Published online Jan 21, 2012. doi: 10.3748/wjg.v18.i3.244
Revised: August 26, 2011
Accepted: October 28, 2011
Published online: January 21, 2012
AIM: To investigate the role of urokinase plasminogen activator (uPA) in cholangiocarcinoma (CCA) invasion and its correlation with clinicopathological parameters.
METHODS: uPA expression in CCA tissue was determined by immunohistochemistry. The level of uPA from two CCA cell lines (HuCCA-1 and KKU-M213) and a non-cancer immortalized cholangiocyte cell line (H69) was monitored by plasminogen-gelatin zymography and western blotting, whereas that of plasminogen activator inhibitor type 1 (PAI-1) protein and uPA receptor (uPAR) mRNA was monitored by western blotting and quantitative real-time reverse transcriptase polymerase chain reaction, respectively. Two independent methods were employed to suppress uPA function: a synthetic uPA inhibitor (B428) and silencing of uPA gene expression using siRNA. In vitro invasion of the uPA-disrupted cells was assessed by Matrigel-coated Transwell assay.
RESULTS: The immunohistochemical study showed that 75.3% (131/174) of CCA tissues expressed uPA. High uPA expression was correlated with lymphatic invasion and metastasis of CCA patients. Plasminogen-gelatin zymography of the conditioned media and cell-surface eluates showed that both CCA cell lines, but not H69, expressed both secreted and membrane-bound forms of uPA. Although the two CCA cell lines, HuCCA-1 and KKU-M213, expressed a relatively high level of uPA and uPAR, the latter exhibited a much lower degree of in vitro invasiveness, correlating with a high expression of PAI-1 in the latter, but not in the former. Suppressing uPA function with a specific uPA inhibitor, B428, or with siRNA against uPA reduced in vitro invasiveness of KKU-M213 cells, demonstrating the requirement for uPA in the invasiveness of CCA cells. Therefore, our in vivo and in vitro studies suggest that uPA is an important requirement for the invasion process of CCA.
CONCLUSION: uPA expression correlates with lymphatic invasion and metastasis in vivo and is required for CCA cell invasion in vitro, suggesting its potential as a therapeutic target.
- Citation: Thummarati P, Wijitburaphat S, Prasopthum A, Menakongka A, Sripa B, Tohtong R, Suthiphongchai T. High level of urokinase plasminogen activator contributes to cholangiocarcinoma invasion and metastasis. World J Gastroenterol 2012; 18(3): 244-250
- URL: https://www.wjgnet.com/1007-9327/full/v18/i3/244.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i3.244
Cholangiocarcinoma (CCA) is a cancer that originates from the biliary epithelium, and it is the second most common form of liver cancer. Although it is a rare malignancy, the incidence and mortality rate has increased worldwide in the past decade[1]. The highest incident rate was observed in Northeast Thailand where there is a high prevalence of liver fluke (Opisthorchis viverrini) infection[2], a group I carcinogen classified by International Agency for Research on Cancer[3]. CCA is considered an incurable disease due to lack of efficient diagnosis, hence most patients at presentation have developed advanced disease with high rate of invasion and metastasis, resulting in a high mortality rate.
Metastasis is a multi-step process that involves spreading of cancer cells from the primary to the secondary site. During this process, cancer cells must invade the surrounding tissue, penetrate the blood or lymphatic vessels, and form a new tumor mass at distant sites. To invade, cancer cells degrade extracellular matrix (ECM) and basement membrane to generate a space for the cells to move out of the original site. This is accomplished by secretion of a variety of matrix-degrading enzymes including matrix metalloproteinases (MMPs) and serine proteases, such as plasminogen activator.
Urokinase plasminogen activator (uPA) is a serine protease that is involved in ECM degradation, cancer invasion and metastasis by regulating the plasminogen/plasmin system. uPA is synthesized as a single-chain proenzyme which is activated by proteolytic cleavage to form the high-molecular-weight two-chain active uPA or the low-molecular-weight uPA through the action of plasmin, kallikrein, or cathepsin B[4]. Active uPA cleaves inactive plasminogen to generate active plasmin, a broad-specific serine protease, which can degrade a variety of ECM proteins. Besides, plasmin and uPA can also activate several types of MMPs which, in turn, degrade ECM. Therefore, uPA amplifies proteolytic cascades in ECM degradation which is crucial for cancer invasion. uPA exerts its effect by binding to the uPA receptor (uPAR), which localizes uPA on the cell surface, enhancing its plasminogen activation capability[5]. This activity is, in turn, negatively regulated by the plasminogen activator inhibitor type 1 and 2 (PAI-1 and -2)[5].
uPA expression has been shown to be upregulated in many cancers, where its expression has been correlated with invasion and metastasis[6]. Although uPA expression has been demonstrated in some CCA cell lines[7,8], there has been no report of uPA expression in clinical specimens, nor a linkage between its expression with the clinical symptoms of CCA patients. In this study, we investigated the possible role of uPA in CCA development in vivo by examining the expression pattern of uPA in clinical samples and relating those findings to the various clinicopathological parameters of CCA patients. In addition, we determined the role of uPA in vitro using siRNA or specific inhibitor to suppress the uPA function and assessed the phenotype of the cells with uPA downregulation in an in vitro Transwell assay.
Archival paraffin-embedded tissue samples were obtained from 174 patients (aged 32-75 years) who underwent liver resection at Srinagarind Hospital, Khon Kaen University, Thailand during 1999-2010. These samples were used to generate the tissue microarray for this retrospective study. All patients were diagnosed with intrahepatic CCA. Vascular, lymphatic and neural or perineural invasion were defined by the presence of tumor cells in the blood vessels, lymphatic vessels and in or around the nerve fibers in the liver, respectively. The study protocol was approved by the Ethical Committee of Khon Kaen University (HE 521209).
Two human CCA cell lines developed from Thai patients, HuCCA-1[9] and KKU-M213 and one human immortalized cholangiocyte cell line, H69, were used. All cell culture materials (medium, serum and antibiotics) were purchased from Gibco Invitrogen (Auckland, New Zealand). The CCA cells were cultured in HAM’s F-12 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mmol/L glutamine, 15 mmol/L HEPES and 14 mmol/L sodium bicarbonate, 100 U/mL penicillin G and 100 U/mL streptomycin. The cells were incubated at 37 °C under a humidified 5% CO2 atmosphere. H69 was grown in Dulbecco’s Modified Eagle’s Medium (DMEM)/DMEM F12 (1:1) supplemented with 10% FBS, hormones and epidermal growth factor, as previously described[10].
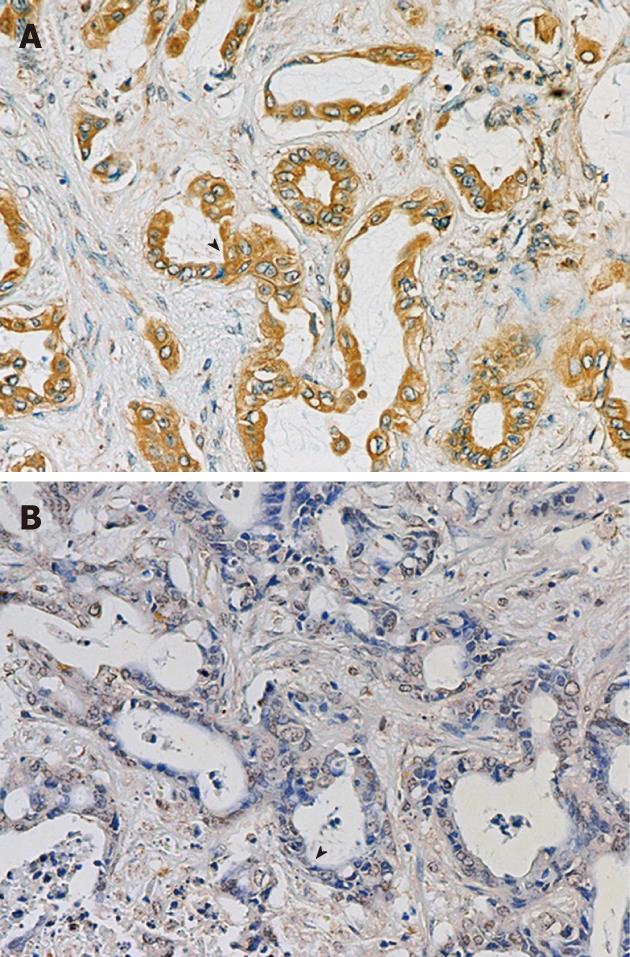
Tissue microarray (TMA) was generated manually from the paraffin-embedded tissues. In brief, the region of interest from each paraffin block was identified on a hematoxylin-eosin-stained slide, after which the slide was aligned with the surface of the original paraffin block to locate the sampling area. The area of interest in the paraffin block was then punched with a 1-mm-diameter needle before each punched tissue was then manually transferred to a new recipient paraffin block to generate a TMA block. Five-micrometer-thick sections were cut from the TMA block, mounted on a silane-coated glass slide, followed by immunohistochemical staining. The specimens were deparaffinized and dehydrated before the endogenous peroxidase activity in the tissue section was blocked with 0.5% H2O2 in methanol for 30 min. After washing with PBS, pH 7.4, and blocking with blocking solution containing 5% normal horse serum in PBS, pH 7.4, for 30 min, the specimens were hybridized with 0.5 μg/mL monoclonal antibody against uPA (Ab No. 3689; American Diagnostica, Stamford, CT, United States), followed by a horseradish peroxidase (HRP)-conjugated secondary antibody (Invitrogen, Carlsbad, CA, United States). The brown color corresponding to the peroxidase activity was developed using diaminobenzidine (Sigma, St Louis, MO, United States). The specimens were counter-stained with Mayer’s hematoxylin. Negative controls were performed in a similar way, omitting the primary antibody. The uPA staining was scored based on signal intensity as follows: negative, weak (+), moderate (++) and strong (+++).
uPA activity secreted from cancer cells into the conditioned medium (CM) was determined by plasminogen-gelatin zymogaphy under non-reducing conditions. Cells (3.5 × 105) were cultured in six-well plates for 2 d, washed twice, and incubated in serum-free medium for 6 h. Proteins in CM were separated by 8% SDS-PAGE containing 10 μg/mL plasminogen (Roche Diagnostics GmbH, Mannheim, Germany) and 1 mg/mL gelatin (Sigma) under non-reducing conditions. After electrophoresis, the gel was washed twice with 2.5% TritonX-100 for 1 h to remove SDS, incubated for 18 h in the reaction buffer containing 100 mmol/L Tris-HCl, pH 7.8, 150 mmol/L NaCl and 1% TritronX-100, followed by staining with 0.25% Coomassie blue and destaining with 45% methanol and 10% acetic acid. A clear band with an estimated molecular weight of 43 kDa represented the uPA activity band. To confirm its PA (not gelatinase) activity, plasminogen-free gelatin zymogram gel was run in parallel as a negative control.
To detect the bound uPA, cells were washed twice with PBS before the bound uPA was eluted by elution buffer [100 mmol/L NaCl and 50 mmol/L glycine-HCl (pH 3.0)]. The eluate was neutralized by adding 0.5 mol/L Tris-HCl, (pH 7.8), at the ratio of eluate:neutralization buffer of 4:1, and analyzed by plasminogen-gelatin zymography.
uPA and PAI-1 protein levels in 40× concentrated CM were determined by western blot analysis using anti-uPA and anti-PAI-1 antibodies (American Diagnostica), respectively. Proteins were separated by 8% SDS-PAGE, transferred to a nitrocellulose membrane, probed with anti-uPA and anti-PAI-1 antibodies, before being hybridized with HRP-conjugated secondary antibodies. The uPA and PAI-1 bands were developed by enhanced chemiluminescence ECL Plus reagent (GE Healthcare, Little Chalfont, Bucks, United Kingdom) and visualized by Fluor Chem SP (Alpha Innotech, San Leandro, CA, United States).
uPA and uPAR mRNA expression was determined by real-time reverse transcriptase polymerase chain reaction (RT-PCR) using ABI 7500 (Applied Biosystems, Foster City, CA, United States). RNA was extracted using RNeasy mini kit (Qiagen, Valencia, CA, United States) following the manufacturer’s protocol. Two micrograms total RNA was converted to cDNA by SuperScript™ IIIReverse Transcriptase kit (Invitrogen, Grand Island, NY, United States) using random hexamer primer, and the cDNA was amplified by real-time PCR in a 20-μL reaction volume containing 0.5 U HotStart Taq polymerase (Qiagen), 1× FastStart Universal SYBR Green Master cocktail (Roche Diagnostics GmbH, Mannheim, Germany) and 4 pmol of each specific primer (5’-TTGCTCACCACAACGACATT-3’ and 5’-ATTTTCAGCTGCTCCGGATA-3’ for uPA[11], 5’-GGTGACGCCTTCAGCATGA-3’ and 5’-CCCACTGCGGTACTGGACAT-3’ for uPAR and 5’-GTAACCCGTTGAACCCCATT-3’ and 5’-CCATCCAATCGGTAGTAGCG-3’ for 18sRNA, as an internal control). The reactions were started with an initial heat activation step, followed by 40 thermal cycles. The mRNA levels among the test cells were analyzed by relative quantification 2–ΔΔCt method.
KKU-M213 cells were transiently transfected with siRNA against uPA (Santa Cruz Biotechnology, Santa Cruz, CA, United States) using siRNA transfection reagent (Santa Cruz Biotechnology) following the manufacturer’s protocol with some modifications. In brief, 6 μL siRNA and 6 μL transfection reagent were separately diluted in 100 μL siRNA transfection medium. The diluted siRNA solution was mixed with the diluted transfection reagent, incubated at room temperature for 15 min, before being added to a six-well plate seeded with 2 × 105 CCA cells in 0.8 mL transfection medium. The level of uPA mRNA was accessed at 48 and 72 h after transfection. Silencer Negative Control siRNA #1 (Ambion, Austin, TX, United States), a non-targeted sequence, was used as the negative control.
The invasiveness of CCA cells was determined using a Matrigel-coated Transwell chamber (8-μm pore size polyvinylpyrrolidone-free polycarbonate filter with 6.5 mm diameter) (Corning Inc., Corning, NY, United States) pre-coated with 30 μg Matrigel (BD Biosciences, San Diego, CA, United States). One hundred thousand transfected cells at 66 h post-transfection in FBS-free media were added to the upper chamber of the Transwell, while 600 μL medium containing 10% FBS was added to the lower chamber. After incubation for 6 h at 37 °C in a CO2 incubator, non-invaded cells were removed from the upper chamber, and invaded cells were fixed and stained for 30 min with 0.5% crystal violet in 25% methanol. The number of invaded cells in five random fields was counted under a light microscope using a 10× objective. Three independent experiments were performed, each done in duplicate.
All statistical analysis was performed using SPSS version 16.0 software. Correlation between uPA expression and clinicopathological factors was analyzed using the χ2 test. Survival analysis was done by Kaplan-Meier and log-rank tests. mRNA and invasion data were expressed as mean ± SE from three independent experiments. Comparison of the data between groups was performed by t test. P < 0.05 was considered significant.
We examined uPA expression in 174 CCA specimens by performing immunohistochemistry on the TMA. One hundred and thirty-one cases (75.3%) expressed uPA, which was mainly localized in cytoplasm of CCA cells (Figure 1). The low uPA expressing cells (negative and +) accounted for 50.6%, whereas those with high uPA expression (++ and +++) constituted 49.4% of the total sample number (Figure 1).
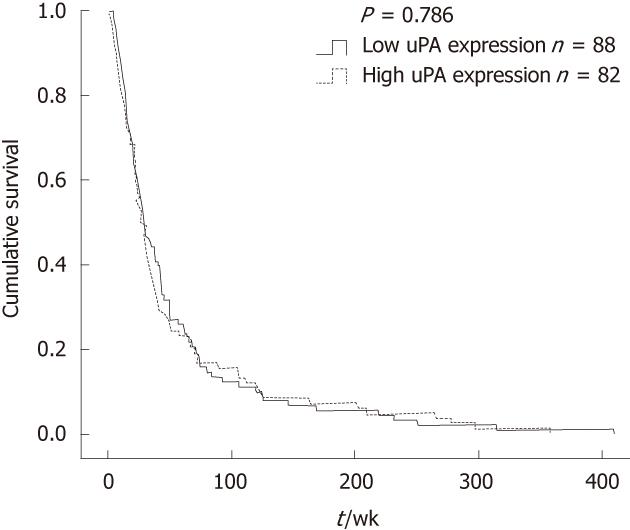
The correlation between uPA expression and clinicopathological parameters is summarized in Table 1. There was no correlation between uPA expression level and age, sex, tumor size, histological type, vascular invasion, neural invasion (Table 1) and patients’ survival (Figure 2). However, uPA expression level in the CCA tissues was positively correlated with lymphatic invasion (P = 0.012) and metastasis (P = 0.048).
| Variables | uPA | P value | |
| Low | High | ||
| Age (yr) | |||
| ≤ 50 | 22 | 23 | 0.793 |
| > 50 | 66 | 63 | |
| Sex | |||
| Male | 61 | 55 | 0.453 |
| Female | 27 | 31 | |
| Tumor size (cm) | |||
| ≤ 5 | 29 | 28 | 0.956 |
| > 5 | 59 | 58 | |
| Histotype group | |||
| Well differentiated | 33 | 31 | 0.065 |
| Moderately differentiated | 16 | 20 | |
| Poorly differentiated | 18 | 6 | |
| Papillary | 20 | 25 | |
| Adenosquamous | 1 | 4 | |
| Gross type | |||
| Mass forming | 71 | 63 | 0.348 |
| Periductal infiltration | 15 | 22 | |
| Mix type1 | 2 | 1 | |
| Vascular invasion (n = 168) | |||
| Absent | 28 | 32 | 0.451 |
| Present | 57 | 51 | |
| Nerve invasion (n = 166) | |||
| Absent | 55 | 46 | 0.154 |
| Present | 28 | 37 | |
| Lymphatic invasion (n = 167) | |||
| Absent | 29 | 15 | 0.0122 |
| Present | 55 | 68 | |
| Metastasis | |||
| Absent | 50 | 36 | 0.0482 |
| Present | 38 | 50 | |
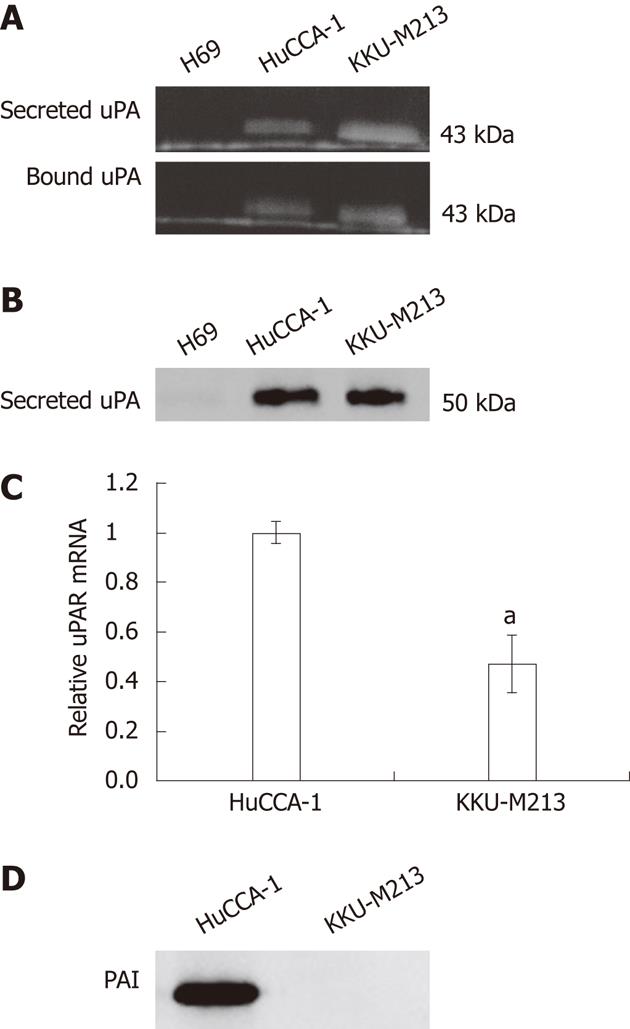
Expression pattern of the secreted form and the membrane-bound form of uPA was investigated using plasminogen-gelatin zymography in the CCA cell lines, HuCCA-1, and KKU-M213, compared with that of an immortalized cholangiocyte cell line, H69. Both CCA cell lines, but not the cholangiocytes, expressed a 43-kDa PA band on the plasminogen–gelatin zymogram (Figure 3A), and this was later confirmed by western blotting using anti-uPA antibody to correspond to uPA (Figure 3B). The discrepancy in the molecular weight of uPA determined by plasminogen–gelatin zymography (43 kDa) and by western blotting (50 kDa) could be accounted for by the different denaturation conditions employed by the two methods, in which the protein was partially denatured by a non-reducing-unheated condition in the zymogram gel, whereas it was completely unfolded by a reducing-heated condition in the western blotting.
The uPA is under positive and negative regulation by uPAR and PAI-1, respectively. Using quantitative real-time PCR, we showed that both CCA cell lines expressed uPAR mRNA, where the expression level in HuCCA-1 was twice the level in KKU-M213 cells (Figure 3C). In addition, we showed by western blotting that PAI-1, a major uPA inhibitor, was secreted abundantly in HuCCA-1 but not in KKU-M213 cells (Figure 3D).
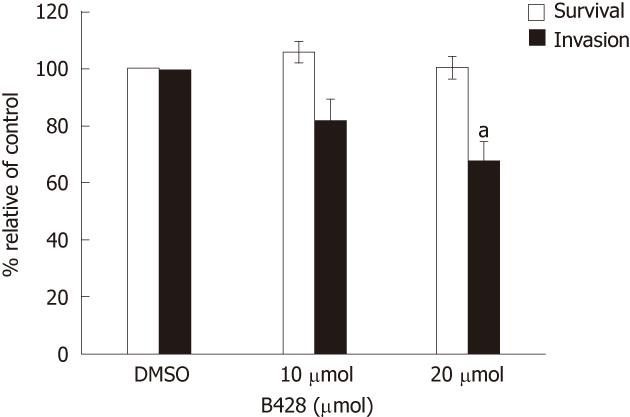
Our immunohistochemical data showed that uPA expression in CCA specimens correlated with invasion and metastasis, thus, we investigated if uPA played a role in vitro by assaying for the invasiveness of the cancer cell line in which the uPA activity was inhibited. We showed that B428, a uPA-specific inhibitor[12], dose dependently reduced the invasiveness of KKU-M213 cells compared with controls (Figure 4). Treatment with 20 μmol/L B428 suppressed in vitro invasion by 32%, whereas uPA activity as determined by plasminogen-gelatin zymography, of which both gel and reaction buffer contained the inhibitor, was reduced by 64%. Suppression of in vitro invasion was not due to toxicity of B428, because the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay showed that B428 had no significant effect on cell survival under these conditions (Figure 4).
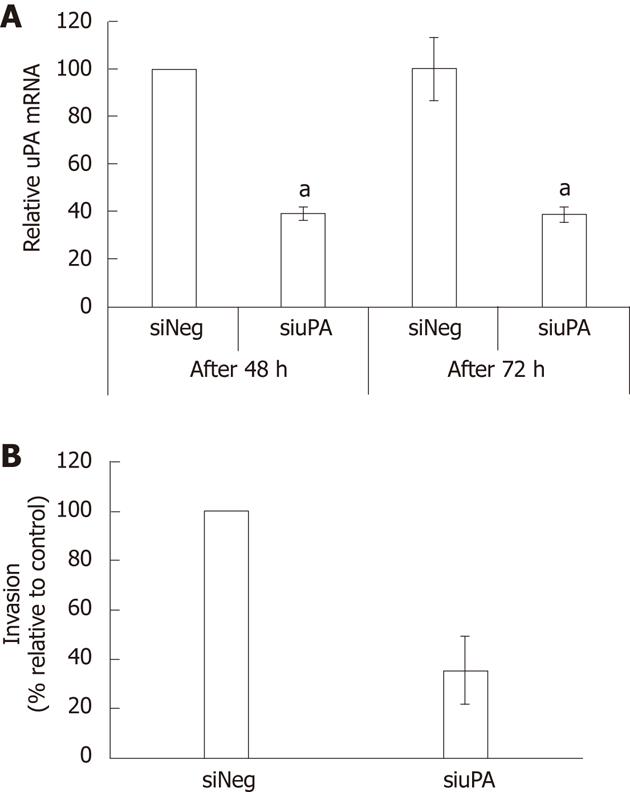
The significance of uPA in the invasiveness of CCA cells was confirmed by silencing of uPA using siRNA to target uPA gene expression in KKU-M213 cells. Using quantitative real-time RT-PCR, we showed that the uPA mRNA level of cells transfected with siuPA was suppressed by about 60% compared to that of cells transfected with siNeg (Figure 5A). In vitro invasion of the uPA-silenced cells was reduced by 66% ± 12% compared to that in the negative controls (Figure 5B). Suppression of uPA activity using two independent methods confirmed that uPA is an important requirement for invasiveness of CCA cells.
One of the key features required for cancer invasion and metastasis is the ability to degrade ECM and basement membrane barrier. This process is accomplished by the action of a variety of proteolytic enzymes, including serine proteases and MMPs, which work in concert[5]. uPA, a plasminogen-specific serine protease, is one of the important proteolytic enzymes contributing to this process.
uPA has been reported to be upregulated in many cancer types, such as breast[13], prostate[14], colorectal[15], gastric[16], and thyroid[17], and its expression level correlates with cancer progression. Here, we used immunohistochemical analysis to demonstrate that uPA expression could be detected in the majority (75.3%) of CCA specimens, and that high level of uPA expression correlated with lymphatic invasion and metastasis in CCA patients. However, we found no significant correlation between uPA expression level and patients’ survival rate. Several clinical features contribute to survival of intrahepatic CCA patients. Large tumor size, multifocal tumors, positive resection margin, gross appearance as mass-forming plus periductular infiltration, invasion and metastasis have been shown as clinicopathological features related to poor prognosis and short survival[18].
The role of uPA in CCA invasion was investigated in vitro using CCA cell lines, HuCCA-1 and KKU-M213, and compared with the non-cancer immortalized cholangiocytes, H69. Plasminogen-gelatin zymography and western blotting demonstrated that both CCA cell lines, but not the non-cancer immortalized cholangiocytes, expressed the cell-surface-bound and the secreted forms of uPA. Although the two cell lines expressed relatively high amounts of uPA, only KKU-M213, but not HuCCA-1 cells exhibited a high degree of invasiveness as previously determined by in vitro Transwell assay[19]. This could probably be explained by the fact that HuCCA-1, but not KKU-M213 cells expressed PAI-1, a uPA inhibitor, thereby attenuating uPA activity to promote matrix degradation and cell invasion. Expression of PAI-1 in HuCCA-1 cells may also explain why the level of cell-surface-bound uPA was similar in the two cell lines, although that of uPAR mRNA was twice as high in HuCCA-1 compared to KKU-M213 cells. Binding of PAI-1 to the uPA-uPAR complex has been reported to promote uPA-uPAR-PAI-1 complex internalization and lysosomal degradation of uPA and PAI-1[6], leading to a reduction of the bound uPA on the cell surface.
Inhibition of uPA or uPAR function by anti-uPA or anti-uPAR antibody or by non-specific serine protease inhibitor has been shown to reduce significantly in vitro invasiveness of papillary thyroid cancer cells[17]. Similarly, silencing of uPA and/or uPAR by siRNA has been shown to suppress in vitro invasion of many cancer types including those of the prostate[20] and breast[21], and glioma[22]. We showed that inhibition of uPA activity by specific uPA inhibitor, B428, or of uPA expression by siRNA efficiently suppressed in vitro invasion of KKU-M213 cells, consistent with a previous report in QBC939 cells, a uPA-expressing CCA cell line, whose invasiveness was markedly reduced by inhibiting plasminogen activation with tranexamic acid or 6-aminocaproic acid[7]. Observations of the importance of uPA in invasion in a variety of cancers suggest a common role of uPA in cancer invasion, including CCA. A drug inhibiting uPA is already undergoing clinical trial for some cancers[23] and if proven successful, could be applied to CCA.
In summary, we have showed by immunohistochemistry that high uPA expression in CCA tissue was correlated with lymphatic invasion and metastasis. Studies in cell lines confirmed the importance of uPA in cell invasiveness, but the presence of PAIs or uPAR can affect uPA-dependent cell invasion. The roles of these factors in modulating CCA invasion and metastasis clearly warrant future investigation.
The authors thank Professor Sirisinha S (Mahidol University, Thailand) for providing us HuCCA-1 cell line and Professor Alpini G (Texas A and M University, TX, United States) and Professor Gores G (Mayo Clinic, MN, United States) for H69 cell line, and Professor Wilairat P and Dr. Kitjaroentham A for critical reading of the manuscript.
Cholangiocarcinoma (CCA), a cancer of the bile duct, is an aggressive cancer with high metastatic and short survival rate. One of the critical properties required for cancer metastasis is the ability to degrade extracellular matrix. Urokinase plasminogen activator (uPA), a key proteolytic enzyme involved for this process, is frequently overexpressed in many types of cancers.
Although uPA has been demonstrated to play an important role in invasion of many cancer types, the correlation between uPA expression and CCA progression has not hitherto been reported.
The authors have demonstrated that high uPA expression in CCA tissue correlated with high lymphatic invasion and metastasis in patients. The importance of uPA in cell invasiveness was confirmed in vitro using uPA-expressing CCA cell lines. In addition, the in vitro study indicated that plasminogen activator inhibitors could have an important impact on uPA-mediated cancer cell invasiveness.
The requirement of uPA in CCA invasiveness highlights uPA as a potential therapeutic target.
Urokinase plasminogen activator (uPA) is a plasminogen-specific serine protease. It converts inactive plasminogen to active plasmin, which degrades extracellular matrix and activates matrix metalloproteinases.
This study investigated uPA expression in CCA tissues and its role in CCA cell invasion in vitro. The authors found that high uPA expression correlated with invasion and metastasis in CCA patients. The design of this research is good and the findings are novel and important, so it is suitable for our readers.
| 1. | Patel T. Cholangiocarcinoma. Nat Clin Pract Gastroenterol Hepatol. 2006;3:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 600] [Cited by in RCA: 605] [Article Influence: 31.8] [Reference Citation Analysis (2)] |
| 3. | Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [PubMed] |
| 4. | Stepanova VV, Tkachuk VA. Urokinase as a multidomain protein and polyfunctional cell regulator. Biochemistry (Mosc). 2002;67:109-118. [PubMed] |
| 5. | Danø K, Behrendt N, Høyer-Hansen G, Johnsen M, Lund LR, Ploug M, Rømer J. Plasminogen activation and cancer. Thromb Haemost. 2005;93:676-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 356] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 6. | Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH. Evolving role of uPA/uPAR system in human cancers. Cancer Treat Rev. 2008;34:122-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 318] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 7. | Wang S, Han B, Duan H. [The role of urokinase type plasminogen activator in invasion of bile duct carcinoma]. Zhonghua Yixue Zazhi. 1996;76:594-596. [PubMed] |
| 8. | Menakongka A, Suthiphongchai T. Involvement of PI3K and ERK1/2 pathways in hepatocyte growth factor-induced cholangiocarcinoma cell invasion. World J Gastroenterol. 2010;16:713-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Sirisinha S, Tengchaisri T, Boonpucknavig S, Prempracha N, Ratanarapee S, Pausawasdi A. Establishment and characterization of a cholangiocarcinoma cell line from a Thai patient with intrahepatic bile duct cancer. Asian Pac J Allergy Immunol. 1991;9:153-157. [PubMed] |
| 10. | Grubman SA, Perrone RD, Lee DW, Murray SL, Rogers LC, Wolkoff LI, Mulberg AE, Cherington V, Jefferson DM. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am J Physiol. 1994;266:G1060-G1070. [PubMed] |
| 11. | Nielsen A, Scarlett CJ, Samra JS, Gill A, Li Y, Allen BJ, Smith RC. Significant overexpression of urokinase-type plasminogen activator in pancreatic adenocarcinoma using real-time quantitative reverse transcription polymerase chain reaction. J Gastroenterol Hepatol. 2005;20:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Towle MJ, Lee A, Maduakor EC, Schwartz CE, Bridges AJ, Littlefield BA. Inhibition of urokinase by 4-substituted benzo[b]thiophene-2-carboxamidines: an important new class of selective synthetic urokinase inhibitor. Cancer Res. 1993;53:2553-2559. [PubMed] |
| 13. | Hurd TC, Sait S, Kohga S, Winston J, Martinick M, Saxena R, Lankes H, Markus G, Harvey S, Gibbs JF. Plasminogen activator system localization in 60 cases of ductal carcinoma in situ. Ann Surg Oncol. 2007;14:3117-3124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Gavrilov D, Kenzior O, Evans M, Calaluce R, Folk WR. Expression of urokinase plasminogen activator and receptor in conjunction with the ets family and AP-1 complex transcription factors in high grade prostate cancers. Eur J Cancer. 2001;37:1033-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Harvey SR, Sait SN, Xu Y, Bailey JL, Penetrante RM, Markus G. Demonstration of urokinase expression in cancer cells of colon adenocarcinomas by immunohistochemistry and in situ hybridization. Am J Pathol. 1999;155:1115-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Kaneko T, Konno H, Baba M, Tanaka T, Nakamura S. Urokinase-type plasminogen activator expression correlates with tumor angiogenesis and poor outcome in gastric cancer. Cancer Sci. 2003;94:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Nowicki TS, Kummer NT, Iacob C, Suslina N, Schaefer S, Schantz S, Shin E, Moscatello AL, Tiwari RK, Geliebter J. Inhibition of uPAR and uPA reduces invasion in papillary thyroid carcinoma cells. Laryngoscope. 2010;120:1383-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Sirica AE, Dumur CI, Campbell DJ, Almenara JA, Ogunwobi OO, Dewitt JL. Intrahepatic cholangiocarcinoma progression: prognostic factors and basic mechanisms. Clin Gastroenterol Hepatol. 2009;7:S68-S78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Treekitkarnmongkol W, Suthiphongchai T. High expression of ErbB2 contributes to cholangiocarcinoma cell invasion and proliferation through AKT/p70S6K. World J Gastroenterol. 2010;16:4047-4054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Pulukuri SM, Gondi CS, Lakka SS, Jutla A, Estes N, Gujrati M, Rao JS. RNA interference-directed knockdown of urokinase plasminogen activator and urokinase plasminogen activator receptor inhibits prostate cancer cell invasion, survival, and tumorigenicity in vivo. J Biol Chem. 2005;280:36529-36540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 21. | Subramanian R, Gondi CS, Lakka SS, Jutla A, Rao JS. siRNA-mediated simultaneous downregulation of uPA and its receptor inhibits angiogenesis and invasiveness triggering apoptosis in breast cancer cells. Int J Oncol. 2006;28:831-839. [PubMed] |
| 22. | Gondi CS, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS. Downregulation of uPA, uPAR and MMP-9 using small, interfering, hairpin RNA (siRNA) inhibits glioma cell invasion, angiogenesis and tumor growth. Neuron Glia Biol. 2004;1:165-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Patient recruitment in phase II breast cancer trial with Mesupron successfully completed. Wilex AG, Munich, Germany, 2011-05-05. Available from: http: //www.wilex.de/News/2011/050511.htm. |
Peer reviewers: Mansour A Parsi, MD, Center for Endoscopy and Pancreatobiliary Disorders, Digestive Disease Institute/A31, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, United States; Michele Reni, MD, Department of Oncology, San Raffaele Scientific Institute, via Olgettina 60, Milan 20132, Italy; Ching Chung Lin, MD, MMS, Division of Gastroenterology, Department of Internal Medicine, Mackay Memorial Hospital, Taipei 111, Taiwan, China
S- Editor Lv S L- Editor Kerr C E- Editor Xiong L