Published online Aug 21, 2012. doi: 10.3748/wjg.v18.i31.4150
Revised: April 27, 2012
Accepted: May 5, 2012
Published online: August 21, 2012
AIM: To find a possible relationship between inflammation and CA19-9 tumor marker by analyzing data from patients with benign jaundice (BJ) and malignant jaundice (MJ).
METHODS: All patients admitted for obstructive jaundice, in the period 2005-2009, were prospectively enrolled in the study, obtaining a total of 102 patients. On admission, all patients underwent complete standard blood test examinations including C-reactive protein (CRP), bilirubin, CA19-9. Patients were considered eligible for the study when they presented obstructive jaundice confirmed by instrumental examinations and increased serum bilirubin levels (total bilirubin > 2.0 mg/dL). The standard cut-off level for CA19-9 was 32 U/mL, whereas for CRP this was 1.5 mg/L. The CA19-9 level was adjusted by dividing it by the value of serum bilirubin or by the CRP value. The patients were divided into 2 groups, MJ and BJ, and after the adjustment a comparison between the 2 groups of patients was performed. Sensitivity, specificity and positive predictive values were calculated before and after the adjustment.
RESULTS: Of the 102 patients, 51 were affected by BJ and 51 by MJ. Pathologic CA19-9 levels were found in 71.7% of the patients. In the group of 51 BJ patients there were 29 (56.9%) males and 22 (43.1%) females with a median age of 66 years (range 24-96 years), whereas in the MJ group there were 24 (47%) males and 27 (53%) females, with a mean age of 70 years (range 30-92 years). Pathologic CA19-9 serum level was found in 82.3% of MJ. CRP levels were pathologic in 66.6% of the patients with BJ and in 49% with MJ. Bilirubin and CA19-9 average levels were significantly higher in MJ compared with BJ (P = 0.000 and P = 0.02), while the CRP level was significantly higher in BJ (P = 0.000). Considering a CA19-9 cut-off level of 32 U/mL, 82.3% in the MJ group and 54.9% in the BJ group were positive for CA19-9 (P = 0.002). A CA19-9 cut-off of 100 U/mL increases the difference between the two groups: 35.3% in BJ and 68.6% in MJ (P = 0.0007). Adjusting the CA19-9 value by dividing it by serum bilirubin level meant that 21.5% in the BJ and 49% in the MJ group remained with a positive CA19-9 value (P = 0.003), while adjusting the CA19-9 value by dividing it by serum CRP value meant that 31.4% in the BJ group and 76.5% in the MJ group still had a positive CA19-9 value (P = 0.000004). Sensitivity, specificity, positive predictive values of CA19-9 > 32 U/mL were 82.3%, 45% and 59.1%; when the cut-off was CA19-9 > 100 U/mL they were, respectively, 68.6%, 64.7% and 66%. When the CA19-9 value was adjusted by dividing it by the bilirubin or CRP values, these became 49%, 78.4%, 69.4% and 76.5%, 68.6%, 70.9%, respectively.
CONCLUSION: The present study proposes CRP as a new and useful correction factor to improve the diagnostic value of the CA19-9 tumor marker in patients with cholestatic jaundice.
- Citation: La Greca G, Sofia M, Lombardo R, Latteri S, Ricotta A, Puleo S, Russello D. Adjusting CA19-9 values to predict malignancy in obstructive jaundice: Influence of bilirubin and C-reactive protein. World J Gastroenterol 2012; 18(31): 4150-4155
- URL: https://www.wjgnet.com/1007-9327/full/v18/i31/4150.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i31.4150
CA19-9 is a tumor marker that increases in pancreatic and biliary malignancy and it has been promoted as a reliable test for the detection of pancreato-biliary malignancy. In pancreatic cancer, CA19-9 has been reported to have 70%-80% sensitivity and 80%-90% specificity in tumor diagnosis, whereas in cholangiocarcinoma without history of sclerosing cholangitis the sensitivity and specificity are, respectively, 77.9% and 76.3%[1-2]. CA19-9 is unfortunately increased not only in patients with pancreatic or biliary cancers but also in benign biliary diseases which often present with jaundice and is therefore often misleading, reducing significantly the diagnostic accuracy of this marker[3-5]. The relationship between CA19-9 and jaundice has been analyzed and studied to find possible adjustments to increase the sensitivity, specificity and predictive value of the test in differential diagnosis of hepatobiliary diseases associated with jaundice. Therefore, some authors have suggested to adjust CA19-9 value by dividing it empirically by the serum bilirubin value[6,7]. Other factors that could influence and alter the values of this tumor marker have not been studied yet. Inflammation contributes to elevating the CA19-9 value and it can be assessed by monitoring the acute-phase proteins: one of these is the C-reactive protein (CRP) which rises in response to infection, injury and neoplasm.
This research was stimulated by our own experience of patients presenting with jaundice of benign etiology who were found to have grossly elevated CA19-9 levels. The purpose of this study was to clarify the clinical interpretation and diagnostic value of an elevated serum CA19-9 level, with special reference to coexistent obstructive jaundice. The present study, first in the literature, analyzes a possible relationship between CA19-9, bilirubin and inflammation, expressed as CRP value, aiming to find a ratio or a better corrective factor to increase predictivity of CA19-9 and reduce the number of misleading false positive results.
All the patients admitted for obstructive jaundice to the Department of Emergency Surgery, Cannizzaro Hospital, University of Catania, Italy, between September 2005 and September 2009, were considered for the present study. The patients were enrolled prospectively, 51 for each group of benign jaundice (BJ) and malignant jaundice (MJ) patients, obtaining a total of 102 patients. At admission, all patients underwent complete standard blood test examinations, including serum bilirubin levels. Patients were considered eligible for the study when they presented obstructive jaundice confirmed by instrumental examinations and increased bilirubin serum levels (total bilirubin > 2.0 mg/dL). Serum levels of CA19-9 and CRP were also measured at the time of admission. The standard cut-off level of CA19-9 was 32 U/mL, whereas for CRP this was 1.5 mg/L. The definitive diagnosis was obtained by instrumental examinations, surgical exploration and pathology. Instrumental examinations included ultrasonography, computed tomography scan, magnetic resonance imaging, endoscopic ultrasound scan and endoscopic retrograde cholangiopancreatography. The CA19-9 level was adjusted by dividing it by the value of serum bilirubin firstly, and then by the CRP value. The patients were divided into 2 groups: MJ and BJ. After the adjustment a comparison between the serum levels in the 2 groups of patients was obtained by Wilcoxon two sample test. Differences for categorical variables were assessed using the chi-square test or Fisher’s exact test when adequate. Spearman correlation between the 3 parameters of CA19-9, bilirubin and CRP in each group of patients was computed. The receiver operating characteristic curve was used to establish the probability that a patient with MJ has a high value of CA19-9. A P value less than 0.05 was considered statistically significant.
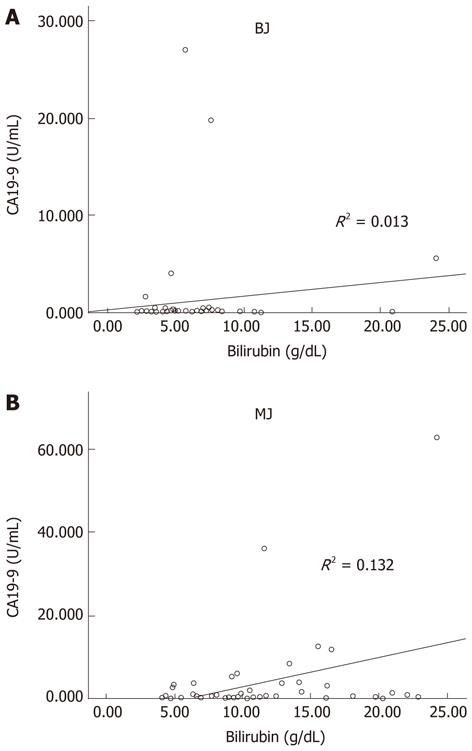
The present longitudinal study analyzed a total of 102 patients: 53 (52%) men and 49 (48%) women with a median age of 69 years (range 24-96 years). In the group of 51 BJ patients there were 29 (56.9%) males and 22 (43.1%) females with a median age of 66 years (range 24-96 years), whereas in the MJ group there were 24 (47%) males and 27 (53%) females, with a median age of 70 years (range 30-92 years). Causes of BJ included: common bile duct (CBD) stones (66.6%), gallbladder stones with cholangitis (15.7%), biliary pancreatitis (15.7%), papillitis (1.9%). Causes of MJ included: pancreatic cancer (49%), bile duct cancer (19.6%), gallbladder cancer (13.7%), ampullary cancer (9.8%), intrahepatic cholangiocarcinoma (1.9%) and other malignancy (5.8%; 2 patients with metastatic nodes in porta hepatis and one patient with peritoneal carcinomatosis from ovarian cancer). Pathologic CA19-9 serum level was found in 82.3% of MJ, but in two patients the CA19-9 was 2.5 U/mL. CA19-9 did not correlate directly with the grade of biliary obstruction either expressed as bilirubin level in BJ and MJ (Figure 1). CRP levels were pathologic in 66.6% of the patients with BJ and in 49.0% with MJ. Mean bilirubin and CA19-9 levels were significantly higher in MJ compared to BJ, while the CRP level was significantly higher in BJ (Table 1). Table 2 reports the serum level of bilirubin, CA19-9 and CRP according to the cause of jaundice. There were no relevant differences in bilirubin according to the cause of jaundice. Comparing the CA19-9 value between patients with pancreatic cancer and patients with other causes of MJ and BJ, no difference was reported. CRP was significantly (P = 0.04) higher in patients with CBD stones than in pancreatic cancer, and patients with BJ and pancreatitis had the highest value of CRP. In MJ the average level of CRP was significantly higher in gallbladder cancer than in bile duct cancer, but not different from that in pancreatic cancer.
| Benign | Malignant | P value | |
| No. of patients | 51 | 51 | |
| Bilirubin serum level (median and range) (g/dL) | 5.2 (2.12-24.03) 95% CI = 5.0-7.2 | 9.7 (4.07-24.25) 95% CI = 9.6-12.5 | < 0.0001 |
| CA19-9 serum level (median and range) (U/mL) | 36 (2-27019) 95% CI = 0-2.508 | 405 (2.5-62974) 95% CI = 699-6282 | 0.02 |
| > 32 (%) | 28/51 (54.9) | 42/51 (82.3) | 0.003 |
| > 100 (%) | 18/51 (35.3) | 35/51 (68.6) | 0.0007 |
| CRP (median and range) (U/mL) | 6.91 (0.1-30) 95% CI = 4.6-8.3 | 2.97 (0.1-13.2) 95% CI = 2.3-4.1 | 0.0002 |
| > 1.5 (%) | 34/51 (66.6) | 25/51 (49.0) | 0.005 |
| > 5 (%) | 21/51 (41.2) | 7/51 (13.7) | 0.004 |
| Cause | No. of cases | Bilirubin(g/dL), median | CA19-9 (U/mL) | CRP (U/mL) | ||||||
| Median | P value1 | > 32 | > 100 | Median | P value2 | > 1.5 | > 5 | |||
| Malignant | 51 | 9.7 | 405 | 42 (8.3) | 35 (68.6) | 2 | 25 (49) | 7 (13.7) | ||
| Pancreatic cancer | 25 (49) | 11.29 | 461 | - | 23 (92) | 19 (76) | 1.96 | 0.04 | 12 (48) | 3 (12) |
| Bile duct cancer | 10 (19.6) | 9.94 | 313 | 0.28 | 7 (70) | 6 (60) | 1.26 | 0.06 | 1 (10) | 0 |
| Gallbladder cancer | 7 (13.7) | 8.94 | 3361 | 0.59 | 6 (85.7) | 5 (71.4) | 4.13a | 0.91 | 5 (71.4) | 2 (28.5) |
| Ampullary cancer | 5 (9.8) | 6.49 | 196 | 0.42 | 4 (80) | 3 (60) | 4.5 | 0.69 | 4 (80) | 1 (0.2) |
| Intrahepatic cholangio carcinoma | 1 (1.9) | 16.13 | 2600 | NA | 1 | 1 | 5.96 | NA | 1 | 1 |
| Others | 3 (5.8) | 7.65 | 16 | 0.53 | 1 | 1 | 4.95 | 0.84 | 2 (66.6) | 1 (33.3) |
| Benign | 51 | 5.2 | 36 | 28 (54.9) | 18 (35.3) | 4.5 | 34 (66.6) | 21 (41.2) | ||
| CBD stones | 34 (66.6) | 5.63 | 42 | 0.10 | 19 (55.8) | 12 (35.2) | 3.47 | - | 22 (64.7) | 11 (32.3) |
| Gallbladder stones with cholangitis | 8 (15.7) | 5.26 | 55 | 0.54 | 4 (50) | 3 (37.5) | 2.31 | 0.73 | 5 (62.5) | 3 (37.5) |
| Biliary pancreatitis | 8 (15.7) | 3.85 | 61 | 0.27 | 4 (50) | 3 (37.5) | 10.33 | 0.04 | 7 (87.5) | 7 (87.5) |
| Chronic papillitis | 1 (1.9) | 6.6 | 35 | NA | 1 | 0 | 0.7 | NA | 0 | 0 |
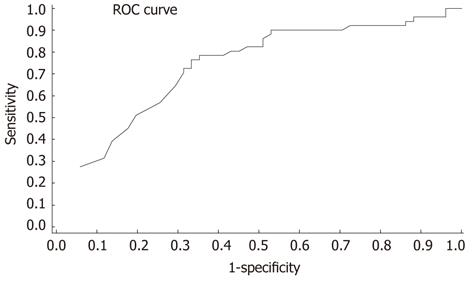
When considering the CA19-9 cut-off level of 32 U/mL, 42 of 51 patients (82.3%) in the malignant group and 28 of 51 (54.9%) in the benign group were positive for CA19-9 (Fisher’s exact test: P = 0.002). Figure 2 shows that the area under the curve or probability that a patient diagnosed with MJ has a major value of CA19-9 compared to a patient diagnosed with BJ was 0.71. Increasing the cut-off level of CA19-9 to 100 U/mL, the difference between the two groups increases: 35.3% in BJ and 68.6% in MJ (Fisher’s exact test: P = 0.0007). Changing the cut-off level alters the sensitivity and specificity as shown in Table 3, but by pushing up the cut-off level in spite of an increase of specificity we have obtained a reduction in the sensitivity of the test.
| Sensitivity | Specificity | PPV | |
| CA19-9 > 32 U/mL | 82.3 | 45.0 | 59.1 |
| CA19-9 > 100 U/mL | 68.6 | 64.7 | 66.0 |
| CA19-9/BIL | 49.0 | 78.4 | 69.4 |
| CA19-9/CRP | 76.5 | 68.6 | 70.9 |
Adjusting the CA19-9 value by dividing it by serum bilirubin level, 11 of 51 patients (21.6%) in the BJ and 26 of 51 patients (51.0%) in the MJ group remained with a positive CA19-9 value (Fisher’s exact test: P = 0.003), increasing the specificity to 78.4% but reducing the sensitivity to 49%.
The second mode of adjustment of the CA19-9 value was performed by dividing it by serum CRP value. Consequently, 16 of 51 patients (31.4%) in the BJ group and 39 of 51 patients (76.5%) in the MJ group remained with a positive CA19-9 value (Fisher’s exact test: P = 0.000004). By this adjustment the sensitivity increases to 76.5%, the specificity to 68.6% and the positive predictive value (PPV) to 70.9%.
The diagnostic role of CA19-9 as a test for the detection of pancreato-biliary malignancy remains poorly defined, because, as in other diagnostic modalities, the utility of CA19-9 has several confounding limitations. Firstly, false positive elevations in CA19-9 exist in benign conditions such as liver diseases (primary sclerosing cholangitis, primary biliary cirrhosis, chronic hepatitis, acute liver failure), obstructive jaundice, pancreatitis[3,5,8-11]. Even diseases not related to the hepatobiliary tract such as interstitial pulmonary disease[12], collagen vascular diseases and, reportedly, heavy tea consumption[4,13], suggest that CA19-9 may be expressed as a marker of a systemic inflammatory response. Furthermore, CA19-9 has also been shown to be upregulated in other malignant tumors including gastric, ovarian, and colorectal carcinoma[14]. However, the most common cause of false positive CA19-9 is obstructive jaundice[10]. Physiologically, biliary epithelial cells secrete mucins carrying the epitope of CA19-9, hence the high level of CA19-9 in serum during the obstructive jaundice, reflecting both inflammatory hypersecretion and leakage of biliary mucins into serum. This process can be reversed by resolution of the jaundice, which is often associated with a fall in CA19-9 greater in benign disease than in malignant[3], because in malignant disease the synthesis of CA19-9 by proliferating cells contributes to the total level in a manner independent from any associated condition[3,5]. In this series patient with malignant jaundice had higher mean levels of bilirubin than those with benign jaundice; additionally, the level of CA19-9 was significantly higher in malignant than in benign, especially if we considered a cut off level > 100 U/mL. Unfortunately, in our study, CA19-9 does not correlate directly with the grade of biliary obstruction either in benign or in malignant jaundice (Figure 1), even if we observed that in patients with benign disease levels of CA19-9 decreased after relief of biliary obstruction (data not shown).
Secondly, the concentration of this tumor marker in the serum may be influenced by the patient’s secretor status, because patients who are genotypically negative for the Lewis blood group antigen (a-, b-), approximately 4%-15% of the general population, do not synthesize CA19-9[15]. As above in the present series the rate of MJ with a CA19-9 value less than 2 U/mL is 3.9%.
Furthermore, not only hyperbilirubinemia can obscure the clinical value of CA19-9, but inflammation can contribute and have a role. CRP, synthesized in hepatocytes, is one of the acute-phase proteins which are components of the innate immune responses that increase after infections, trauma, burns, tissue infarction, inflammatory process and tumors. In general, increased CRP levels in malignant disease could also be caused by an inflammatory response to tumor invasion[16]. Padillo et al[17], analysing CRP in 24 jaundice patients, found CRP levels significantly higher in patients with cancer. Differently from that study, this series showed the CRP serum levels are higher in benign obstructive jaundice than in malignant. Indeed, Table 2 reports that CRP is significantly (P = 0.04) higher in patients with CBD stones than those with pancreatic cancer; in particular, patients with BJ and pancreatitis have the highest value of CRP. Malignancy such as gallbladder cancer and intrahepatic cholangiocarcinoma present values of CRP comparable to BJ, probably due to an intensive inflammatory response to the tumor.
In our series, 54.9% of patients with benign jaundice had positive CA19-9 levels (cut-off 32 U/mL), and 35.5% had CA19-9 value over 100 U/mL; therefore, although the overall increase of the tumor marker in benign jaundice was inferior compared to that observed in malignancies, there was an overlap of values between cancer and non-cancer causes. This resulted in a low accuracy of CA19-9 to diagnose pancreatic-biliary malignancies in patients with jaundice, especially since this marker is not able to distinguish pancreatic carcinoma from other malignancy or other benign causes of jaundice, as reported in Table 2, differing from what has been shown in other studies[14,18]. Even when considering a cut-off level of 100 U/mL, the specificity is still 64.7%. As a result of this diagnostic overlap, the American Society of Clinical Oncology does not currently advocate its use for screening, evaluation of resectability or disease follow-up[19]. For this reason some authors suggested to push up the cut-off level to 300 U/mL in presence of cholangitis and cholestasis to increase CA19-9 specificity, but this was associated with a significant decrease of sensitivity[20]. To achieve a specificity of 100%, cut-off levels greater than 1000 U/mL should be considered[21]. We recorded ten BJ patients with CA19-9 level greater than 300 U/mL and five BJ patients with more than 1000 U/mL, and all of them had serum bilirubin level lower than 8 mg/dL. These data can explain why there is no difference in the mean CA19-9 value between each cause of BJ and pancreatic cancer jaundice (Table 2). Indeed, multiple reports of patients with extremely high CA19-9 values in patients with BJ can be found in the literature[22-24]. Several studies have shown that the association of elevated levels of CA19-9 with the diagnosis of cancer is significantly obscured in the face of obstructive jaundice, and because the bilirubin level correlates with CA19-9, they suggest that this value should be adjusted for hyperbilirubinemia[10,11]. In our study CA19-9 does not correlate directly with the grade of biliary obstruction, either in BJ or in MJ.
Hence, based on the knowledge that in benign jaundice high levels of CA19-9 are an expression of obstruction and inflammation and CRP levels are higher in this group of patients, the most appropriate adjusting factor could be the CRP and not the bilirubin value. Indeed, 54.9% of patients with BJ had a CA19-9 level greater than 32 U/mL, and using a CA19-9 cut-off of 100 U/mL, the specificity of the test to detect malignancy is increased, with little decrease in the sensitivity. But the majority of benign jaundice patients have a CA19-9 value < 100 U/mL, so by adjusting this value with the CRP it is possible to increase the reliability of the test. As shown in Table 3 using the bilirubin as adjusting factor, even if the specificity reaches 78.4%, the sensitivity falls down to 49%; instead the CRP value better reflects the inflammatory status, obtaining 76.5% sensitivity, 68.6% specificity and 70.9% PPV. Certainly, the CA19-9 value has to be considered as an adjunctive value to the patient’s history in the diagnosis of patients with obstructive jaundice, and its value, even after adjustment, should be helpful in planning the type and priority of further investigations with regard to the liver, bile ducts and pancreas.
In conclusion, the present study proposes adjusting to the CA19-9/CRP ratio as a new diagnostic tool in patients with cholestatic jaundice, which has not been reported in similar studies in the literature. This simple ratio can significantly increase the specificity and the positive predictive value of CA19-9 in the differential diagnosis between malignant and benign jaundice. Other complementary studies of these markers are essential for diagnosis of malignant tumors when jaundice is present.
CA19-9 is a tumor marker which is increased in both benign and malignant hepatobiliary diseases. Previous studies have shown that CA19-9 has high sensitivity and specificity in pancreatic cancer. However, this tumor marker has been found to be elevated in benign biliary diseases as well. Hence the accuracy of CA19-9 is unreliable. The authors have tried to analyze the relationship between CA19-9, bilirubin and CRP levels to predict the accuracy of CA19-9 in malignant obstructive jaundice.
After experiencing patients presenting with jaundice of benign etiology and having grossly elevated CA19-9 levels, the authors aimed to clarify the clinical interpretation and diagnostic value of an elevated serum CA19-9 level, with special reference to coexistent obstructive jaundice. CA19-9 is demonstrated to be influenced by bilirubin level, but other factors such as inflammation could influence and alter the values of this tumor marker. The research hotspot is how the inflammation influences the level of CA19-9.
In the past, the accuracy of CA19-9 level was improved by dividing it by bilirubin level, but the high level of CA19-9 in serum during obstructive jaundice reflects also an inflammatory hypersecretion. The inflammation can be assessed by monitoring the acute-phase proteins: one of these is the C-reactive protein (CRP). In this regard, in the present study, the CA19-9 level was adjusted by dividing it by the value of serum bilirubin and by the CRP value to look for a ratio or a better corrective factor to increase predictivity of CA19-9 and reduce the amount of misleading false positives. In fact, this adjustment increases the sensitivity to 76.5%, the specificity to 68.6% and the positive predictive value to 70.9%.
The study results suggest that considering inflammation as a reliable factor which increases the CA19-9 level in benign disease, abnormal tumor marker values in these patients can be corrected using the CRP in order to reduce the false positive results. In this way, it is possible to concentrate efforts in planning the type and priority of further investigations in the liver, bile ducts and pancreas in patients affected by malignancy.
CA19-9: Tumor marker that increases in pancreatic and biliary malignancy, as physiologically biliary epithelial cells secrete mucins carrying the epitope of CA19-9; CRP: Synthesized in hepatocytes, is one of the acute-phase proteins, which are components of the innate immune responses that increase after infections, trauma, burns, tissue infarction, inflammatory process and tumors.
This is a good longitudinal study in which authors analyze the relationship between CA19-9, bilirubin and CRP levels to predict the accuracy of CA19-9 in malignant obstructive jaundice. The results are interesting and suggest that the inflammation influences the CA19-9 value and reducing this confounding factor can help to better identify patients with malignant obstructive jaundice.
| 1. | Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 630] [Article Influence: 31.5] [Reference Citation Analysis (1)] |
| 2. | John AR, Haghighi KS, Taniere P, Esmat ME, Tan YM, Bramhall SR. Is a raised CA 19-9 level diagnostic for a cholangiocarcinoma in patients with no history of sclerosing cholangitis ? Dig Surg. 2006;23:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Marrelli D, Caruso S, Pedrazzani C, Neri A, Fernandes E, Marini M, Pinto E, Roviello F. CA19-9 serum levels in obstructive jaundice: clinical value in benign and malignant conditions. Am J Surg. 2009;198:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Kim HJ, Kim MH, Myung SJ, Lim BC, Park ET, Yoo KS, Seo DW, Lee SK, Min YI. A new strategy for the application of CA19-9 in the differentiation of pancreaticobiliary cancer: analysis using a receiver operating characteristic curve. Am J Gastroenterol. 1999;94:1941-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Mann DV, Edwards R, Ho S, Lau WY, Glazer G. Elevated tumour marker CA19-9: clinical interpretation and influence of obstructive jaundice. Eur J Surg Oncol. 2000;26:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 216] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Kang CM, Kim JY, Choi GH, Kim KS, Choi JS, Lee WJ, Kim BR. The use of adjusted preoperative CA 19-9 to predict the recurrence of resectable pancreatic cancer. J Surg Res. 2007;140:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 7. | Ortiz-González J, Alvarez-Aguila NP, Medina-Castro JM. Adjusted carbohydrate antigen 19-9. Correlation with histological grade in pancreatic adenocarcinoma. Anticancer Res. 2005;25:3625-3627. [PubMed] |
| 8. | Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 288] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Qin XL, Wang ZR, Shi JS, Lu M, Wang L, He QR. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: in comparison with CEA. World J Gastroenterol. 2004;10:427-432. [PubMed] |
| 10. | Ong SL, Sachdeva A, Garcea G, Gravante G, Metcalfe MS, Lloyd DM, Berry DP, Dennison AR. Elevation of carbohydrate antigen 19.9 in benign hepatobiliary conditions and its correlation with serum bilirubin concentration. Dig Dis Sci. 2008;53:3213-3217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Mery CM, Duarte-Rojo A, Paz-Pineda F, Gómez E, Robles-Díaz G. [Does cholestasis change the clinical usefulness of CA 19-9 in pacreatobiliary cancer?]. Rev Invest Clin. 2001;53:511-517. [PubMed] |
| 12. | Kodama T, Satoh H, Ishikawa H, Ohtsuka M. Serum levels of CA19-9 in patients with nonmalignant respiratory diseases. J Clin Lab Anal. 2007;21:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Howaizi M, Abboura M, Krespine C, Sbai-Idrissi MS, Marty O, Djabbari-Sobhani M. A new cause for CA19.9 elevation: heavy tea consumption. Gut. 2003;52:913-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Morris-Stiff G, Teli M, Jardine N, Puntis MC. CA19-9 antigen levels can distinguish between benign and malignant pancreaticobiliary disease. Hepatobiliary Pancreat Dis Int. 2009;8:620-626. [PubMed] |
| 15. | Vestergaard EM, Hein HO, Meyer H, Grunnet N, Jørgensen J, Wolf H, Orntoft TF. Reference values and biological variation for tumor marker CA 19-9 in serum for different Lewis and secretor genotypes and evaluation of secretor and Lewis genotyping in a Caucasian population. Clin Chem. 1999;45:54-61. [PubMed] |
| 16. | Morley JJ, Kushner I. Serum C-reactive protein levels in disease. Ann N Y Acad Sci. 1982;389:406-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 265] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Padillo FJ, Muntane J, Montero JL, Briceño J, Miño G, Solorzano G, Sitges-Serra A, Pera-Madrazo C. Effect of internal biliary drainage on plasma levels of endotoxin, cytokines, and C-reactive protein in patients with obstructive jaundice. World J Surg. 2002;26:1328-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Fujioka S, Misawa T, Okamoto T, Gocho T, Futagawa Y, Ishida Y, Yanaga K. Preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels for the evaluation of curability and resectability in patients with pancreatic adenocarcinoma. J Hepatobiliary Pancreat Surg. 2007;14:539-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313-5327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1133] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 20. | Kim HR, Lee CH, Kim YW, Han SK, Shim YS, Yim JJ. Increased CA 19-9 level in patients without malignant disease. Clin Chem Lab Med. 2009;47:750-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Steinberg W. The clinical utility of the CA 19-9 tumor-associated antigen. Am J Gastroenterol. 1990;85:350-355. [PubMed] |
| 22. | Lowe D, Lee J, Schade R, Chaudhary A. Patient with markedly elevated CA 19-9 not associated with malignancy. South Med J. 2006;99:306-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Akdoğan M, Saşmaz N, Kayhan B, Biyikoğlu I, Dişibeyaz S, Sahin B. Extraordinarily elevated CA19-9 in benign conditions: a case report and review of the literature. Tumori. 2001;87:337-339. [PubMed] |
| 24. | Korkmaz M, Ünal H, Selçuk H, Yilmaz U. Extraordinarily elevated serum levels of CA 19-9 and rapid decrease after successful therapy: a case report and review of literature. Turk J Gastroenterol. 2010;21:461-463. [PubMed] |
Peer reviewer: Dr. Ashok Kumar, Department of Surgical Gastroenterology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Raebareli Road, Lucknow 226014, India
S- Editor Wu X L- Editor Logan S E- Editor Li JY