INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common solid tumor in the world and accounts for about 500 000 deaths per year[1]. Long-term outcomes after hepatic resection remain unsatisfactory because of the high incidence of postoperative recurrence[2]. Although intrahepatic metastases are the most common type of recurrence, extrahepatic metastases from HCC have an important impact on long-term survival[3]. The most frequent site of hematogenous metastases is the lung, followed by the adrenal gland and skeleton[4,5]. However, lymph node (LN) metastases from HCC are uncommon, with an reported prevalence of 2.2% in a series of Japanese patients who underwent hepatic resection[4]. LN metastases are usually associated with systematic metastases, and there is currently no standard treatment[6]. The 5-year survival rate of patients with LN metastases from HCC is about 20%[7], but successful resection of a solitary LN metastasis is expected to result in better outcomes[8]. We describe the surgical resection of a solitary para-aortic LN metastasis from HCC.
CASE REPORT
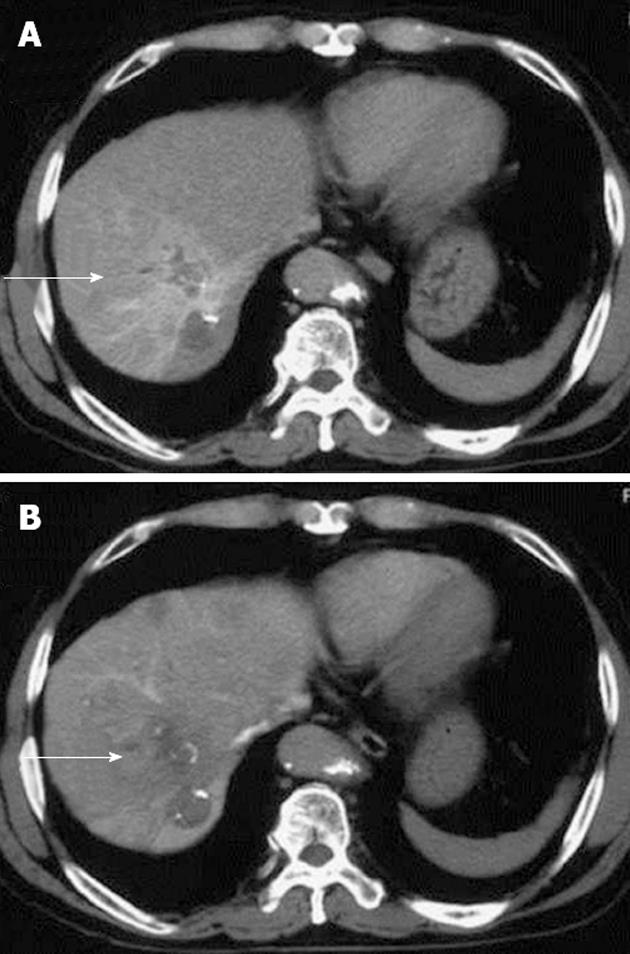
A 65-year-old Japanese man with B-type liver cirrhosis was admitted for the evaluation of a liver tumor. He had already undergone radiofrequency ablation, transcatheter arterial chemoembolization, and percutaneous ethanol injection therapy for HCC. Despite treatment, HCC remained viable. We therefore considered surgical resection. The patient had undergone the repair of an abdominal aortic aneurysm with a vascular prosthesis 1 year previously. Laboratory examinations revealed the serum hemoglobin concentration to be 14.7 g/dL (normal, 14 to 17 g/dL); the platelet count was 9.7 × 104/μL (normal, 12 to 38 × 104/μL); the total bilirubin level was 2.4 mg/dL; the direct bilirubin level was 1.9 mg/dL; the albumin level was 4.1 g/dL; the serum creatinine level was 1.24 mg/dL (normal, < 1.2 mg/dL); and the prothrombin time was 90.8%, which indicated Child-Pugh class A disease. The indocyanine green clearance rate of 15 min was 3.5% (normal, < 10%). The serum concentration of PIVKA-2 was 1226 mAU/mL (normal, < 37 mAU/mL), whereas the serum concentration of alpha-fetoprotein (AFP) was 563.7 ng/mL (normal, < 10 ng/mL). Enhanced computed tomography (CT) of the abdomen revealed a tumor, 20 mm in diameter, in segment 4 of the left lobe on early-phase images, with washout on late-phase images. Abdominal CT angiography revealed a diffuse high-density area, including lesions previously treated by radiofrequency ablation, in segment 8 of the right hepatic lobe. The high-density area was visible on arterial-phase images and washed out on late-phase images (Figure 1). The tumors in both segments were diagnosed as viable HCCs. We performed hepatic right paramedian sectionectomy with partial resection of the left paramedian section of the liver. During the operation, a tumor thrombus was found in a peripheral portal vein in segment 8. Histopathologically, the tumor contained broad areas of necrosis and fibrosis, and the HCC had a moderate-grade funicular structure. Poorly differentiated HCC was present in some parts of the tumor. The diagnosis was moderately to poorly differentiated HCC (T2N0M0, pStage II UICC TNM classification). Peripheral portal vein invasion was detected (Figure 2). The postsurgical course was uneventful, and the patient was discharged on postoperative day 14. After the operation, the serum concentration of PIVKA-2 decreased to 439 mAU/mL, whereas the concentration of AFP fell to 25.5 ng/mL after the operation. Upper gastrointestinal endoscopy revealed the presence of mild esophageal varices without gastric varices (Li, Cw, F1, RC0, according to the General Rules for Recording Endoscopic Findings of Esophagogastric Varices[9]).
Figure 1 An abdominal computed tomography angiographic scan, showing a diffuse high-density area.
A: An abdominal computed tomography angiographic scan including a lesion previously treated by radiofrequency ablation in segment 8 of the right hepatic lobe, in the arterial phase (arrow); B: The area was washed out on late-phase images (arrow).
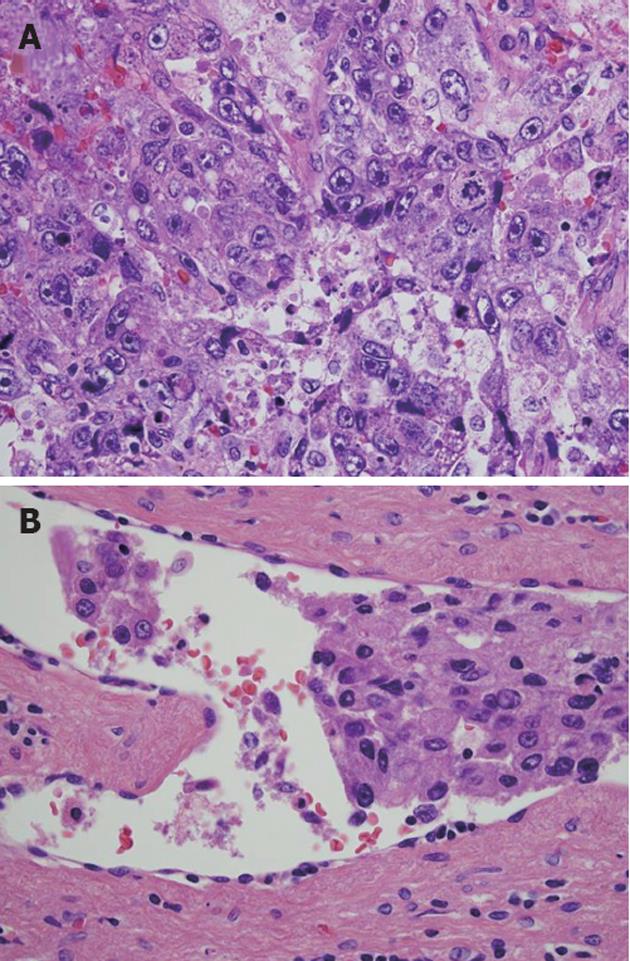
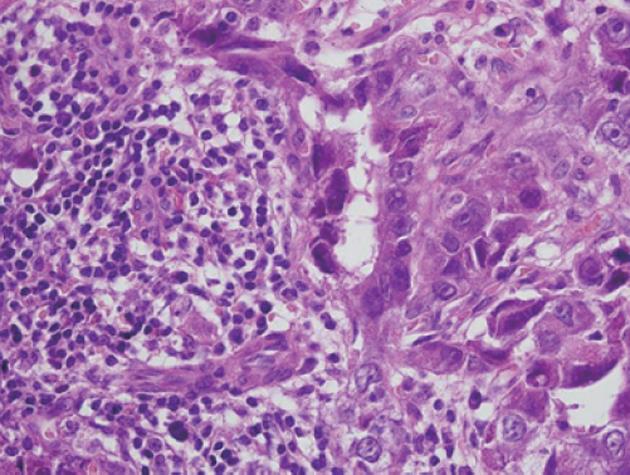
Figure 2 Histopathological examination revealed the presence of considerable necrosis and fibrosis in the tumor.
The hepatocellular carcinoma (HCC) was graded as moderate-grade funicular type. A: Poorly differentiated HCC was present in some regions; B: Peripheral portal vein invasion was detected (hematoxylin and eosin, ×600).
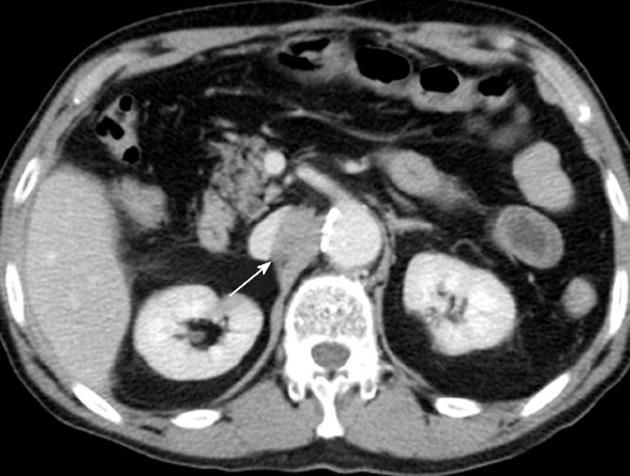
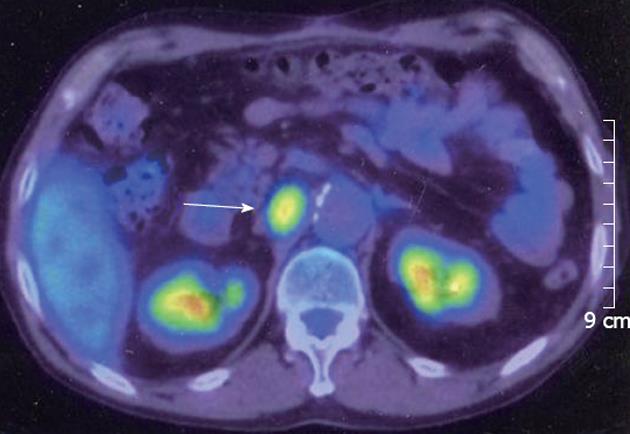
Six months later, serum concentrations of PIVKA-2 (507 mAU/mL) and AFP (189 ng/mL) rose again. Enhanced CT of the abdomen revealed a tumor (20 mm in diameter with slight enhancement) on the right side of the abdominal aorta (Figure 3). Magnetic resonance imaging of the abdomen revealed a high-intensity tumor in the right para-aortic region on T2-weighted images. Fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET) revealed an increased standard uptake value (SUV) in this tumor (Figure 4). There was no evidence of recurrence in any other region. Upper-gastrointestinal endoscopy and colonoscopy showed no evidence of a malignant tumor in the gastrointestinal tract. The tumor was diagnosed as a para-aortic LN metastasis from HCC because of the increased tumor marker level and increased SUV on FDG-PET. We performed lymphadenectomy. At the time of the operation, the surface of the tumor was smooth and slightly adhesive. Histopathological examination showed that the tumor was largely necrotic, with poorly differentiated HCC on its surface (Figure 5). The invasion of lymphocytes was noted. The tumor was diagnosed as a para-aortic LN metastasis from HCC. Recovery was uneventful, and the patient was discharged on postoperative day 17. After 6 mo, the levels of tumor markers remained normal, with no evidence of recurrence.
Figure 3 An enhanced computed tomographic scan of the abdomen revealed a tumor (20 mm in diameter, arrow) on the right side of the abdominal aorta.
Figure 4 Fluorine-18 fluorodeoxyglucose positron emission tomography revealed that the tumor had an elevated standard uptake value (arrow).
Figure 5 Histopathological examination revealed that the tumor consisted of a broad region of necrosis with poorly differentiated hepatocellular carcinoma on its surface (hematoxylin and eosin, ×600).
DISCUSSION
LN metastasis is an important risk factor for the progression and recurrence of many malignant tumors[10]. LN status is an established prognostic factor in oncologic surgery, with a major impact on long-term survival[7]. LN metastasis also plays an important role in the outcomes of patients with HCC, in whom its presence is related to poorer survival and a higher risk of tumor recurrence[7]. Even after lymphadenectomy, patients with LN metastases still have poorer disease-free survival and overall survival than those without LN metastases[11].
The most common site of LN metastases from HCC is the hepatic pedicle node, followed by the retropancreatic space and common hepatic artery station. These nodes appear to be key stations for lymphatic spread from liver tumors toward regional and more distant nodes[12]. However, solitary para-aortic LN metastasis from HCC is relatively rare. Some HCCs can lead to what has been termed “skip LN metastases”, LN metastases at distant sites without metastases in the hepatoduodenal ligament[13-16]. Moreover, peritumoral vascular or lymphatic invasion, tumor multifocality, or distal obstruction of the lymphatics by tumor metastasis can change the direction of lymph flow to alternative routes[17]. In our patient, skipping LN metastasis might have been caused by liver cirrhosis or an operation altering the direction of lymph flow.
Previous studies have reported that patients with more advanced intrahepatic tumors or vessel invasion at initial diagnosis are more likely to have extrahepatic metastases[18]. A primary tumor size of > 5 cm has been associated with the presence of extrahepatic metastasis in HCC[19]. In our patient, the primary HCC was associated with peripheral portal vein invasion.
No standard treatment for extrahepatic metastases developing after hepatic resection of HCC is available[3]. Most patients with recurrent HCC have multiple extrahepatic metastases. The median survival time after the diagnosis of extrahepatic metastases is about 5 mo[20]. Patients with a solitary metastasis from a controlled intrahepatic tumor can be treated surgically, and good outcomes have been reported[8]. In our patient, the para-aortic LN metastasis was solitary, and there was no tumor in the residual liver or evidence of other extrahepatic metastases. We therefore resected the solitary LN metastasis.
A recent study reported that FDG-PET is a useful imaging technique for identifying extrahepatic metastases[19]. The sensitivity of FDG-PET for the detection of extrahepatic metastases was 79%. The detection rate was influenced by the size of metastatic lesions: it was 83% for metastatic lesions larger than 1 cm and 13% for lesions less than or equal to 1 cm[21]. FDG-PET is considered a very useful noninvasive imaging technique for diagnosis, staging and monitoring the treatment response in a variety of malignant tumors[22,23]. In patients with HCCs that produce AFP, an unexplained rise of the serum AFP level after treatment is an early sign of tumor recurrence or extrahepatic metastasis[24]. One study reported that tumor restaging by FDG-PET can detect and localize disease recurrence among patients with no or mild symptoms and elevated levels of tumor markers[25]. As well as being a useful tool for the preoperative staging of HCC, FDG-PET is also better than conventional diagnostic modalities for follow-up, especially staging and re-staging after hepatectomy[24]. In our patient, the serum AFP level increased after hepatectomy, and para-aortic LN metastasis was detected on FDG-PET. We therefore recommend FDG-PET for the screening of metastasis from HCC after hepatectomy.
In conclusion, our experience suggests that a solitary para-aortic LN metastasis from HCC can be treated surgically.
ACKNOWLEDGMENTS
The Department of Surgery for Organ and Biological Regulation, Nippon Medical School, performed the histological examination of the hepatocellular carcinoma and lymph node metastases and played a crucial role in producing this manuscript.
Peer reviewer: Dr. Masayuki Ohta, Faculty of Medicine, Oita University, 1-1 Idaigaoka, Hasama-machi, Oita 879-5593, Japan
S- Editor Gou SX L- Editor A E- Editor Li JY