies in each well were counted and the colony formation fraction was calculated as follows: colony number/(number of cells seeded × plating efficiency), where plating efficiency is equivalent to the colony number divided by the number of cells seeded in the drug-free medium.
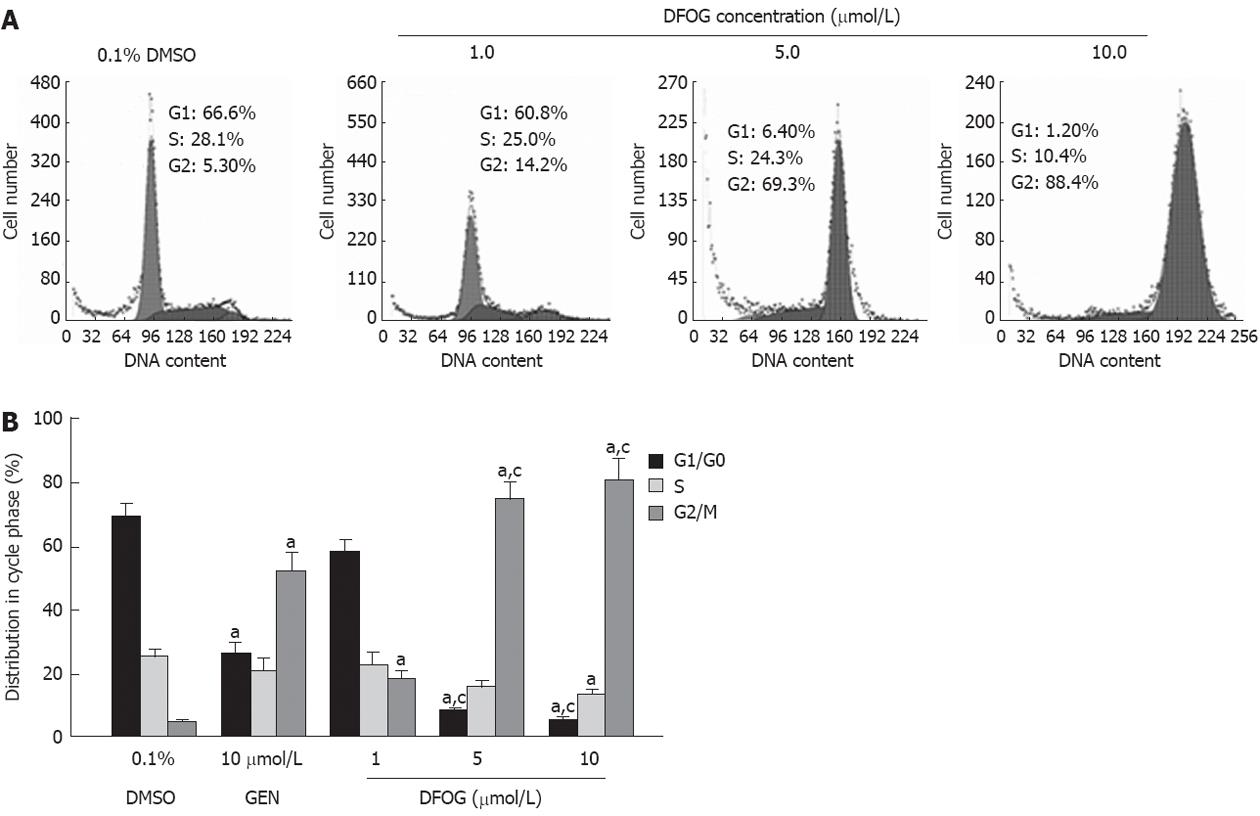
Cells were plated in 6-well plates at a density of 1 000 000 cells/well for 24 h, prior to the addition of various concentrations (1, 5 and 10 μmol/L) of DFOG and 10 μmol/L genistein. After 24 h of treatment, cells were harvested, and DNA content was stained for 15 min at 37 °C with a solution containing 0.4% Triton X-100 (Sigma), 50 μg/mL of propidium iodide (Sigma), and 2 μg/mL of DNase-free RNase (Roche, United States). The cells were then analyzed for cell cycle perturbation using a FACSCalibur (FACS 420, Becton Dickinson, United States). The CellQuest program was used to quantitate the distribution of cells in each cell cycle phase: G1, S and G2/M.
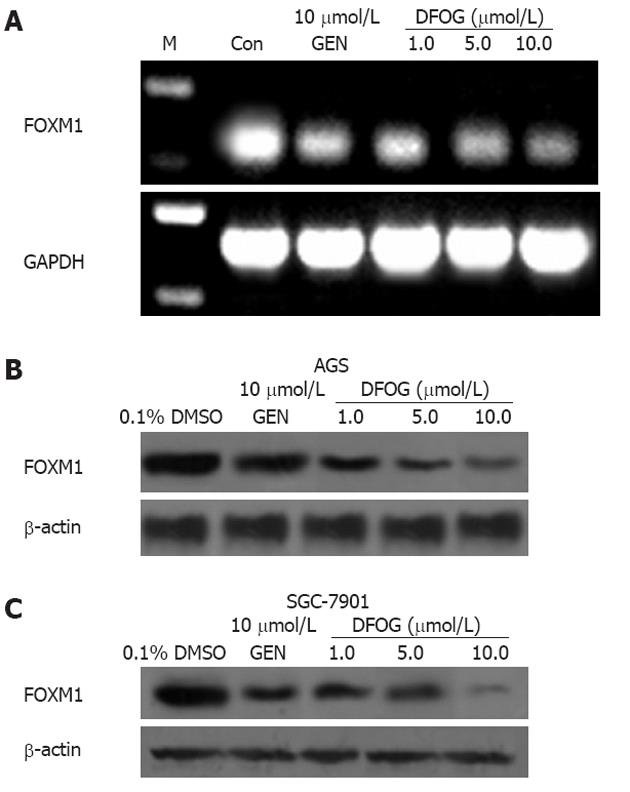
Total RNA was extracted using Trizol reagent (Life Technologies, Gaithersburg, MD, United States). The integrity of the RNA was checked by 2% agarose gel electrophoresis. Approximately 2 μg RNA was reversely transcribed following the protocol of the Super Script™ first-strand synthesis system (Invitrogen Corporation, Carlsbad, CA, United States). The cDNAs encoding FoxM1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes were amplified by polymerase chain reaction (PCR) as follows: denaturation at 94 °C for 30 s, annealing at 63 °C for 30 s and elongation at 72 °C for 45 s. Primer sequence was designed using Primer. For FoxM1, the forward primer was 5’-AACCGCTACTTGACATTGG-3’ and reverse primer 5’-GCAGTGGCTTCATCTTCC-3’. A housekeeping gene, GAPDH was used as the internal control. The forward primer was 5’-ACCCAGAAGACTGTGG ATGG-3’, and the reverse primer was 5’-TGCTGTAGCCAAATTCGTTG-3’. PCR products were analyzed by agarose (2%) gel electrophoresis.
FOXM1 small interfering RNA (siRNA) and control siRNA were obtained from Santa Cruz Biotechnology. The FOXM1 cDNA plasmid was purchased from OriGene Technologies Inc (Rockvile, MD, United States). Human gastric cancer AGS cells were transfected with FOXM1 siRNA and cDNA, respectively, using Lipofectamine 2000 (Invitrogen) as described by Wang et al[20].
Western blotting analysis was carried out as previously described[21]. Cells were lysed in lysis buffer by incubation for 20 min at 4 °C. The protein concentration was determined using the Bio-Rad assay system (Bio-Rad, Hercules, CA, United States). Total proteins were fractionated using sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto Polyvinylidene fluoride membrane (Millipore, United States). Anti-FOXM1, anti-CDK1, anti-cyclin B, anti-p27KIP1, anti-cdc25B and anti-β-actin rabbit polyclonal antibodies were used as primary antibodies. The signals were detected using an ECL Advance Western blotting analysis system (Amersham Pharmacia Biotech Inc., Piscataway, NJ, United States).
The SPSS 15.0 software package (SPSS Inc, Chicago, IL, United States) was used for the statistical analysis. Data were expressed as mean ± SD. The means of multiple groups were compared with one-way analysis of variance, after the equal check of variance, and the pairwise comparisons among the means were performed using the least significant difference method. Statistical comparison was also performed with two-tailed t test when appropriate. A P < 0.05 was considered as statistically significant.
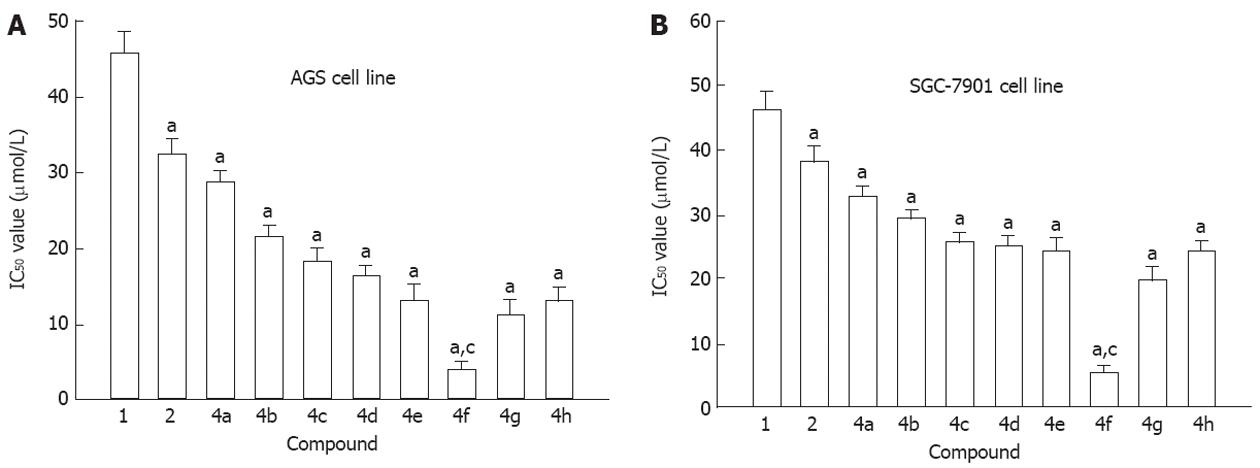
First, we examined the effects of genistein and the genistein analogues on the viability of AGS and SGC-7901 cells using MTT assay. Nine of difluoromethylated geni-stein analogues had higher effective antitumor activities than genistein. Among the aforementioned analogues, DFOG showed the strongest activity against AGS and SGC-7901 in vitro (Figure 1A and B). IC50 of DFOG was 3.9 μmol/L for AGS cells and 5.2 μmol/L for SGC-7901 cells, the potency of DFOG was 11.7 and 8.9 as much as that of the lead compound, genistein (IC50 was 45.9 μmol/L for AGS cells and 46.3 μmol/L for SGC-7901 cells).
Next, we tested the effects of DFOG on cell growth by clonogenic assay. DFOG treatment resulted in a significant inhibition of colony formation of AGS cells compared with controls (Figure 2A and B). Similar results were observed in SGC-7901 cells (data not shown). These data suggests that DFOG inhibits the growth of gastric cancer cells.
To assess whether the loss of cell survival could in part be attributed to the induction of cell cycle arrest, we evaluated the effects of DFOG treatment on the distribution in cell phase using flow cytometry with propidium iodide staining. As shown in Figure 3A and B, in gastric cancer cell line AGS, DFOG treatment caused a significant accumulation of cells in the G2/M phase and a marked decrease in the G1/G0 phase compared with control cells. Similar results were observed in SGC-7901 cells (data not shown). These results provided convincing data that DFOG could induce the growth inhibition and arrest of cell cycle in G2/M phase in gastric cancer cells.
The studies by Wang et al[18] have shown that FOXM1 signaling is over-expressed in pancreatic cancer and is involved in promotion of cell growth, and thus considered as a putative target for drug development. Therefore, we investigated whether DFOG could regulate FOXM1 signaling pathway. FOXM1 mRNA and protein expression in AGS cell line treated with DFOG and genistein for 24 h were decreased in a concentration-dependent manner (Figure 4A and B). We also found that FOXM1 protein expression was down-regulated by DFOG and genistein in SGC-7901 cells (Figure 4C).
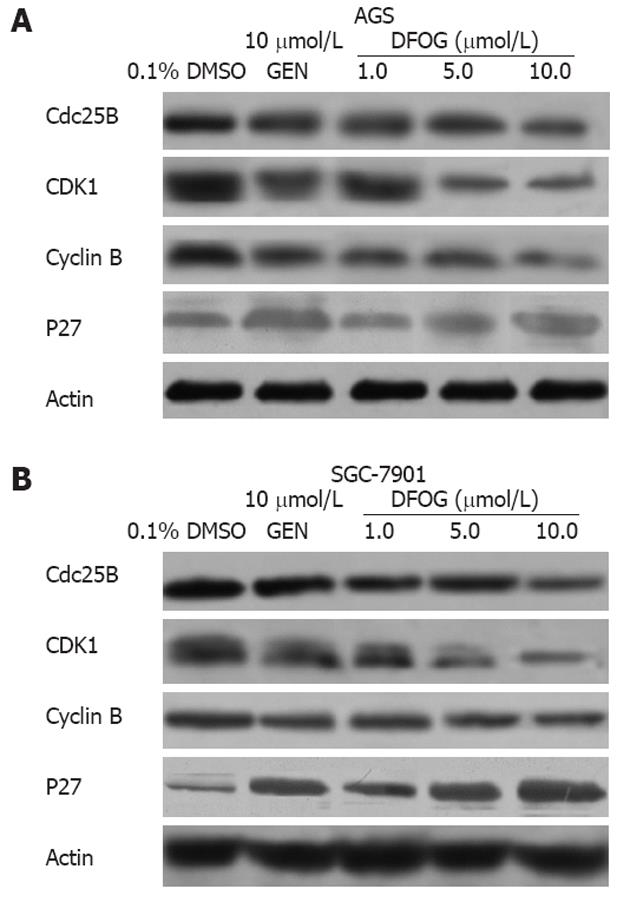
It is well known that FOXM1 has several downstream target genes, such as CDK1, Cdc25B, cyclin B, and p27KIP1. We used Western blotting analysis to determine the expression of these genes, and found that DFOG and genistein inhibited the expression of CDK1, Cdc25B, cyclin B, and increased p27KIP1 at the protein levels in AGS and SGC-701 cells (Figure 5A and B).
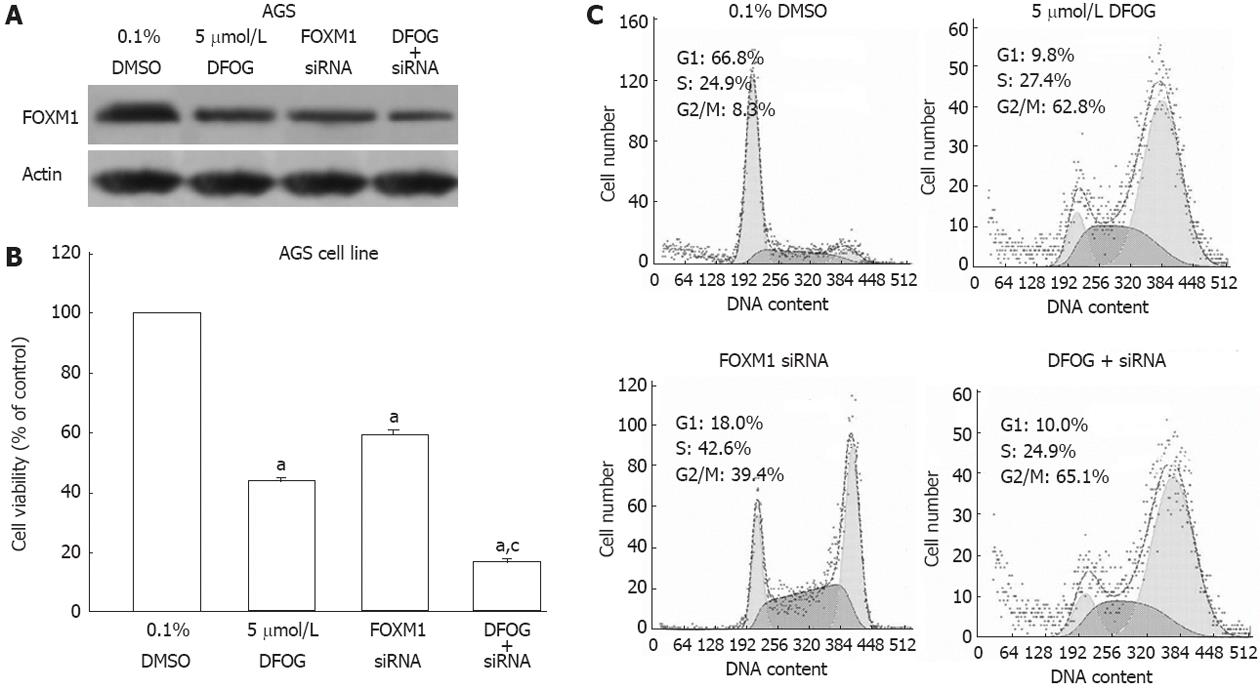
Down-regulation of FOXM1 by siRNA transfection showed less expression of FOXM1 protein in AGS cells, as confirmed by Western blot (Figure 6A).The down-regulation of FOXM1 expression significantly inhibited cell viability induced by DFOG (Figure 6B). DFOG plus FOXM1 siRNA inhibited cell growth to a greater degree compared with DFOG alone. FOXM1 siRNA transfection induces arrest of cell cycle in G2/M phase in AGS cells (Figure 6C). These results provide some molecular evidences suggesting that the DFOG-induced inhibition of the growth is mediated via inactivation of FOXM1 in gastric cancer cells.
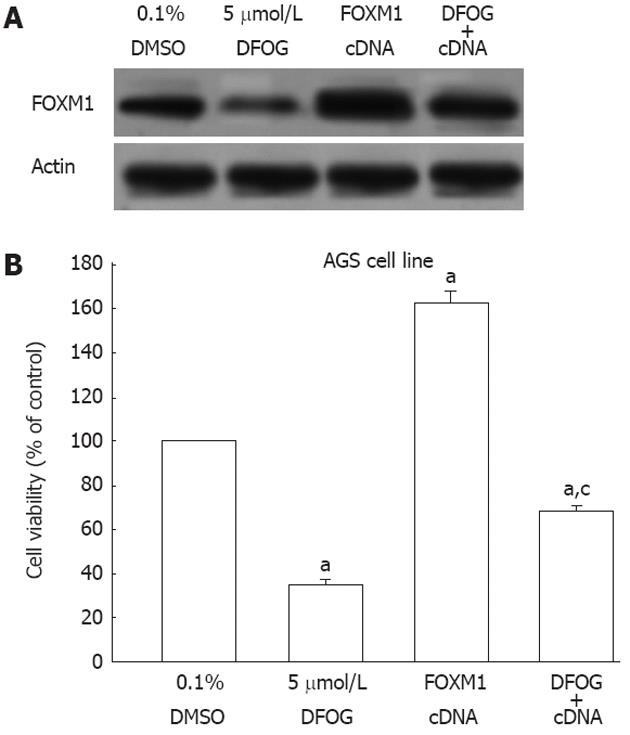
Up-regulation of FOXM1 by cDNA transfection showed over-expression of FOXM1 protein in AGS cells, as confirmed by Western blotting (Figure 7A). The over-expression of FOXM1 rescued DFOG-induced cell viability inhibition to a certain degree (Figure 7B). These findings suggested that DFOG-inhibited cell growth is in part attributed to inactivation of FOXM1 signaling pathway in gastric cancer cells.
FOXM1 signaling has been demonstrated to maintain a balance between cell proliferation, differentiation and apoptosis, suggesting that abnormal activation of FOXM1 gene is one of the characteristics of human cancers[22]. Increasing studies have shown over-expression of FOXM1 gene in human cancer cells and tissues[14,23,24]. Thus, the development of agents targeting FOXM1 is likely to have a significant therapeutic impact on the treatment of human cancers, including gastric cancer. FOXM1 could be down-regulated by some drugs, such as antibiotic thiazole compound Siomycin A, thiostrepton, and epidermal growth factor receptor inhibitor gefitinib[25-27]. These observations clearly suggest that chemical compounds that target FOXM1 may act as anti-cancer drugs[27]. Wang et al[18] have shown that genistein may inhibit FOXM1 activation in pancreatic cancer cells, leading to apoptotic cell death. In the present study, we used two human gastric cancer cell lines, AGS and SGC-7901, which have high expression of FOXM1, and found that DFOG, a novel synthetic genistein analogue, and genistein could induce significant growth inhibition in the two cell lines, as evidenced by both MTT and clonogenic assay. Furthermore, DFOG and genistein inhibited the expression of FOXM1 and its target genes. Therefore, DFOG and genistein could mediate the cell growth inhibition partly via inactivation of FOXM1. Down-regulation of FOXM1 by siRNA together with DFOG treatment inhibited cell growth to a greater degree in AGS cells as compared with DFOG treatment alone. Up-regulation of FOXM1 by cDNA transfection showed over-expression of FoxM1 protein as confirmed by Western blotting analysis, and this over-expression in FOXM1 attenuated DFOG-induced cell growth inhibition in AGS cells. In view of these findings, we strongly believe that inactivation of FOXM1 by DFOG and genistein results in the down-regulation of its target genes, which are mechanistically linked with DFOG and genistein induced cell growth inhibition.
Genistein has been found to induce G2 arrest and inhibit proliferation in a variety of cancer cell lines[28]. Because down-regulation of FOXM1 by DFOG reduced cell growth, we postulated that cell growth inhibition might result from cell cycle arrest in any specific phase of the cell cycle. We did find that FOXM1 down-regulation increased cell population in the G2/M phase and decreased cells in the G1/G0 and S phase. We also observed a marked reduction in cyclin B, Cdc25B and CDK1 expression in DFOG-treated cells. It is known that Cdc25B, cyclin B and CDK1 are the major effectors of the G2/M checkpoint response[29]. Diminished G1-S progression and growth rate was associated with increased expression of the CdkI protein p21CIP and p27KIP1, which have negative effects on cell cycle machinery by binding to various cyclin-Cdk complexes and inhibiting their activities[24,30]. In our study, the decreased cyclin B, cdc25B and Cdk1 and the increased expression of CdkI proteins, p27KIP1, were strongly correlated with the altered cell cycle distribution phenotype and growth suppression. These results suggest that FOXM1 affects the gastric cancer cell cycle by regulating the expression levels of some cyclins (cyclin B), CDK (CDK1), CDK modulator (cdc25B) and CDK inhibitors (p27KIP1).
In summary, we presented experimental evidence which strongly supports the role of DFOG, a novel synthetic genistein analogue, as an anti-tumor agent mediated through inactivation of FOXM1 signaling pathway. However, further in-depth studies are needed to identify how DFOG could regulate the FOXM1 pathway, and to assess the anti-tumor activity mediated by the inactivation of FOXM1 either by genistein and DFOG or other synthetic compounds in pre-clinical animal models for the successful treatment of gastric cancer in the future. It is also tempting to speculate that the inactivation of FOXM1 together with the treatment of gastric tumor cells with conventional agents could be a useful strategy toward better treatment of human malignancies, especially gastric cancer.
Gastric cancer is one of the most common malignancies in the world and its incidence and mortality rank first in China. Recent data indicate that the mortality of gastric cancer in China tends to increase and it severely threatens the health and life of people. New therapeutic agents for this malignant disease are urgently needed.
Genistein, 5,7,4’-trihydroxylisoflavone, a major component of soybean products, has been reported to possess anticancer activities. The authors synthesized a series of difluoromethoxylated genistein analogues and determined their protective effects against vascular endothelial cells. There have been few studies focusing on the anticancer effect of fluorinated genistein analogues.
The authors investigated whether the inhibitory effects of genistein and the novel synthetic genistein analogue 7-difluoromethoxyl-5, 4’-di-n-octylgenistein (DFOG) on the growth of gastric cancer cells could be attributed to modulation of Forkhead Box M1 (FOXM1) activity. It was found that DFOG and genistein down-regulated the FOXM1 expression and its downstream genes, including Cdc25B, CDK1, cyclin B and up-regulated p27KIP1, resulting in the inhibition of gastric cancer cell growth.
These results provide strong evidences for the first time to support that FOXM1 is a rational target in gastric cancer, and the targeted inactivation of FOXM1, especially by genistein and its analogue DFOG, and provided new insight into strategies for better prevention of tumor progression and/or treatment of gastric cancer.
Genistein, 5,7,4’-trihydroxylisoflavone, as one of the active constituents of soybean products, has been reported to possess anti-cancer activities. The DFOG is a novel synthetic genistein analogue.
The novel anti-cancer drugs are mandatory to overcome cancer cells resistant to conventional anti-cancer drugs. In this paper the authors identified DFOG to be a good anti-cancer drug in gastric cancer cells. In addition, they also obtained the results to show FOXM1 as one of target molecules of DFOG. All data presented in this paper is acceptable.
| 1. | Cunha-Rodrigues M, Portugal S, Prudêncio M, Gonçalves LA, Casalou C, Buger D, Sauerwein R, Haas W, Mota MM. Genistein-supplemented diet decreases malaria liver infection in mice and constitutes a potential prophylactic strategy. PLoS One. 2008;3:e2732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Zhou HB, Chen JM, Cai JT, Du Q, Wu CN. Anticancer activity of genistein on implanted tumor of human SG7901 cells in nude mice. World J Gastroenterol. 2008;14:627-631. [PubMed] |
| 3. | Koo H, Schobel B, Scott-Anne K, Watson G, Bowen WH, Cury JA, Rosalen PL, Park YK. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J Dent Res. 2005;84:1016-1020. [PubMed] |
| 4. | Refsnes M, Thrane EV, Låg M, Thoresen GH, Schwarze PE. Mechanisms in fluoride-induced interleukin-8 synthesis in human lung epithelial cells. Toxicology. 2001;167:145-158. [PubMed] |
| 5. | Zheng X, Meng WD, Xu YY, Cao JG, Qing FL. Synthesis and anticancer effect of chrysin derivatives. Bioorg Med Chem Lett. 2003;13:881-884. [PubMed] |
| 6. | Zheng X, Cao JG, Meng WD, Qing FL. Synthesis and anticancer effect of B-ring trifluoromethylated flavonoids. Bioorg Med Chem Lett. 2003;13:3423-3427. [PubMed] |
| 7. | Zheng X, Cao JG, Liao DF, Liu HT. Systhesis and Anticancer Effect of gem-Difluoromethylenated Chrysin Derivatives. Zhongguo Huaxve Kuaibao. 2006;17:1439-1442. |
| 8. | Fu XH, Wang L, Zhao H, Xiang HL, Cao JG. Synthesis of genistein derivatives and determination of their protective effects against vascular endothelial cell damages caused by hydrogen peroxide. Bioorg Med Chem Lett. 2008;18:513-517. [PubMed] |
| 9. | Huang B, Wang Z, Sun Z, Zhao B, Xu H. A novel insight of sentinel lymph node concept based on 1-3 positive nodes in patients with pT1-2 gastric cancer. BMC Cancer. 2011;11:18. [PubMed] |
| 10. | Takayama S, Wakasugi T, Funahashi H, Takeyama H. Strategies for gastric cancer in the modern era. World J Gastrointest Oncol. 2010;2:335-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Zeng J, Wang L, Li Q, Li W, Björkholm M, Jia J, Xu D. FoxM1 is up-regulated in gastric cancer and its inhibition leads to cellular senescence, partially dependent on p27 kip1. J Pathol. 2009;218:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Wierstra I, Alves J. FOXM1, a typical proliferation-associated transcription factor. Biol Chem. 2007;388:1257-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 248] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Costa RH. FoxM1 dances with mitosis. Nat Cell Biol. 2005;7:108-110. [PubMed] |
| 14. | Petrovic V, Costa RH, Lau LF, Raychaudhuri P, Tyner AL. FoxM1 regulates growth factor-induced expression of kinase-interacting stathmin (KIS) to promote cell cycle progression. J Biol Chem. 2008;283:453-460. [PubMed] |
| 15. | Wang IC, Chen YJ, Hughes DE, Ackerson T, Major ML, Kalinichenko VV, Costa RH, Raychaudhuri P, Tyner AL, Lau LF. FoxM1 regulates transcription of JNK1 to promote the G1/S transition and tumor cell invasiveness. J Biol Chem. 2008;283:20770-20778. [PubMed] |
| 16. | Chen YJ, Dominguez-Brauer C, Wang Z, Asara JM, Costa RH, Tyner AL, Lau LF, Raychaudhuri P. A conserved phosphorylation site within the forkhead domain of FoxM1B is required for its activation by cyclin-CDK1. J Biol Chem. 2009;284:30695-30707. [PubMed] |
| 17. | Park HJ, Carr JR, Wang Z, Nogueira V, Hay N, Tyner AL, Lau LF, Costa RH, Raychaudhuri P. FoxM1, a critical regulator of oxidative stress during oncogenesis. EMBO J. 2009;28:2908-2918. [PubMed] |
| 18. | Wang Z, Ahmad A, Banerjee S, Azmi A, Kong D, Li Y, Sarkar FH. FoxM1 is a novel target of a natural agent in pancreatic cancer. Pharm Res. 2010;27:1159-1168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Ai XH, Zheng X, Tang XQ, Sun L, Zhang YQ, Qin Y, Liu HQ, Xia H, Cao JG. Induction of apoptosis of human gastric carcinoma SGC-7901 cell line by 5, 7-dihydroxy-8-nitrochrysin in vitro. World J Gastroenterol. 2007;13:3824-3828. [PubMed] |
| 20. | Wang Z, Banerjee S, Kong D, Li Y, Sarkar FH. Down-regulation of Forkhead Box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells. Cancer Res. 2007;67:8293-8300. [PubMed] |
| 21. | Yang XH, Zheng X, Cao JG, Xiang HL, Liu F, Lv Y. 8-Bromo-7-methoxychrysin-induced apoptosis of hepatocellular carcinoma cells involves ROS and JNK. World J Gastroenterol. 2010;16:3385-3393. [PubMed] |
| 22. | Laoukili J, Stahl M, Medema RH. FoxM1: at the crossroads of ageing and cancer. Biochim Biophys Acta. 2007;1775:92-102. [PubMed] |
| 23. | Francis RE, Myatt SS, Krol J, Hartman J, Peck B, McGovern UB, Wang J, Guest SK, Filipovic A, Gojis O. FoxM1 is a downstream target and marker of HER2 overexpression in breast cancer. Int J Oncol. 2009;35:57-68. [PubMed] |
| 24. | Gemenetzidis E, Bose A, Riaz AM, Chaplin T, Young BD, Ali M, Sugden D, Thurlow JK, Cheong SC, Teo SH. FOXM1 upregulation is an early event in human squamous cell carcinoma and it is enhanced by nicotine during malignant transformation. PLoS One. 2009;4:e4849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | McGovern UB, Francis RE, Peck B, Guest SK, Wang J, Myatt SS, Krol J, Kwok JM, Polychronis A, Coombes RC. Gefitinib (Iressa) represses FOXM1 expression via FOXO3a in breast cancer. Mol Cancer Ther. 2009;8:582-591. [PubMed] |
| 26. | Radhakrishnan SK, Bhat UG, Hughes DE, Wang IC, Costa RH, Gartel AL. Identification of a chemical inhibitor of the oncogenic transcription factor forkhead box M1. Cancer Res. 2006;66:9731-9735. [PubMed] |
| 27. | Bhat UG, Halasi M, Gartel AL. FoxM1 is a general target for proteasome inhibitors. PLoS One. 2009;4:e6593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Li M, Zhang Z, Hill DL, Chen X, Wang H, Zhang R. Genistein, a dietary isoflavone, down-regulates the MDM2 oncogene at both transcriptional and posttranslational levels. Cancer Res. 2005;65:8200-8208. [PubMed] |
| 29. | Zhou BB, Bartek J. Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nat Rev Cancer. 2004;4:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 370] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 30. | Bhat UG, Halasi M, Gartel AL. Thiazole antibiotics target FoxM1 and induce apoptosis in human cancer cells. PLoS One. 2009;4:e5592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
Peer reviewers: Soon-Sun Hong, Professor, Inha University School of Medicine, Clinical Research Center, 2F, Jeongsuk Bld, 3-ga, Shinheung-dong, Incheon 400-712, South Korea; Hikaru Nagahara, Professor, Department of Gastroenterology, Aoyama Hospital, Tokyo Women’s Medical University, 2-7-13 Kitaaoyama, Minato-ku, Tokyo 107-0061, Japan
S- Editor Gou SX L- Editor Ma JY E- Editor Li JY