MATERIALS AND METHODS
Patients
A total of 69 patients (36 males and 33 females, age range 24-68 years, mean age 42.5 years) were diagnosed with gastric stromal tumors originating from the muscularis propria by gastroscopy and endoscopic ultrasonography from January 2008 to March 2011. Among the 69 cases, 19 tumors were located at the gastric antrum, 24 tumors were located at the gastric corpus, and 26 tumors were located at the gastric fundus. Each patient exhibited only one tumor, and no metastasis was detected via computed tomography (CT) examination. Routine blood tests, tests of blood coagulation function and hepatic and renal function, electrocardiograms and abdominal CTs were performed before the endoscopic therapy, and each patient’s written informed consent was obtained.
Instruments
The following instruments were used: Electronic gastroscope (Olympus GIF-Q260J, Olympus company, Japan), hyaline cap (D-201-11304, Olympus company, Japan), spiculiform cutting knife (KD-1 L-1, Olympus company, Japan), IT knife (KD-611 L, Olympus company, Japan), hook knife (KD-620 LR, Olympus company, Japan), injection needle (NM-200 L-0525, Olympus company, Japan), snare (AS-1-S, ASJ-1-S, COOK company, United States), hot biopsy forceps (FD-410 LR, Olympus company, Japan), hemostatic clip (HX-610-90, Olympus company, Japan; HX-600-135, Olympus company, Japan; Boston ResolutionTM, Boston company, United States), high frequency electric knife (ERBE VIO 200S, ERBE company, Germany) and Argon Plasma Coagulation instrument (ERBE APC2, ERBE company, Germany).
Endoscopic therapeutic methods
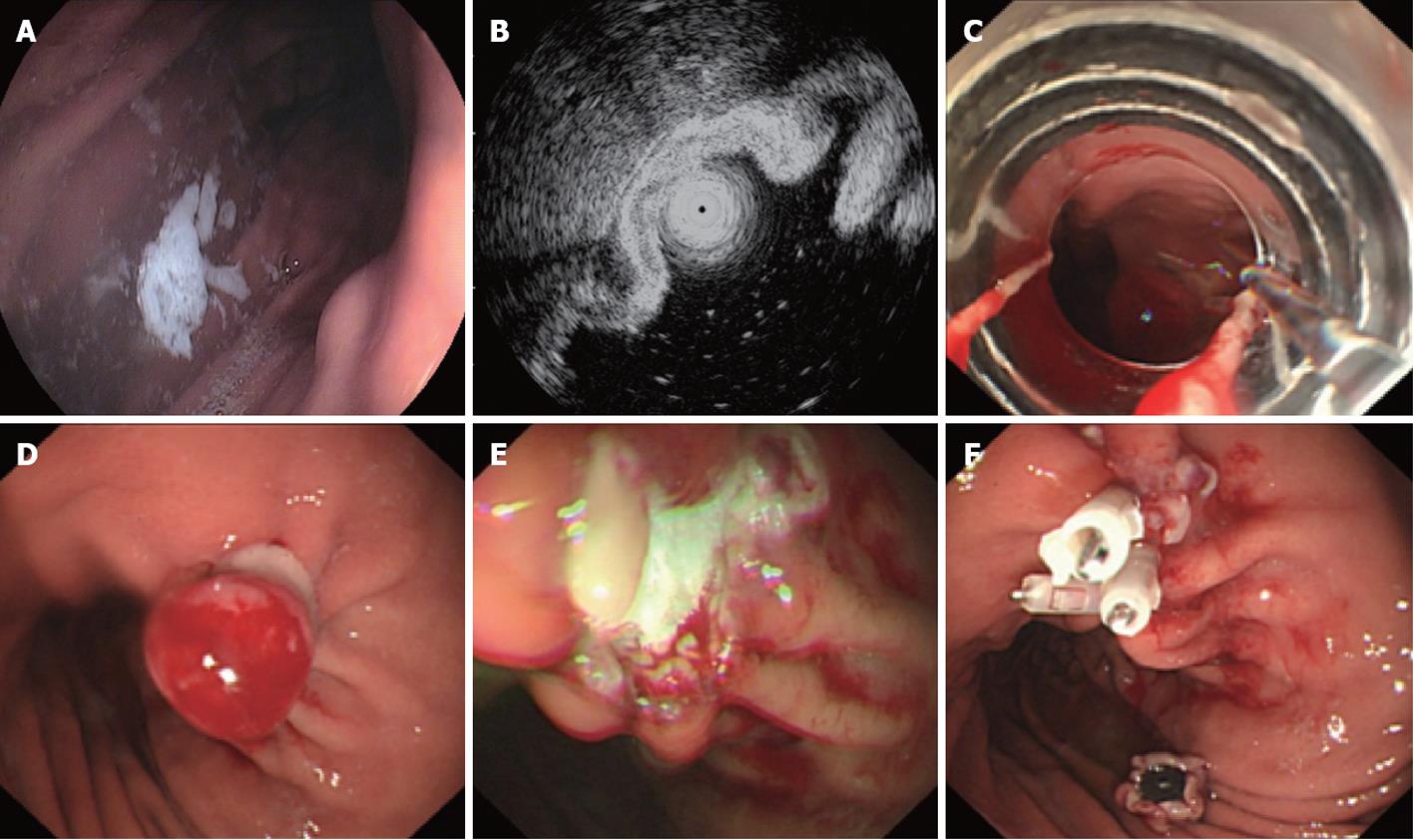
Endoscopic ligation and resection: Endoscopic ligation and resection (ELR) was used for gastric stromal tumors of which the size was less than 1.2 cm. A COOK ligator was assembled on the tip of the gastroscope, and the gastroscope was inserted into the stomach. The ligator was aimed at the tumor, and sufficient aspiration was applied to the tumor that the whole tumor entered the ligator, at which time the rubber band on the ligator was released to ligate the tumor. The purpose of the ligation was to manipulate the gastric stromal tumor into the shape of a polypoid, after which a snare was used to hitch the tumor, and it was resected. The resected tumor was removed for pathologic diagnosis, and the stump was closed with metal clips. If the procedure was complicated by perforation, the perforation was generally small and could be closed with metal clips (Figure 1).
Figure 1 Endoscopic ligation and resection treatment for a gastric stromal tumor less than 1.
2 cm in size originating from the muscularis propria. A: Submucosa lesion at the posterior wall of the gastric corpus; B: Endoscopic ultrasound shows that the lesion originates from the muscularis propria; C: COOK ligator aimed at the lesion, ready to ligate; D: The ligated stromal tumor was in the shape of a polypoid with deuto-stem; E: A snare was used to cut the tumor above the rubber band; F: The wound surface was closed with metal clips.
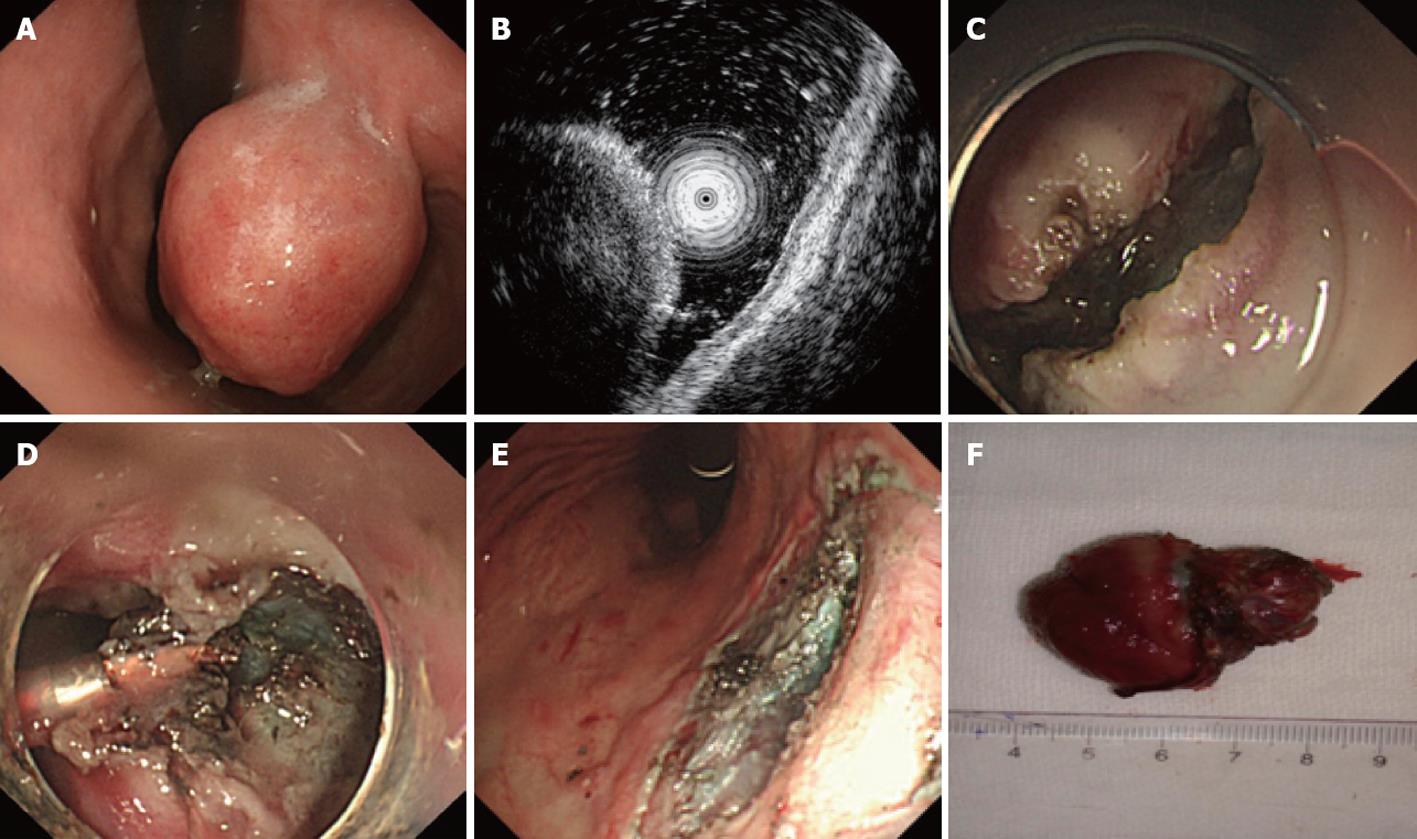
Endoscopic submucosal excavation: Endoscopic sub-mucosal excavation (ESE) was performed for gastric stromal tumors originating from the muscularis propria by the following steps. (1) Marking: Argon plasma coagulation (APC) was used to mark the border of the gastric stromal tumor; (2) Submucosal injection: A mixture of indicarminum, adrenalin and physiological saline (2-3 mL of indicarminum, 1 mL of adrenalin and 100 mL of physiological saline) was injected at the lateral of the mark; (3) Dissection: the mucosa was dissected with a needle knife; (4) Excavation: the submucosa was dissected with a needle knife; when the tumor was exposed, dissection was performed around the margin of the tumor, and when the dissection was finished, the tumor could be resected wholly with a snare; and (5) Wound surface handling: for small vessels bleeding on the surface, hot biopsy forceps or APC could be used for hemostasis, and if necessary, metal clips could be used (Figure 2).
Figure 2 Endoscopic submucosal excavation treatment for a gastric stromal tumor originating from the muscularis propria that is larger than 1.
2 cm. A: Submucosa lesion at the gastric corpus; B: Endoscopic ultrasound shows that the lesion originates from the muscularis propria; C: The mucosa of the stromal tumor was cut after submucosal injection; D: Dissection with an IT knife; E: The excavated wound surface, showing that no perforation occurred; F: The resected stromal tumor (4 cm in size).
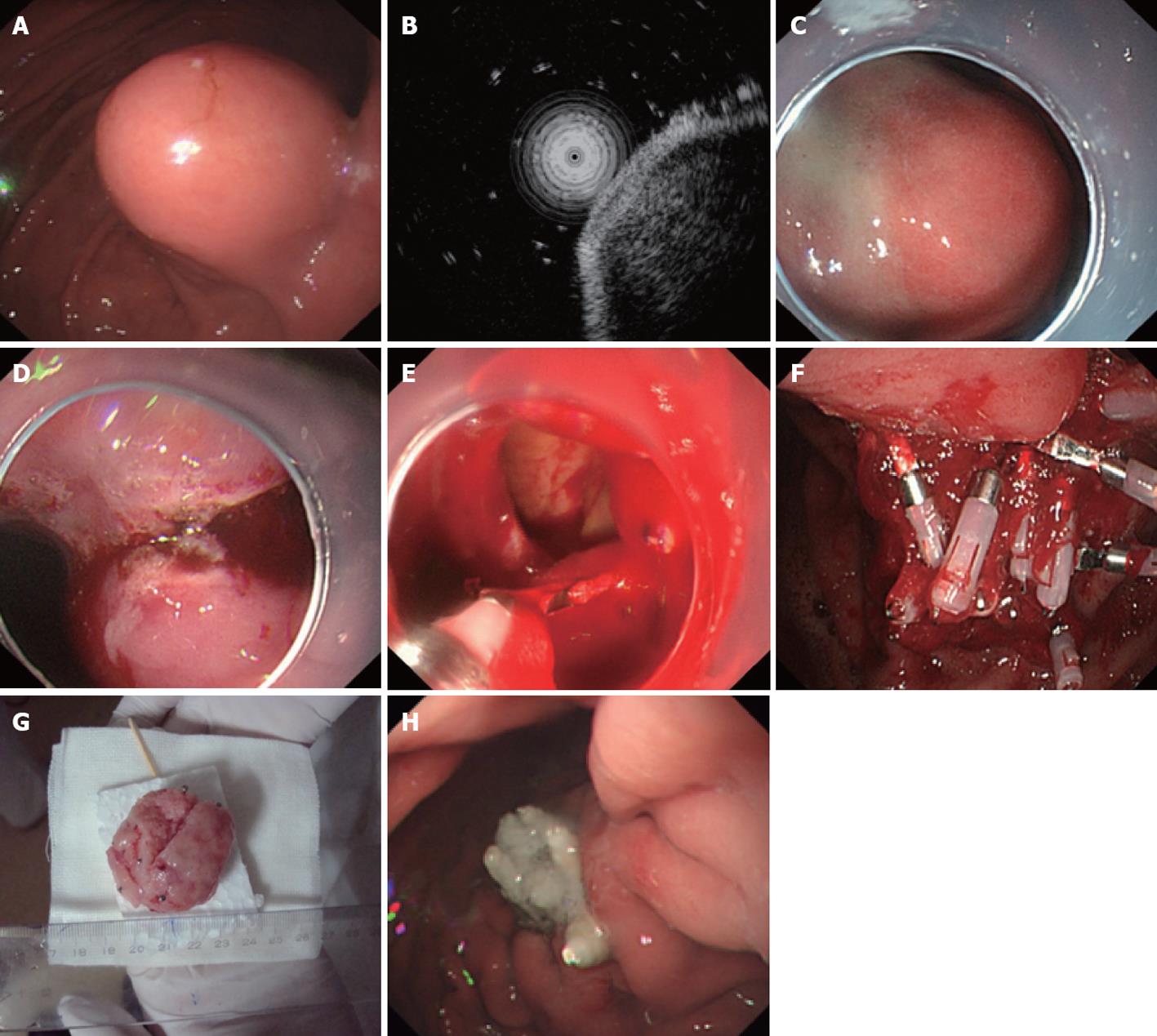
Endoscopic full-thickness resection: A hyaline cap was assembled on the tip of the gastroscope before the endoscopic full-thickness resection (EFR) procedure, and intravenous anesthesia was performed using propofol for patients. EFR was then performed using the following steps. (1) Pre-cutting the mucosa and submucosa around the gastric stromal tumor: APC was used to mark the margin of the tumor; a mixture of indicarminum, adrenalin and physiological saline (2-3 mL of indicarminum, 1 mL of adrenalin and 100 mL of physiological saline) was injected at the lateral portion of the mark, using approximately 2-3 mL of the mixture for each injected point; the mucosa and submucosa around the gastric stromal tumor were pre-cut with a hook knife, and thus the tumor was exposed; (2) Dissection of the muscularis propria around the gastric stromal tumor: a hook knife or IT knife was used to dissect the tumor from the muscularis propria to the serous membrane; (3) Dissection of the serous membrane around the tumor: typically, the tumor was in compact adhesions with the serous membrane; thus the tumor could not be directly dissected with an IT knife, so instead, the serous membrane was cut around the tumor with a needle knife or hook knife, and an “artificial” perforation was made; (4) Full resection of the tumor: the liquid in the stomach was aspirated completely, and an IT knife or hook knife was used to cut the serous membrane around the tumor, after which the tumor was completely resected; and (5) Closure of the wound surface of the stomach: a metal clip was used to close the wound surface from the rim to the center. If the wound surface defect was too large to be closed with a metal clip directly, negative pressure aspiration was used to aspirate the omentum majus into the stomach, and then a metal clip was used to close the wound surface defect (Figure 3).
Figure 3 Endoscopic full-thickness resection treatment for a gastric stromal tumor originating from the muscularis propria larger than 1.
2 cm. A: Submucosa lesion at the gastric corpus; B: Endoscopic ultrasound shows that the lesion originates from the muscularis propria; C: Submucosal injection of the mixture of indicarminum, adrenalin and physiological saline; D: Dissection with an IT knife; E: “Artificial” perforation after gastric stromal tumor resection, closed with metal clips; F: Many clips used to close the wound defect; G: The resected tumor without mucosa (5 cm in size); H: The perforation healed 9 d after endoscopic full-thickness resection.
Specimen handling
The resected specimens were fixed in neutral formalin for pathologic diagnosis. Immunohistostaining of CD34, CD117, Dog-1, S-100 and smooth muscle actin (SMA) was performed for specimens suspected of being gastric stromal tumors[6-10].
Postoperative handling
For patients without perforation, the measures of fasting, infection prevention and limiting acid with proton pump inhibitors were sufficient. For patients with “artificial” perforation, if pneumoperitoneum was serious, evacuation was performed during and after the operation. The evacuating needle was inserted into the lower right quadrant to release abdominal distension. A semi-reclining position was recommended after the operation, along with the use of fasting and gastrointestinal decompression, to allow the perforation to heal. Abdominal pain, abdominal distension and signs of peritoneal irritation after the operation were carefully observed. Meglumine diatrizoate was recommended to be taken orally three days after the operation. To confirm whether there was extravasation of the contrast media and to observe stomach motivation, ultrasonic examination was conducted to observe whether there was hydrops in the abdominal cavity and pelvic cavity. Gastroscopy was performed one month after the operation to observe the conditions of the wound surface and whether there was residual or recurrence.
RESULTS
Effects of endoscopic therapy
ELR: Thirty-eight patients with gastric stromal tumors with a size of less than 1.2 cm underwent ELR, in which the tumor was ligated wholly and then resected. The procedure was complicated by perforations in 3 patients, and all of the wound surfaces and perforations were closed with metal clips. The patients recovered with medical treatment, and no patient needed surgical operation.
ESE: Eighteen patients with gastric stromal tumors in which the size of the specimen resected was more than 1.5 cm underwent ESE. The tumors were resected successfully, and no perforations complicated the procedure.
EFR: Thirteen patients with gastric stromal tumors, for which the size of the specimen resected was more than 2.0 cm, underwent EFR. The tumors were resected successfully, but “artificial” perforation occurred in all of the patients. All of the perforations were closed with metal clips, and meglumine diatrizoate upper gastroenterography three days after the operation showed that there was no extravasation of the contrast media. Gastroscopy showed that the wound surface of the stomach was nearly healed on the 9th day after the operation (Figure 3H).
Immunohistostaining
The positive expression rates of CD34, CD117, Dog-1, S-100 and SMA in the 69 patients who were diagnosed with gastric stromal tumors by gastroscopy were 81.2% (56/69), 82.6% (57/69), 82.6% (57/69), 0% and 17.4% (12/69), respectively, which demonstrated that, among the 69 patients, there were 12 leiomyomas, which express SMA; the other 57 patients had gastric stromal tumors.
Average stay
The average stay for all of the patients was 8.4 ± 2.4 d.
Residual or recurrence of the tumor
Gastroscopy and abdominal CT scans were performed one month after the operation; these procedures showed that the wound surfaces had healed, and there was no residual or recurrence.
DISCUSSION
Stromal tumor is the most common mesenchyme-originated tumor; as it has potential cancerous tendencies, gastric stromal tumor is recommended to be resected[11-15]. For gastric stromal tumors originating from the muscularis propria in which the size was less than 1.2 cm, ELR was the ideal method[16]. After ligation, each tumor was in the shape of a polypoid with a deuto-stem, which made snare resection easy; additionally, a pathologic diagnosis could be obtained with the resected tumor. Because the wound surface in the stomach was not large, perforation would not generally occur; however, if perforation did occur, it could be closed with metal clips. With the medical treatment of fasting, gastrointestinal decompression and proton pump inhibitors, the patients would recover. There were 38 patients in this study in whom the tumor size was less than 1.2 cm; these patients were treated with ELR. Moreover, 3 of these patients were complicated by perforation; the perforations were closed with metal clips, and no surgical assistance was required. Because the texture of the stromal tumor was hard, it could not be as easily aspirated as mucosa or vascular tissue, so the ligation for the stromal tumor required a long period of aspiration. According to our experience, when the stromal tumor was ligated, a red-colored sign occurred when the tumor was aspirated, and at least another one-minute maximum aspiration was necessary to ensure that the rubber band could fully hitch the tumor.
ESE is another effective measure for the treatment of tumors originating from the submucosa or muscularis propria. For tumors of 3.0 cm or less in size originating from the muscularis propria, the achievement ratio of ESE was high and the patient suffered less, whereas the complication rate was low. ESE was able to excavate the whole tumor successfully, and a pathologic diagnosis could be performed using the resected specimen, which was able to provide a systemic and safe therapeutic method for each patient. In this study, there were 18 patients with gastric stromal tumors originating from the muscularis propria who underwent ESE; all of the tumors were excavated wholly, and no perforations occurred.
For patients with gastric stromal tumors originating from the muscularis propria, the perforation rate of endoscopic therapy is high; therefore, the traditional treatment method is surgical operation or therapeutic laparoscopy, especially for stromal tumors larger than 2 cm[17-20]. A total of 13 patients in this study underwent EFR, and perforation occurred in all of the patients. The sizes of the 13 tumors were all larger than 2 cm, all of the perforations were closed with metal clips, the abdominal cavity pressure decreased with the use of abdominal paracentesis, all of the patients recovered with medical treatment, and no patients needed surgical help. The key point in avoiding complications was the use of endoscopic metal clips for oversewing: according to our experience, for larger perforations, the perforation should be closed with metal clips from rim to center, known as oversewing, and even some normal mucosa should be sewed up to decrease the perforation. The key point for successful EFR is to mend the perforation successfully, avoiding surgical mending and postoperative peritonitis[21,22]. The most widely used method to mend perforation is metal clip sutura[23,24]. For small perforations, one or several metal clips could oversew the perforation completely. For larger perforations, as the span of the metal clip is limited, the gas in the stomach must be aspirated properly to reduce the perforation, and several metal clips should be used to close the perforation. If the perforation is too large to be oversewn with metal clips directly, the retina mending method is recommended[25,26]. The gas in the stomach should be aspirated with continuous negative pressure until the adipose tissue outside the gastric wall covers the perforation, at which point the perforation is closed with metal clips. The other essential point for EFR is to avoid too much gastric juice entering the abdominal cavity; to prevent postoperative infection, strict hemostasis for the wound surface should be performed, but repeat rinse hemostasis should be avoided during the procedure[27]. The gas and liquid in the stomach should be aspirated fully before cutting the serous membrane. Postoperative gastrointestinal decompression, proton pump inhibitors and antibiotics were effective measures to prevent abdominal cavity infection, and no complications of peritonitis or peritoneal abscesses occurred in this study.
Pneumoperitoneum caused by perforation after EFR can influence the field of vision in the stomach, making endoscopic operation difficult; thus, during EFR, repeated abdominal touch was necessary. If the pressure in the abdominal cavity increased, prompt evacuation was needed; the puncture site was in the right lower quadrant, and the needle was a commonly used 20 mL injection needle. After puncture, the abdomen was compressed to exhaust the air, the needle was detained until the perforation was closed and the pneumoperitoneum had clearly improved, and the needle was removed when it was confirmed that there was no further gas exhausting from the abdominal cavity.
It is difficult to distinguish gastric stromal tumors from leiomyomas and schwannomas based on cell shape. The clinical diagnosis of this tumor type mainly depends on histopathology, immunohistochemistry (CD34- and CD117-positive) and protocols in molecular biology[28,29]. CD34 is the antigen marker of myeloid stem cells; it resides in the marrow hematopoietic stem cell tissue. CD117 is the product of the c-kit oncogene; it is the receptor of tyrosine kinase growth factor. The Dog-1 gene is located at chromosome 11q13; it has 26 exons and is 114 Kb in length; the protein has 8 trans-membrane functional areas; it may be a chloride channel regulated by calcium ions, and it is currently recognized as the most sensitive and specific marker for gastrointestinal stromal tumors (GIST)[30,31]. Typical leiomyomas and schwannomas do not express CD34; SMA-positive expression is the typical characteristic of leiomyoma, and S-100-positive expression is the typical characteristic of schwannomas[32]. The positive expression rates of CD34, CD117, Dog-1, S-100 and SMA in the 69 patients were 81.2% (56/69), 82.6% (57/69), 82.6% (57/69), 0% and 17.4% (12/69), respectively, which demonstrated that, among the 69 patients, there were 12 leiomyomas, which express SMA, and the other 57 patients had gastric stromal tumors confirmed with immunohistochemistry. Currently, the main treatment method for GISTs is surgical operation; the majority of benign GISTs in this study had a good prognosis after resection, and there was no recurrence in a short time. GISTs are not sensitive to radiotherapy or chemotherapy. For malignant GISTs that could not be completely resected or with recurrence, patients could take imatinib for therapy. Imatinib is an inhibitor of tyrosine kinase; it has significant therapeutic effects on malignant GISTs that express CD117[33-37].
With the maturation of EFR, more and more gastric stromal tumors originating from the muscularis propria would avoid surgical operations, sharing the benefits provided by therapeutic endoscopy techniques.
COMMENTS
Background
The traditional method for the treatment of gastric stromal tumor originating from the muscularis propria was laparoscopic or surgical operation; in recent years, with the development of endoscopic techniques and instruments, endoscopic therapy for gastric stromal tumors originating from the muscularis propria has been made possible.
Research frontiers
Gastric stromal tumor is the most common mesenchymal tissue-originated tumor in the digestive tract; based on the tissue of origin, it can be defined as originating from the muscularis mucosae or the muscularis propria. As the location of the tumor originating from the muscularis mucosae was superficial, endoscopic resection or ligation was commonly performed. In contrast, the location of a tumor originating from the muscularis propria was deep, especially when the tumor grew outside gastric wall, perforation often occurred during endoscopic therapy, and the tumor could not be resected easily and thoroughly. Therefore, this tissue of origin was often considered to be a contraindication for endoscopic therapy, and the traditional treatment for such tumors was surgical or laparoscopic operation. In recent years, on the basis of sufficient clinical practice, endoscopic treatment for gastric stromal tumors originating from the muscularis propria has become possible.
Innovations and breakthroughs
Depending on the size of gastric stromal tumors originating from the muscularis propria, three types of endoscopic therapy could be used to treat the tumor. These techniques were endoscopic ligation and resection, endoscopic submucosal excavation and endoscopic full-thickness resection. The wound surface and the perforation of the gastric wall were closed with metal clips. These results showed that some gastric stromal tumor originated from muscularis propria can be treated successfully with endoscopic techniques, which could replace some surgical operations.
Applications
The study results suggest that some gastric stromal tumors originating from the muscularis propria could be treated successfully with endoscopic techniques, which could replace some surgical operations.
Terminology
Gastrointestinal stromal tumor (GIST): This term indicates a particular type of lobus intermedius-originating tumor in the gastrointestinal tract; usually CD117 was positively expressed. Histologically, this tumor type is often composed of spindle cells and epithelioid cells. With respect to immunophenotype, it expresses the c-kit protein, which is driven by mutated c-kit or platelet-derived growth factor receptors. It is recognized that GIST originate from the intestinal cells of Cajal in gastrointestinal tract, which are in the shape of a network structure distributed in the muscular layer of gastrointestinal tract; this network is the pacemaker of slow-wave activity in the gastrointestinal tract and participates in the mechanism of exertional disease and tumor formation in the gastrointestinal tract.
Peer review
This is a good paper on endoscopic resection of gastric stromal tumors and leimomyomas. The figures are excellent.