Published online Jul 14, 2012. doi: 10.3748/wjg.v18.i26.3379
Revised: July 28, 2011
Accepted: May 12, 2012
Published online: July 14, 2012
AIM: To investigate the effect of age on severity of acute pancreatitis (AP) using biochemical markers, histology and expression of the protective pancreatitis-associated proteins (PAPs).
METHODS: AP was induced via intraductal injection of 4% sodium taurocholate in young and old rats. Sera and pancreata were assayed at 24 h for the parameters listed above; we also employed a novel molecular technique to assess bacterial infiltration using polymerase chain reaction to measure bacterial genomic ribosomal RNA.
RESULTS: At 24 h after induction of AP, the pancreata of older animals had less edema (mean ± SE histologic score of young vs old: 3.11 ± 0.16 vs 2.50 ± -0.11, P < 0.05), decreased local inflammatory response (histologic score of stromal infiltrate: 3.11 ± 0.27 vs 2.00 ± 0.17, P < 0.05) and increased bacterial infiltration (174% ± 52% increase from sham vs 377% ± 4%, P < 0.05). A decreased expression of PAP1 and PAP2 was demonstrated by Western blotting analysis and immunohistochemical staining. There were no differences in serum amylase and lipase activity, or tissue myeloperoxidase or monocyte chemotactic protein-1 levels. However, in the most-aged group, serum C-reactive protein levels were higher (young vs old: 0.249 ± 0.04 mg/dL vs 2.45 ± 0.68 mg/dL, P < 0.05).
CONCLUSION: In older animals, there is depressed PAP expression related to a blunted inflammatory response in AP which is associated with worsened bacterial infiltration and higher C-reactive protein level; this may explain the more aggressive clinical course.
- Citation: Fu S, Stanek A, Mueller CM, Brown NA, Huan C, Bluth MH, Zenilman ME. Acute pancreatitis in aging animals: Loss of pancreatitis-associated protein protection? World J Gastroenterol 2012; 18(26): 3379-3388
- URL: https://www.wjgnet.com/1007-9327/full/v18/i26/3379.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i26.3379
Acute pancreatitis (AP) is a disease with significant impact in the United States. In 2003 there were about 226 000 admissions for this diagnosis, at a cost of over $2.2 billion[1]. The incidence of pancreatitis is higher[2], and its severity is worse[3] in the older patient. While Frey et al[3] showed that the increased comorbidities associated with age account, in part, for the worsened severity, he and others also showed that age is an independent prognostic indicator of survival, specifically in patients over the age of 70 years[3-5].
The mechanism that puts the aged pancreas, and aged patient, at risk for severity in pancreatitis is unknown. Possibilities include: the immune response of the organism to the injury is impaired; the host organ response to the injury (e.g., lung or renal) is worsened; or protective mechanisms from the pancreas are depressed. The latter two concepts are supported by the observation that the aged patient with pancreatitis has a higher incidence of systemic complications, while there is a decreased local complication rate[4].
We have recently defined that pancreatitis-associated proteins (PAPs) are proteins innate to the pancreas which are induced during AP. PAPs are members of the pancreatic regenerating (Reg) family of proteins, which are calcium-dependant lectins; all are secretory proteins[6-8]. PAPs are homologues of pancreatic reg I, and in rats, mice and man, localize to the same chromosome[7,9-12]. However, while reg I is constitutively expressed in normal pancreas, PAP mRNA expression is very low. PAPs are highly induced during AP[7,13].
PAPs are endogenous protectors against pancreatic injury. We have shown that targeted inhibition of the three rat PAP isoforms, PAP1, 2 and 3, exacerbated AP[14,15]. They likely protect by immunomodulation[16-18]. In particular, PAP2 protects by inducing macrophages, and PAP1 has other anti-inflammatory and anti-apoptotic effects[19].
Loss of protection by PAP with aging may put patients at risk for pancreatitis. The normal expression of the PAP homologue pancreatic regeneration gene I (reg I), decreases in aging[20], resulting in impairment of its function in the aged animal. We postulate that PAP levels are also depressed in aging, resulting in less protection in AP and more severe disease. However, since baseline PAP levels are minimal in the normal pancreas, we needed to measure the extent of its induction after stimulation.
In this study, we used sodium taurocholate (NaT) to induce AP and determined: (1) the extent of pancreatitis in young and old rats; (2) the extent of PAP expression in young and old animals with pancreatitis; and (3) how PAP expression relates to severity of pancreatitis in the older animals. We employed a novel molecular method to measure bacterial infiltration.
Sprague Dawley male rats weighing 260-1000 mg (Charles River, Wilmington, MA) were divided follows: animals in the young group had an average age of 91.9 ± 3.7 d (range 82-111 d, n = 10), and those in the older group averaged 339 ± 17.9 d (range 291-546 d, n = 21). Each group had 10 unoperated and 10 sham-operated animals as controls.
Animals were fed standard laboratory chow, given water ad libitum, and were randomly assigned to control or experimental groups. All animal studies were approved by the Division of Animal and Laboratory Resources, SUNY Downstate Medical Center.
AP was induced with 4% NaT, by retrograde injection into the pancreatic duct as previously described[14]. Briefly, under nembutal anesthesia (50 mg/kg intraperitoneally, Abbott Laboratories, North Chicago, IL), a midline incision was performed. The common bile duct was identified and cannulated in a retrograde direction with PE-10 tubing (Fisher Scientific, Pittsburgh, PA) through the ampulla of Vater via small needle puncture of the duodenum. The bile duct was ligated to prevent the flow of bile, and NaT was infused into the pancreatic duct at a rate of 1 mL/kg over 30 min. The abdomen was then closed with monofilament suture, and the animals allowed to recover.
Animals assigned to the sham group were anesthetized under the same protocol as the sodium taurocholate group. They were then subjected to a midline incision, pancreaticoduodenal manipulation, and then similarly closed and recovered.
The animals were sacrificed 24 h after surgery. Swab cultures of the pancreata were taken, after which whole blood was taken via an 18-Gauge syringe in the inferior vena cava. The pancreas was then subdivided into 30 mg portions for pathology (10% formalin), RNA isolation (liquid nitrogen and rapid isolation), and protein isolation.
Qualitative cultures were performed in the clinical microbiology laboratory at SUNY Downstate, for which samples were plated on agar plates for 48-72 h.
To calculate edema, a pancreatic sample was immediately weighed on a coverslip and placed in a 37 °C dry oven. These samples were then re-weighed at 72 h on the same scale. The wet-to-dry ratio was then calculated.
Other pancreas samples were homogenized in 25 mmol/L Tris/0.5% Triton (Sigma, St. Louis, MO) in a glass homogenizer and centrifuged at 12 000 g for 10 min at 4 °C. After aspiration of the lipid layer, the supernatant was poured into a 1.5 mL tube and the pellet discarded. Proteins were assessed by the Bradford Assay (Bio-Rad Laboratories, Inc., Richmond, CA) and all samples were diluted to 2 mg/mL in phosphate buffered saline (PBS) (Lonza, Walkersville, MD), treated with protease inhibitor cocktail (Sigma) and stored at -20 °C until analyzed.
Pancreas samples were flash frozen in liquid nitrogen and immediately processed using 600 μL Trizol reagent (Fisher Scientific, Piscataway, NJ). The samples were homogenized and mixed with 75% ethanol. The tubes were centrifuged at 10 000 g, and the clear lysate was applied to an RNeasy column (Qiagen, Valencia, CA). They were centrifuged at 10 000 g for 15 s and then rinsed with wash buffer, and subjected to DNase digestion.
Whole blood was collected in red-top tubes without anticoagulant (Fisher, Piscataway, NJ) and allowed to clot for 30 min at room temperature. Tubes were centrifuged at 1800 rpm for 20 min. Sera were collected and stored at -80 °C until analyzed.
Serum amylase activity was measured using 4,6-ethylidene (G7)-p-nitrophenyl (G1)->1D-maltoheptaoside as the substrate. Serum lipase was measured by the Clinical Laboratories, SUNY Downstate Medical Center. Similarly, C-reactive protein (CRP) levels were determined in the clinical laboratory using a conventional immunoassay system and Beckman nephelometer (Beckman Coulter, Inc., Galway, Ireland).
Pancreatic protein was assessed for rat monocyte chemotactic protein-1 (MCP-1) using the Rat MCP-1 ELISA Kit for tissue lysate (RayBiotech, Inc., Norcross, GA) and myeloperoxidase (MPO) by the rat MPO ELISA kit (Cell Sciences, Canton, MA) according to the manufacturer’s protocol.
One-step real-time quantitative reverse-transcriptase-polymerase chain reaction (PCR) for PAP 1, 2, 3, and reg I was performed as previously described[13,14] using the LightCycler 480 (Roche, Indianapolis, IN) with β-actin as an endogenous control to standardize the amount of sample RNA added to a reaction. Primers and probes were designed as previously for PAP 1, 2, and 3[13,14]; the primers for reg I were 5’-TACAGCTGCCAATGTCTGGATT-3’ (forward), 5’-CAGTGTCCCAGGATTTGTAGAGA-3’ (reverse), and 5’-ATCCCAAAAATAATCGCCGCTGGC-3’ (probe) (Applied Biosystems, Bedford MA). One hundred nanograms of total RNA was used to set up 20 μL real-time quantitative PCRs that consisted of 1 × Master Mix, 500 nmol/L forward and reverse primers, and 250 nmol/L TaqMan probe (Roche). Polymerase chain reaction amplification was performed with the temperature profile of: 3 min at 45 °C, 7 min at 95 °C, and 45 cycles of 5 s at 95 °Cand 30 s at 60 °C. Assays were performed in triplicate. Data were analyzed with the relative standard curve method. Standard curves of the genes of interest and β-actin were prepared with six ten-fold dilutions of total RNA from the sample that was expected to have the highest amount of mRNA for the gene of interest. For each reaction tube, the amount of target or endogenous reference was determined from the standard curves. The mean amount of each sample was calculated from the triplicate data and was normalized by division by the mean quantity of β-actin RNA for the same sample. The mean and standard error of each treated group were calculated from the normalized value for each rat in the group.
Bacterial 16s ribosomal DNA from pancreatic tissue was amplified by PCR as described[21], using probes specific for gram-positive cocci (GPC) and gram-negative rods (GNR). The primers are specific for bacterial 16sRNA, a gene highly conserved in bacterial strains. Briefly, 0.2 μg of total DNA extracted from each pancreas tissue isolated above was added to pre-decontaminated PCR cocktail for PCR amplification with the primers. The product is a 370 bp fragment.
After SDS-polyacrylamide gel electrophoresis (15%), 15 μg and 30 μg protein/well was electrophoretically transferred to nitrocellulose (Nytan; Schleicher and Schuell, Keene, NH) and monoclonal antibody to reg 1[22] was used at 1:10 000 dilution, polyclonal antibodies to PAP 1, and PAP 2[22] were used at 1:1000 dilution. Secondary antibody to IgG at 1:100 000 dilution was used for detection of primary antibody.
Densitometry was performed using the Bio-Rad Gel Documentation System with Quantity One software. Units are presented as Intensity (INT) × area (mm2).
The head of the pancreas from each rat was fixed in 10% buffered formaldehyde solution (Fisher Scientific, Pittsburgh, PA). Four to six micron section slides were generated for each pancreas, collected and stained with hematoxylin and eosin (H and E). Using previously described criteria, the histological severity of pancreatitis was examined in a blinded fashion by our pathologist. Using a scale ranging from zero (representing the least), to four (representing the greatest) severity, the degree of leukocytic infiltration (acinar and stromal) and tissue necrosis (acinar and stromal), edema and hemorrhage were determined for each specimen.
Immunohistochemical analysis was done as described in[23] using PAP1 and 2 antibodies at 1:1500 dilution.
There were three mortalities after surgery to induce pancreatitis; 2 in the young and one in the old group. Where appropriate, we show data from shams and unoperated controls for clarity and comparisons. Where noted, we performed subgroup analysis of the older animals, dividing them into: subgroup (a) aged, whose average age was 256.3 ± 2.1 d (n = 10); subgroup (b) very aged, 372.5 ± 0.4 d (n = 9); and subgroup (c) most aged, 544 ± 2.8 d (n = 3). The latter group had the single mortality of the older group.
Twenty four hours after induction of NaT pancreatitis, older animals had slightly less edema, as measured by ratio of wet to dry weight, when compared to younger ones (1.35 ± 0.04 vs 1.52 ± 0.08, P = 0.07). By comparison, sham-operated animals had 1.12 ± 0.03 wet to dry ratios; unoperated controls had 1.01 ± 0.08 (all statistically significantly lower than the NaT group).
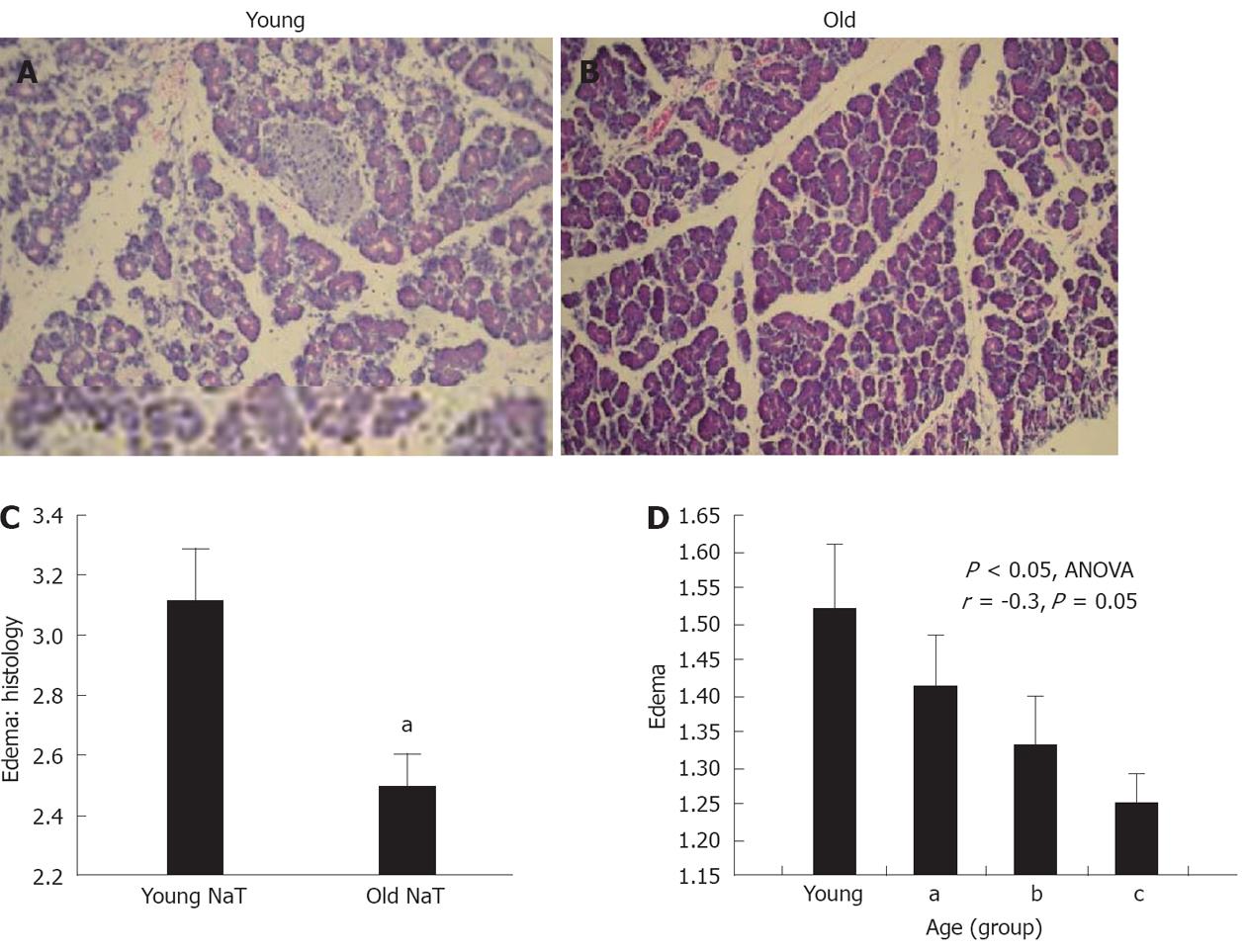
Histopathologic examination also showed that the extent of edema was significantly depressed in the older group (Figure 1A-C, P < 0.05) When subgroups of age (subgroups A-C), were subjected to analysis of variance (ANOVA), there was a statistically significant decrease (Figure 1D, P = 0.02), and significant negative correlation (r = -0.3, P = 0.05) with aging.
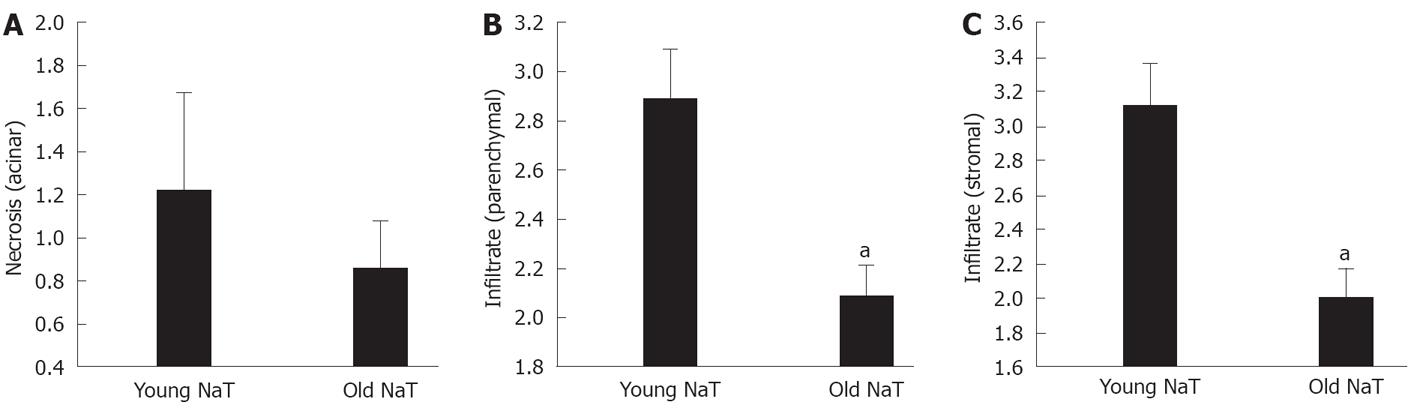
Histopathologic examination also showed that the extent of parenchymal and stromal inflammatory cell infiltrates was depressed in aging (Figures 2A and B, 3B and C). The extent of necrosis (acinar, peripheral acinar or fat necrosis), cellular vacuolization and hemorrhage was not different in the older animals (Figure 3A).
When compared to sham-operated controls, animals with NaT pancreatitis showed significant increases in amylase (628 ± 63 μg/L vs 7919 ± 2022 μg/L, respectively, P < 0.05), and lipase (37.7 ± 16 μg/L vs 864 ± 260 μg/L, respectively, P < 0.05). Also, pancreatic levels of MPO were very high (202 ± 49 ng/mL vs 2014 ± 628 ng/mL, respectively, P < 0.05), as were levels of MCP-1 (2172 ± 463 pg/mL vs 4508 ± 251 pg/mL, respectively, P < 0.05). However, when values from old and young NaT animals were compared, no differences were noted (Table 1).
| Young rats | Old rats | |
| Amylase (μg/L) | 12 536 ± 6141 | 5941 ± 1162 |
| Lipase (μg/L) | 1288 ± 755 | 682 ± 194 |
| MPO (ng/mL) | 1962 ± 538 | 2066 ± 1219 |
| MCP-1 (pg/mL) | 4312 ± 350 | 4730 ± 376 |
| CRP (mg/dL) | 0.25 ± 0.04 | 0.61 ± 0.19 |
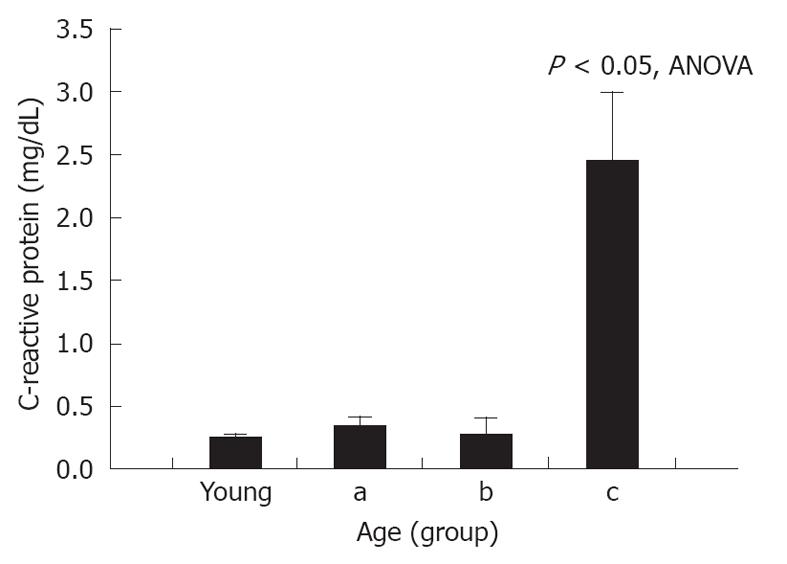
Serum CRP levels in control, unoperated animals were 0.17 ± 0.07 mg/dL. A significant increase in CRP was seen after NaT (0.25 ± 0.04 mg/dL, respectively, P = 0.05 compared to unoperated controls); but when young and old NaT animals were directly compared, no difference was seen between younger and older groups (Table 1). However, subgroup analysis of age groups showed that serum CRP was statistically higher in the most-aged (subgroup c) (Figure 4) (2.45 ± 0.69 mg/dL, P < 0.001 ANOVA compared to all groups, P < 0.001 compared to young, Student’s t test). This phenomenon was noted only for CRP, not for any of the other markers measured above (amylase, lipase, MPO, MCP-1).
Real time PCR of mRNA isolated from rats with pancreatitis showed that reg I, PAP1, PAP2 and PAP3 all increased when compared to controls (not shown). The increase was similar to that which we have observed previously[13,14] with reg I increasing twofold, and PAP1, 2, and 3 increasing 50-100 fold (not shown). Western analysis showed that protein levels increased but only minimally for reg I and by a factor of 3-8 fold for PAP1 and 2.
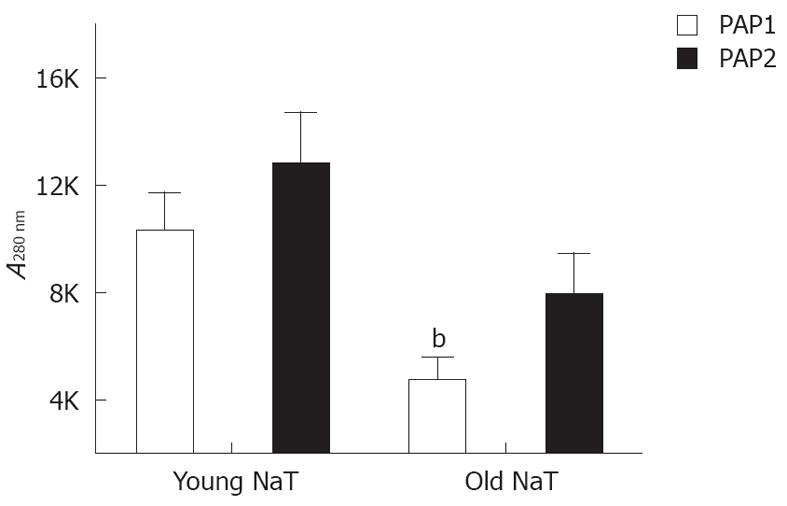
When young and old NaT animal mRNAs were compared, no differences were noted for pancreatic reg I, PAP1 and PAP2 mRNA (Table 2); and only PAP3 levels were significantly decreased. Conversely, when protein levels were compared, older animals had lower levels of PAP1 (P < 0.05); PAP2 proteins were depressed as well (P = 0.07) (Figure 5). PAP3 proteins were not measured since antibodies were not readily available.
| Young rats | Old rats | |
| Reg1 | 0.727 ± 0.19 | 1.27 ± 0.22 |
| PAP1 | 119 ± 46 | 116 ± 18 |
| PAP2 | 690 ± 267 | 475 ± 142 |
| PAP3 | 57.8 ± 19 | 26.4 ± 4.3a |
Since we have previously shown in vitro that PAP2 directly activates leukocytes[17], we postulated that its expression would correlate with MPO or MCP-1 activity. While there were no correlations between mRNAs or proteins and MCP-1 levels, a significant positive correlation of PAP2 mRNA levels with MPO protein was observed (r = 0.81, P = 0.004).
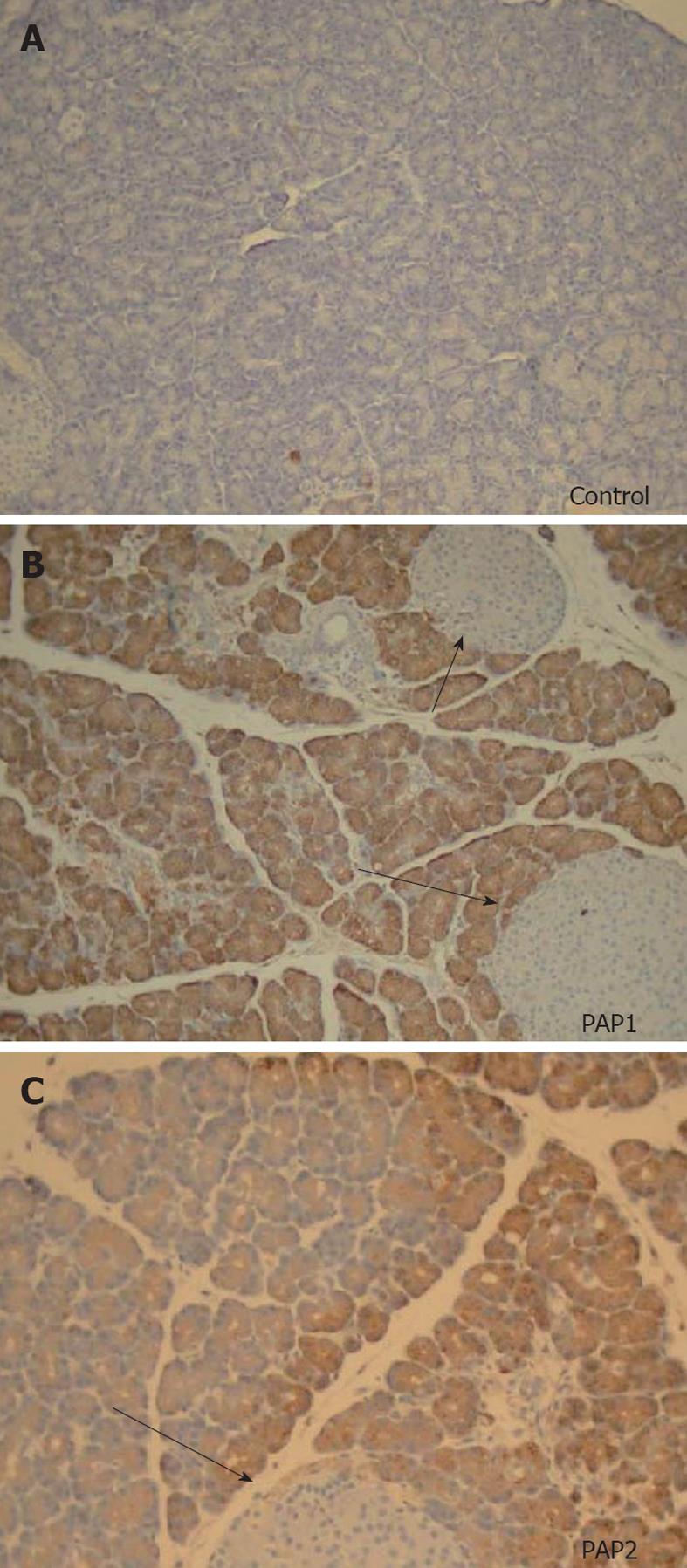
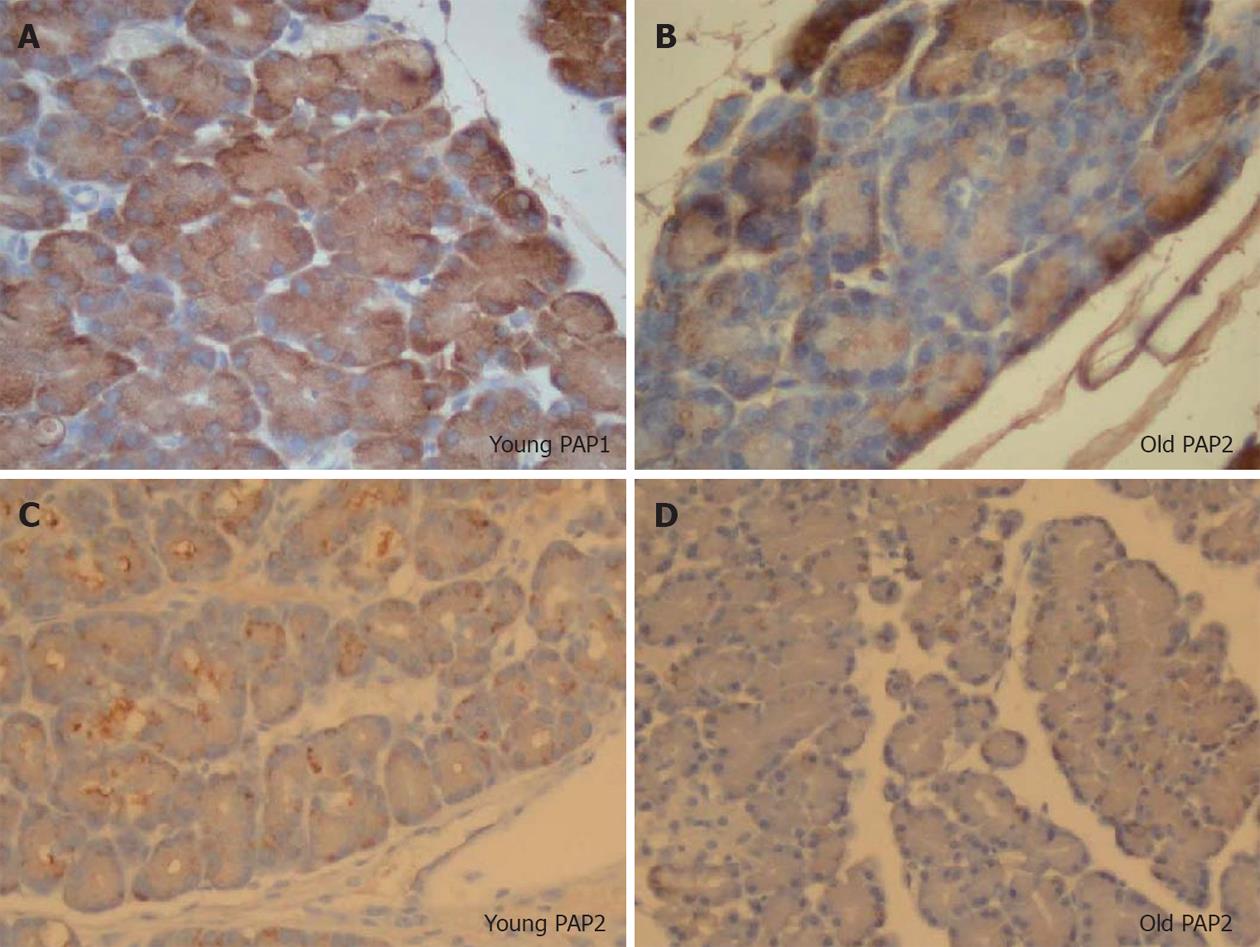
PAP1 and 2 immunohistochemistry showed no staining in normal pancreas, and positive staining pattern in the acinar cells (Figure 6A-C) after induction of NaT pancreatitis. Similar to our observation in the Western analysis, both PAP1 and 2 immunohistochemistry showed more intensity in the younger animals when compared to older (Figure 7); preparation of the slides was done side by side.
In young animals, PAP1 staining in AP was typically intracellular, uniform, and oriented in a base-to-apical intensity (Figures 6B, 7A). In contrast, PAP2 staining in AP was heterogenous (Figures 6C, 7C): it too was intracellular, but was occasionally visible as dense, clumped proteins towards the base of the cells.
In older animals the staining patterns reversed: PAP1 staining became more heterogenous (Figure 7B), and the pattern for PAP2 became more homogenous (Figure 7C).
Interestingly, in many sections, PAP2 staining was found in areas which corresponded to parenchymal and stromal inflammatory infiltrates (Figure 8).
Qualitative cultures of the pancreata from almost all the animals showed colonization by GPC and GNR, with no difference between old and young animals. For example, 5 of 9 young animals with NaT pancreatitis grew GPC, while 8 of 9 grew GNR; only one animal had no culture positive. In the older group, 8 of 19 grew GPC, while 18 of 19 grew GNR; only one had no culture positive.
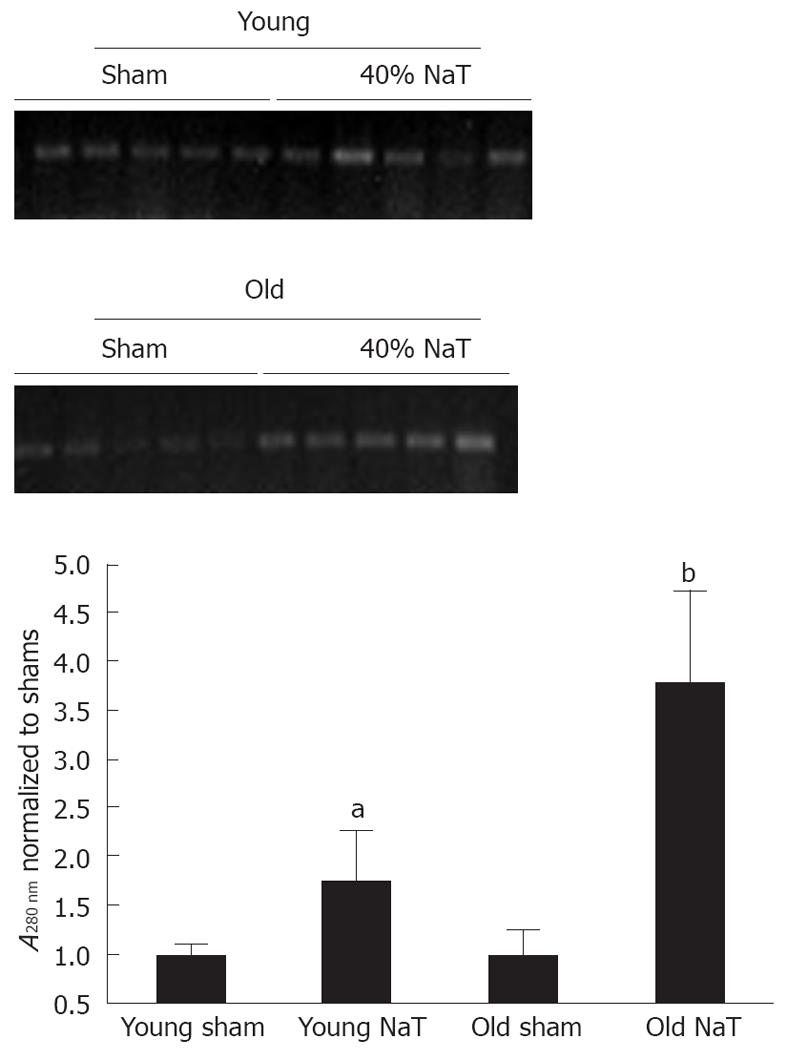
In order to accurately quantitate bacterial infiltration of the pancreas, we employed a novel molecular technique to discern the amount of bacterial genomic ribosomal RNA (rRNA) in the pancreata. As shown in Figure 9, compared to sham-operated animals, there was an increase in bacterial genomic rRNA in both the young and old NaT animals, and a more intense amount of bacterial rRNA in the older animals when compared to young.
In the present study, we found that 24 h after induction of AP, the pancreata of older animals have less edema, decreased inflammatory response and increased bacterial infiltration. This is associated with decreased expression of PAPs as measured by Western analysis and immunohistochemistry. While there were no differences between young and old animals in serum amylase and lipase activity, in the most-aged subgroup (c), there was an increase in CRPs.
These data suggest that the local response to AP is less severe in the older animals, and the systemic response may be worse. PAPs are endogenous protectors against pancreatic injury[14-18], and it appears that the decrease in PAPs may not be related to the decreased inflammatory infiltrate. As will be described below, the effect may be a result of an altered balance of PAP 1 and 2. The decrease of PAPs may also result in increased bacterial infiltration into the pancreas, and ultimately a worsened outcome.
While the pancreas progressively atrophies with age, its reserve capacity results in minimal clinical changes. Studies comparing baseline and stimulated levels of pancreatic enzymes have shown no significant differences[24,25]. However, the regenerative capacity of the pancreas is diminished[26,27], as evidenced by decreased recovery from pancreatitis. At the molecular level, this has been attributed to decreased phosphorylation of AkT[27].
Very little is known about the aged pancreas in AP. As we have observed in animals, in humans there seems to be a blunted local response and an exaggerated systemic one. Fan et al[4] noted decreased local complications but increased systemic complications, leading to death. Kimura et al[28] described elderly patients with high mortality from AP, but autopsy evidence revealed only mild interstitial pancreatitis, where the inflammation was centered in the ductal and interstitial tissues regions.
We postulated that as animals age, there would be loss of protective mechanisms from the pancreas which would lead to worsened fate after pancreatitis. We focused on the PAP family since we and others have shown that they are endogenous proteins which are protective in AP[14-16,29]. We know that expression of other genes of the PAP family decrease with aging[20], and we formulated experiments to determine whether PAPs decreased too.
We showed that in older rats subjected to NaT, there is blunted PAP1 and 2 protein response in both Western analysis and immunohistologic staining patterns. How does this correlate with the histologic and biochemical data above?
PAP1’s protective activity may be local or systemic. Locally, it has both mitogenic[30] and anti-apoptotic[31] effects, which affect regeneration after pancreatitis. PAP1 is a known anti-inflammatory agent[16], and a potent modulator of lung injury in pancreatitis[29,32]. Its loss with aging may worsen the systemic response after acute pancreatitis.
However, our results differ from others who observed increased local inflammatory infiltrates in PAP1 knockout mice[19]. Our model differs not only in that we employed a different rodent species, but in the fact that other proteins, such as PAP2, 3 and other inflammatory modulators, are likely also depressed.
PAP2’s protective activity, on the other hand, is likely local. It can directly activate macrophages[17], and macrophage activation has been shown to be protective in pancreatitis[33-35]. PAP2 is a more potent immunomodulator than PAP1[36]. In our present study we even noted that PAP2-positive pancreatic cells co-localize with leukocytes (Figure 8); this was not observed with PAP1. We also noted a positive correlation of PAP2 with pancreatic MPO activity. Activation of macrophages can induce MPO activity[37,38], and it is possible that a decrease of PAP2 will be associated with decreased macrophages or macrophage activity, which is detrimental for the pancreas and host. The decrease in PAP2 may really be the reason for the decreased leukocyte infiltration seen in older animals.
Finally, PAP proteins directly interact with bacteria. They can bind gram-negative[7] and gram-positive bacteria[39], and we have discovered a bacteriocidal action of PAP1 and 2 (unpublished results). Our data suggest there is more bacterial infiltration in the older pancreata, as evidenced by increased quantity of bacterial rRNA. The blunted PAP responses may result in more acute pancreatic infection.
Increased bacterial colonization could lead to an increased systemic response to the injury, which is worsened by decreased PAP1 level. It is possible that PAPs are endogenous bacteriostatic or bacteriocidal agents that protect against infection during AP. Whether the loss of PAP with aging leads to increased clinical pancreatic infection remains to be seen. To study this, longer term experiments along with qualitative analysis of bacterial species will be necessary.
In conclusion, this is the first study to show a physiologic difference in AP between young and old animals, and the first to show that endogenous pancreatic molecules are related to the diathesis of the disease. In older animals, there is decreased edema and leukocyte infiltration, but increased bacterial infiltration and even serum CRP level. All are associated with decreased endogenous PAPs in the pancreas. Further studies are planned on the direct effect of all three PAPs in modulating the severity of pancreatitis, by focusing on their local immunomodulatory effect and effect on bacterial infiltrates, as well as the systemic response of the host.
The authors further recognize the technical assistance of Ehab Hassanain, MD and Okiremute Oyiborhoro, BS.
Acute pancreatitis (AP) is a disease with significant impact and expense worldwide. In the United States, the cost is significant, and only recently has the impact of this disease on the elderly begun to emerge.
The mechanism that puts the aged pancreas, and aged patient, at risk for severity in pancreatitis is currently being explored. Impaired immune system, host organ response to the injury or protective mechanisms from the pancreas are possibilities. A molecular mechanism is the likely source.
The authors recently showed that pancreatitis-associated proteins (PAPs) are protective in pancreatitis. The authors postulated that their expression would be depressed in elderly animals with pancreatitis, resulting in worsened disease.
By understanding how PAPs affect the outcome of pancreatitis, the authors suggest that physicians can then employ strategies to enhance their expression in elderly patients with the disease and prevent complications. The authors also employed a new way of monitoring the infiltration of bacteria into the pancreas using polymerase chain reaction (PCR), which is much more sensitive than culture techniques.
PAPs are members of the Reg family of pancreatic regenerative proteins. They have been shown to be important in pancreatic disease, including AP and gastrointestinal malignancies. Expression was monitored by standard techniques such as Western blot and immunohistochemical staining, as well as PCR. Myeloperoxidase and monocyte chemotactic protein-1 analysis was used to monitor inflammation. Bacterial infiltration was monitored by PCR, using their 16s ribosomal RNA as the unique target.
The overall goal of the paper is relevant. The data presented are solid and credible. The results are interesting and clinically important.
| 1. | Fagenholz PJ, Fernández-del Castillo C, Harris NS, Pelletier AJ, Camargo CA. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas. 2007;35:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 228] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Fagenholz PJ, Castillo CF, Harris NS, Pelletier AJ, Camargo CA. Increasing United States hospital admissions for acute pancreatitis, 1988-2003. Ann Epidemiol. 2007;17:491-497. [PubMed] |
| 3. | Frey C, Zhou H, Harvey D, White RH. Co-morbidity is a strong predictor of early death and multi-organ system failure among patients with acute pancreatitis. J Gastrointest Surg. 2007;11:733-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Fan ST, Choi TK, Lai CS, Wong J. Influence of age on the mortality from acute pancreatitis. Br J Surg. 1988;75:463-466. [PubMed] |
| 5. | Gardner TB, Vege SS, Chari ST, Pearson RK, Clain JE, Topazian MD, Levy MJ, Petersen BT. The effect of age on hospital outcomes in severe acute pancreatitis. Pancreatology. 2008;8:265-270. [PubMed] |
| 6. | Keim V, Iovanna JL, Rohr G, Usadel KH, Dagorn JC. Characterization of a rat pancreatic secretory protein associated with pancreatitis. Gastroenterology. 1991;100:775-782. [PubMed] |
| 7. | Iovanna J, Orelle B, Keim V, Dagorn JC. Messenger RNA sequence and expression of rat pancreatitis-associated protein, a lectin-related protein overexpressed during acute experimental pancreatitis. J Biol Chem. 1991;266:24664-24669. [PubMed] |
| 8. | Zenilman ME, Tuchman D, Zheng Q, Levine J, Delany H. Comparison of reg I and reg III levels during acute pancreatitis in the rat. Ann Surg. 2000;232:646-652. [PubMed] |
| 9. | Frigerio JM, Dusetti NJ, Keim V, Dagorn JC, Iovanna JL. Identification of a second rat pancreatitis-associated protein. Messenger RNA cloning, gene structure, and expression during acute pancreatitis. Biochemistry. 1993;32:9236-9241. [PubMed] |
| 10. | Frigerio JM, Dusetti NJ, Garrido P, Dagorn JC, Iovanna JL. The pancreatitis associated protein III (PAP III), a new member of the PAP gene family. Biochim Biophys Acta. 1993;1216:329-331. [PubMed] |
| 11. | Suzuki Y, Yonekura H, Watanabe T, Unno M, Moriizumi S, Miyashita H, Okamoto H. Structure and expression of a novel rat RegIII gene. Gene. 1994;144:315-316. [PubMed] |
| 12. | Narushima Y, Unno M, Nakagawara K, Mori M, Miyashita H, Suzuki Y, Noguchi N, Takasawa S, Kumagai T, Yonekura H. Structure, chromosomal localization and expression of mouse genes encoding type III Reg, RegIII alpha, RegIII beta, RegIII gamma. Gene. 1997;185:159-168. [PubMed] |
| 13. | Kandil E, Lin YY, Bluth MH, Zhang H, Levi G, Zenilman ME. Dexamethasone mediates protection against acute pancreatitis via upregulation of pancreatitis-associated proteins. World J Gastroenterol. 2006;12:6806-6811. [PubMed] |
| 14. | Zhang H, Kandil E, Lin YY, Levi G, Zenilman ME. Targeted inhibition of gene expression of pancreatitis-associated proteins exacerbates the severity of acute pancreatitis in rats. Scand J Gastroenterol. 2004;39:870-881. [PubMed] |
| 15. | Lin YY, Viterbo D, Mueller CM, Stanek AE, Smith-Norowitz T, Drew H, Wadgaonkar R, Zenilman ME, Bluth MH. Small-interference RNA gene knockdown of pancreatitis-associated proteins in rat acute pancreatitis. Pancreas. 2008;36:402-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Vasseur S, Folch-Puy E, Hlouschek V, Garcia S, Fiedler F, Lerch MM, Dagorn JC, Closa D, Iovanna JL. p8 improves pancreatic response to acute pancreatitis by enhancing the expression of the anti-inflammatory protein pancreatitis-associated protein I. J Biol Chem. 2004;279:7199-7207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Viterbo D, Bluth MH, Lin YY, Mueller CM, Wadgaonkar R, Zenilman ME. Pancreatitis-associated protein 2 modulates inflammatory responses in macrophages. J Immunol. 2008;181:1948-1958. [PubMed] |
| 18. | Viterbo D, Bluth MH, Mueller CM, Zenilman ME. Mutational characterization of pancreatitis-associated protein 2 domains involved in mediating cytokine secretion in macrophages and the NF-kappaB pathway. J Immunol. 2008;181:1959-1968. [PubMed] |
| 19. | Gironella M, Folch-Puy E, LeGoffic A, Garcia S, Christa L, Smith A, Tebar L, Hunt SP, Bayne R, Smith AJ. Experimental acute pancreatitis in PAP/HIP knock-out mice. Gut. 2007;56:1091-1097. [PubMed] |
| 20. | Bluth M, Mueller CM, Pierre J, Callender G, Kandil E, Viterbo D, Fu SL, Sugawara A, Okamoto H, Zenilman ME. Pancreatic regenerating protein I in chronic pancreatitis and aging: implications for new therapeutic approaches to diabetes. Pancreas. 2008;37:386-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Greisen K, Loeffelholz M, Purohit A, Leong D. PCR primers and probes for the 16S rRNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J Clin Microbiol. 1994;32:335-351. [PubMed] |
| 22. | Viterbo D, Callender GE, DiMaio T, Mueller CM, Smith-Norowitz T, Zenilman ME, Bluth MH. Administration of anti-Reg I and anti-PAPII antibodies worsens pancreatitis. JOP. 2009;10:15-23. [PubMed] |
| 23. | Bluth MH, Patel SA, Dieckgraefe BK, Okamoto H, Zenilman ME. Pancreatic regenerating protein (reg I) and reg I receptor mRNA are upregulated in rat pancreas after induction of acute pancreatitis. World J Gastroenterol. 2006;12:4511-4516. [PubMed] |
| 24. | Gullo L, Priori P, Daniele C, Ventrucci M, Gasbarrini G, Labò G. Exocrine pancreatic function in the elderly. Gerontology. 1983;29:407-411. [PubMed] |
| 25. | Gullo L, Ventrucci M, Naldoni P, Pezzilli R. Aging and exocrine pancreatic function. J Am Geriatr Soc. 1986;34:790-792. [PubMed] |
| 26. | Greenberg RE, McCann PP, Holt PR. Trophic responses of the pancreas differ in aging rats. Pancreas. 1988;3:311-316. [PubMed] |
| 27. | Watanabe H, Saito H, Rychahou PG, Uchida T, Evers BM. Aging is associated with decreased pancreatic acinar cell regeneration and phosphatidylinositol 3-kinase/Akt activation. Gastroenterology. 2005;128:1391-1404. [PubMed] |
| 28. | Kimura W, Ohtsubo K. Clinical and pathological features of acute interstitial pancreatitis in the aged. Int J Pancreatol. 1989;5:1-10. [PubMed] |
| 29. | Folch-Puy E, García-Movtero A, Iovanna JL, Dagorn JC, Prats N, Vaccaro MI, Closa D. The pancreatitis-associated protein induces lung inflammation in the rat through activation of TNFalpha expression in hepatocytes. J Pathol. 2003;199:398-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Zenilman ME, Magnuson TH, Swinson K, Egan J, Perfetti R, Shuldiner AR. Pancreatic thread protein is mitogenic to pancreatic-derived cells in culture. Gastroenterology. 1996;110:1208-1214. [PubMed] |
| 31. | Malka D, Vasseur S, Bödeker H, Ortiz EM, Dusetti NJ, Verrando P, Dagorn JC, Iovanna JL. Tumor necrosis factor alpha triggers antiapoptotic mechanisms in rat pancreatic cells through pancreatitis-associated protein I activation. Gastroenterology. 2000;119:816-828. [PubMed] |
| 32. | Heller A, Fiedler F, Schmeck J, Lück V, Iovanna JL, Koch T. Pancreatitis-associated protein protects the lung from leukocyte-induced injury. Anesthesiology. 1999;91:1408-1414. [PubMed] |
| 33. | Browder IW, Sherwood E, Williams D, Jones E, McNamee R, DiLuzio N. Protective effect of glucan-enhanced macrophage function in experimental pancreatitis. Am J Surg. 1987;153:25-33. [PubMed] |
| 34. | Nakamichi I, Habtezion A, Zhong B, Contag CH, Butcher EC, Omary MB. Hemin-activated macrophages home to the pancreas and protect from acute pancreatitis via heme oxygenase-1 induction. J Clin Invest. 2005;115:3007-3014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Perides G, Laukkarinen J, Weiss ER, Duffeld J, Steer ML. Monocytes-macrophages mediate recovery from acute pancreatitis. Pancreas. 2007;35:422. [DOI] [Full Text] |
| 36. | Hassanian E, Bluth MH, Viterbo D, Wei L, Mueller CM, Zenilman ME. Structurally intact Pap and Reg proteins are critical for their immunologic, but not mitogenic, effects. Pancreas. 2008;37:474. [DOI] [Full Text] |
| 37. | Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879-891. [PubMed] |
| 38. | Rodrigues MR, Rodriguez D, Russo M, Campa A. Macrophage activation includes high intracellular myeloperoxidase activity. Biochem Biophys Res Commun. 2002;292:869-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Peer reviewer: Julian Swierczynski, MD, PhD, Professor, Department of Biochemistry, Medical University of Gdansk, 80-211 Gdansk, Poland
S- Editor Cheng JX L- Editor Logan S E- Editor Zheng XM