Published online Jun 14, 2012. doi: 10.3748/wjg.v18.i22.2793
Revised: October 19, 2011
Accepted: May 12, 2012
Published online: June 14, 2012
AIM: To determine if the observed paracellular sucrose leak in Barrett’s esophagus patients is due to their proton pump inhibitor (PPI) use.
METHODS: The in vivo sucrose permeability test was administered to healthy controls, to Barrett’s patients and to non-Barrett’s patients on continuous PPI therapy. Degree of leak was tested for correlation with presence of Barrett’s, use of PPIs, and length of Barrett’s segment and duration of PPI use.
RESULTS: Barrett’s patients manifested a near 3-fold greater, upper gastrointestinal sucrose leak than healthy controls. A decrease of sucrose leak was observed in Barrett’s patients who ceased PPI use for 7 d. Although initial introduction of PPI use (in a PPI-naïve population) results in dramatic increase in sucrose leak, long-term, continuous PPI use manifested a slow spontaneous decline in leak. The sucrose leak observed in Barrett’s patients showed no correlation to the amount of Barrett’s tissue present in the esophagus.
CONCLUSION: Although future research is needed to determine the degree of paracellular leak in actual Barrett’s mucosa, the relatively high degree of leak observed with in vivo sucrose permeability measurement of Barrett’s patients reflects their PPI use and not their Barrett’s tissue per se.
- Citation: Farrell C, Morgan M, Tully O, Wolov K, Kearney K, Ngo B, Mercogliano G, Thornton JJ, Valenzano MC, Mullin JM. Transepithelial leak in Barrett's esophagus patients: The role of proton pump inhibitors. World J Gastroenterol 2012; 18(22): 2793-2797
- URL: https://www.wjgnet.com/1007-9327/full/v18/i22/2793.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i22.2793
The sucrose permeability test is designed to measure paracellular (non-transcellular) leak of sucrose from the lumen of the upper gastrointestinal (GI) tract into the vasculature because sucrose is markedly hydrophilic and lacks any significant affinity for any carbohydrate transport proteins on the cell surface[1]. Moreover sucrose is destroyed enzymatically in the duodenum, and ceases to exist as a disaccharide after the early-mid portion of the small bowel. Sucrose leak would therefore be detected only while the probe (sucrose) still exists chemically in the upper GI lumen. This limits potential sites of leak to the esophageal, gastric and early intestinal mucosa. However, considerations of surface area and contact time (after oral consumption of the sucrose) argue that the sucrose permeability test results reflect primarily the gastroduodenal mucosa and less so the esophageal mucosa which has the smallest surface area (no villi or folds-except in Barrett’s) and the shortest contact time.
Still, Barrett’s patients are believed not to have a histological mucosal defect other than in the esophagus. Therefore when in previous work our group published that patients with a known diagnosis of Barrett’s esophagus (BE) manifested a greater magnitude of transepithelial sucrose leak across the upper gastrointestinal tract than that seen in healthy control subjects, the strong difference was ascribed to the presence of Barrett’s metaplasia in these patients[2]. In other words, a transepithelial leak was presumed to exist in the Barrett’s metaplasia.
In this current work we revisited that conclusion by asking whether considerations other than the simple presence of Barrett’s metaplasia were the reason for the increased leak observed in BE patients. Aside from the presence of Barrett’s tissue, the foremost distinction about BE patients is their chronic, long-term use of acid suppression medications, most notably proton pump inhibitors (PPIs). We therefore posed the question of whether the leak we observed in BE patients was traceable to their regular use of PPI medications or to the presence of Barrett’s metaplasia. Results indicated that the observed leak is due to PPI use by these patients.
Patients with a prior known history of Barrett’s esophagus or healthy controls with no current or history of upper GI disease were recruited by a gastroenterologist in a tertiary care teaching hospital bordering the suburbs of Philadelphia, PA. Diagnosis of BE was made by endoscopic exam and only after biopsies were documented to possess goblet cell metaplasia. All enrolled subjects gave informed consent and the study was approved by the Main Line Hospitals Institutional Review Board committee.
Test subjects were recruited without regard to gender or ethnicity. Exclusion criteria were: diabetes mellitus, steroid use, prior gastric or esophageal surgery, age < 18 years, current GI bleeding, weight loss, intractable nausea and vomiting or renal insufficiency. PPI use comprised omeprazole, esomeprazole, lansoprazole, pantoprazole and rabeprazole.
All test subjects consumed in their homes a chilled solution of 100 g of sucrose in 200 cc of water containing 5 g of a citric acid-based flavoring agent at bedtime. An 8 h (overnight) urine sample was collected in a container with 5 mL of 10% thymol in isopropanol and mixed. For patients undergoing upper endoscopic examination, the sucrose permeability test was performed either before the procedure or at least two weeks later to avoid potential effects of endoscope trauma on epithelial barrier tissue. The total urine volume was measured and recorded. The concentration of sucrose in the urine sample was then measured by an enzymatic/spectrophotometric assay after prior desalting of the urine sample by anion and cation exchange resins[3]. The total amount of sucrose in the urine in mg was determined by multiplying the urine volume in mL by the sucrose concentration in mg/mL. This equates to the amount of sucrose which leaked out of the upper GI lumen.
Test subjects were instructed to refrain from solid food for at least 2 h prior to the sucrose permeability test and 8 h after testing. Specific foods were however not prohibited. Test subjects were also instructed to refrain from alcohol or non-steroidal anti-inflammatory medications for 24 h prior to testing and for 8 h after testing. Brushing teeth or flossing was proscribed before testing and until at least 20 min after testing.
Data are reported throughout as the mean + SE. Experimental and control groups are compared throughout by unpaired Student’s t tests, with statistical significance being ascribed when P < 0.05.
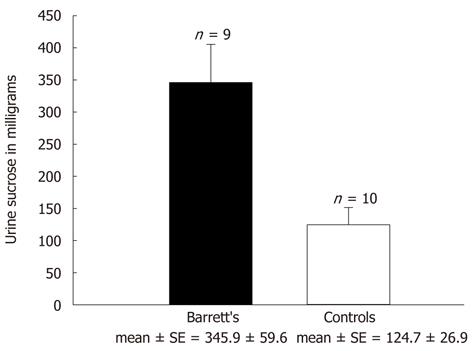
Sucrose permeability testing was conducted on 19 patients, 9 carrying a diagnosis of BE and 10 being healthy controls. The 9 BE patients were on PPI therapy at the time of their testing while the healthy controls were receiving no acid-suppressive medication. As was shown in previous studies from our group[2], a significantly greater sucrose leak was observed in the BE patients (345.9 ± 59.6 mg) compared to the healthy controls (124.7 ± 26.9 mg) (Figure 1). This difference was statistically significant with a P < 0.003. Following this confirmation of an increased transepithelial sucrose leak in BE patients, the etiology of the leak (as a result of BE itself or of PPI use) was pursued.
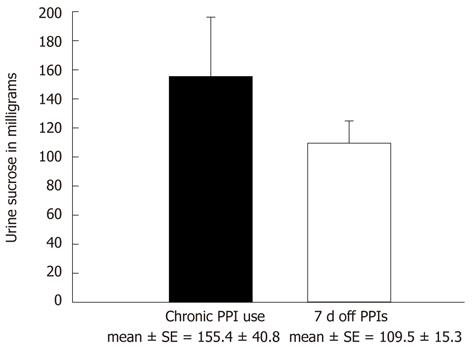
First, a second group of BE patients was studied to evaluate the effect of cessation of their PPI therapy on their observed sucrose leak. Thirty-eight BE patients underwent sucrose permeability testing while on a continuous PPI regimen. They then performed a second sucrose permeability test after having stopped their PPIs for 7 d, in order to look for any change in observed leak. Patients were allowed to consume antacid medications during this period but not PPIs or H-2 receptor antagonists. Any patients reporting very difficult reflux symptoms were allowed to leave the study. The mean sucrose leak decreased by approximately 30% from 155.4 ± 40.8 mg to 109.5 ± 15.3 mg after PPI medications were temporarily stopped for this 7 d period (Figure 2). These findings were however not statistically significant, with a P = 0.283 (paired Student’s t-test). As before, a high SE was found for patients on PPI therapy, indicating a considerable amount of variability. The sucrose leak observed in the BE patients after their PPIs were discontinued for 7 d decreased to nearly the same level observed in Figure 1 for healthy controls not taking PPIs.
Since BE exists in a wide range of segment lengths, and a (passive diffusion) transepithelial leak due specifically to Barrett’s metaplasia should correlate with the surface area of the Barrett’s metaplasia, we investigated whether sucrose leak in the Barrett’s patients correlated with length of segment of the Barrett’s tissue. Endoscopically, characteristic findings of reddish/velvety Barrett’s mucosa are defined as short segment BE if < 3 cm and long segment BE if ≥ 3 cm in length. Data from 49 BE patients were examined. Greater leak was not observed in patients with long-segment Barrett’s. Mean leak was actually greater in short-segment Barrett’s (209 vs 148.9). Analysis of sucrose leak between the two segment groups showed similar medians of 130.5 ± 40.4 mg (short) and 121.0 ± 21.4 mg (long) (P = 0.36) with no statistically significant difference between them.
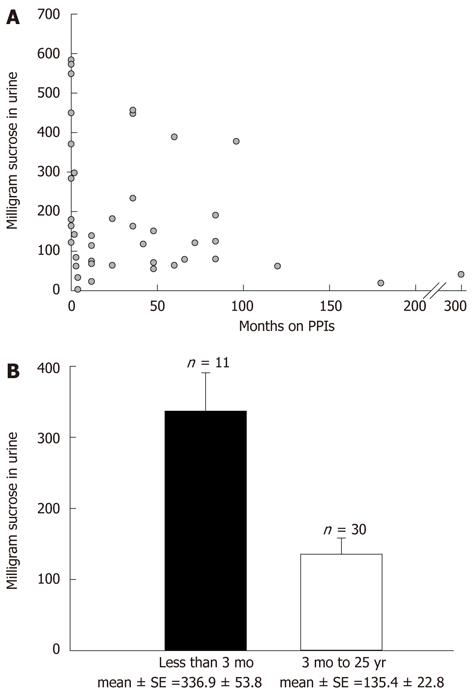
Due to the magnitude of sucrose leak variability that we observed among patients (especially among patients on PPIs), other potential confounding sources of variability were explored. First, the issue of potential effect of duration of PPI use on observed transepithelial sucrose leak was investigated. In a non-BE population taking PPIs on a regular, long-term basis (gastroesophageal reflux disease patients not known to have Barrett’s), the sucrose leak was found to vary as a function of the duration of PPI use. Specifically, the magnitude of leak correlated inversely with the length of time on PPI medications (Figure 3A). A statistically significant difference in leak was observed in patients on PPIs for < 3 mo (336.9 ± 53.8 mg) in comparison to those on PPIs for 3 mo to 25 years (135.4 ± 22.8 mg) (P < 0.0003; Figure 3B). The PPI-induced leak thus appeared to exhibit tachyphylaxis, and this would lead to wider observed variation in leak data if one did not correct for duration of PPI therapy.
Discontinuation of long-term PPI use followed by resumption of PPIs did not result in re-induction of leak. In a separate group of 14 patients on long-term PPI therapy, PPI use was discontinued for 7 d, and was then reinstituted. Sucrose permeability testing was again utilized to assess upper gastrointestinal leak 7 d after resumption of PPI use. A significant leak did not re-occur in these patients who had previously been on long-term PPI treatment (Table 1). This is in sharp contrast to patients taking PPIs for the first time, where induced sucrose leak is substantial[3].
| On PPIs | 7 d off PPIs | 7 d after PPI resumption | |
| Median | 87.8 | 101.9 | 63.0 |
| mean ± SE | 116.6 ± 16.5 | 142.7 ± 34.2 | 101.5 ± 21.0 |
When our clinical studies with Barrett’s patients were begun in 2005, we did not anticipate increased barrier leak being induced by PPIs. In fact, the opposite was expected due to PPIs’ documented ability to allow for mucosal (both gastric and esophageal) healing from inflammation and micro-ulceration by means of suppression of acid secretion and subsequent elevation of the pH of gastric luminal contents. Therefore when we first noted a transmucosal leak in BE patients we ascribed the observed leak to the presence of Barrett’s metaplasia, as BE patients typically do not have other upper GI pathology[2]. When we later observed that Barrett’s patients’ transmucosal sucrose leak did not correlate with the length of the Barrett’s segment and the surface area of the Barrett’s metaplasia, we began to question if the source of the leak was in fact the metaplasia. We realized that PPI medications were the other common characteristic-in addition to the metaplasia itself-of all the Barrett’s patients studied.
We then began a series of clinical studies to ask whether PPIs in fact could induce upper GI leak in healthy controls free of upper GI disease, and found that PPIs indeed have this effect[3]. We further explored that phenomenon in an animal model (Sprague Dawley rat), observing that exposure of rat gastric corpus to omeprazole can lead to an immediate increase in transmucosal permeability which is bidirectional, concentration-dependent and size-specific[4-6]. This work confirmed the earlier findings by Hopkins et al[7] that omeprazole induced leak to 14C-mannitol in rat gastric corpus. These previous findings together with our current observation (that the sucrose leak seen in Barrett’s patients decreases when their PPI medications are discontinued) suggests that PPI use, not the presence of BE per se, is the cause of the upper GI leak seen in BE patients.
Note however that this still does not address whether Barrett’s epithelium is or is not paracellularly leaky. It simply means that the clinical, in vivo, sucrose permeability test which is employed here is reflecting the effects of PPIs on upper GI barrier function. To determine whether Barrett’s metaplasia is leaky will likely require Ussing chamber-type permeability studies with actual Barrett’s tissue. This could be difficult to perform since the major source of Barrett’s tissue that is large enough in surface area for typical Ussing studies is esophagectomy surgery for adenocarcinoma. Here, adjacent Barrett’s tissue may be available but it can be considerably modified/eliminated by radiation and chemotherapy prior to surgery, which is now a current, common practice in esophageal adenocarcinoma management. This is therefore clearly a situation where Ussing studies using Barrett’s biopsy tissue (readily available through upper endoscopic screening) would be highly useful[8-10].
An unanswered question from our earlier study on sucrose leak in Barrett’s patients is that not only was sucrose leak in Barrett’s patients significantly greater than sucrose leak in healthy controls, but it was also greater than the leak measured in patients with chronic gastroesophageal reflux disease (GERD)[1]. As both GERD and Barrett’s patient groups would be receiving PPI therapy, it is unclear why leak in the Barrett’s group would be quantitatively and significantly different if PPIs were the cause of leak in both patient groups. One possible explanation is that our patient population happened to be too small in the earlier study to get a fully accurate representation, and that sucrose permeability testing can carry intrinsically high variability, being affected by certain dietary constituents as well as certain over-the-counter drugs such as aspirin[11-13].
The sucrose leak that we observed in the Barrett’s cohort, or any of our patient groups taking PPIs, was not only high in absolute value but was associated with a large variance and high standard error of the mean. This led us to examine whether the PPI-induced leak was stable over time, since the length of the duration on PPI therapy was a frequent variable in our studies. As shown in Figure 3, the effect of PPIs on leak is indeed not stable over time, but in fact decreases over long-term PPI use. In contrast, a similar tachyphylactic aspect to PPI activity has not been observed for PPI-inhibition of acid secretion[14]. The inability of PPIs to induce a major transmucosal, molecular leak (> 200 mg of sucrose in the described test) after long-term PPI use has been interrupted for 7 full days, suggests that long-term PPI use has caused modification of intracellular signaling pathways and/or cell/tissue structural aspects that mitigate against reintroduction of leak.
In summary, sucrose leak observed in Barrett’s esophagus patients appears to be due to the use of PPIs by these patients, not due to their Barrett’s metaplasia. This PPI-induced upper GI leak appears to diminish during long-term PPI therapy. After long-term use of PPIs, leak is difficult to re-induce even after short interruption of PPI therapy. Finally, variability associated with sucrose permeability testing in a clinical population may necessitate use of relatively large patient groups to support one’s conclusions.
The authors are very grateful for the clinical coordination efforts of Ms. Yvette Mercer and Ms. Mary Sue Whitby throughout these studies. The kind work of Ms. Renee Mercer, Ms. Laurie Trudgeon and Ms. Maureen Metzler in obtaining patient clinical data and contact information is gratefully appreciated. The ever excellent assistance of Ms. Kate Ciavarelli and Ms. Kristin Hayden in the preparation of this manuscript is deeply appreciated.
In earlier work, the research group has shown that Barrett’s esophagus (BE) patients manifest a barrier leak to the paracellular probe, sucrose, in their upper gastrointestinal tract. This phenomenon could be due to a molecular-level leak in the Barrett’s metaplasia, or to some other aspect of the Barrett’s patient cohort. This study was an attempt to determine if the common medication of all BE patients, proton pump inhibitors (PPIs), is responsible for the phenomenon.
The phenomenon of PPI-induced gastric barrier leak is certainly an unexpected side-effect of PPI therapy. The mechanism for the phenomenon remains unknown. More importantly, the clinical implications of the phenomenon are as yet poorly understood.
If one adopts a stance that a PPI-induced gastric barrier leak is clinically benign, it is possible that the phenomenon could be useful in drug delivery. However a greater understanding of the characteristics of the leak are needed (what can actually permeate through the leak), as well as a full realization of the clinical implications (if any). These could range from localized inflammation to altered kinetics of uptake of other (oral) medications into the bloodstream.
Barrier function refers here to the ability of the gastric mucosa to separate the stomach luminal compartment from interstitial fluid and bloodstream. Barrier leak can result from either injured/dying/detaching epithelia or altered (and leaky) epithelial tight junctions.
This study found that BE manifested a greater magnitude of transepithelial sucrose leak across the upper gastrointestinal tract than that seen in healthy control subjects, previously. In the present study, they determined if the observed paracellular sucrose leak in BE patients is due to their proton pump inhibitors use. This subject is a new topic in the research area and may contribute to the literature.
| 1. | Meddings JB, Sutherland LR, Byles NI, Wallace JL. Sucrose: a novel permeability marker for gastroduodenal disease. Gastroenterology. 1993;104:1619-1626. [PubMed] |
| 2. | Mullin JM, Valenzano MC, Trembeth S, Allegretti PD, Verrecchio JJ, Schmidt JD, Jain V, Meddings JB, Mercogliano G, Thornton JJ. Transepithelial leak in Barrett's esophagus. Dig Dis Sci. 2006;51:2326-2336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Mullin JM, Valenzano MC, Whitby M, Lurie D, Schmidt JD, Jain V, Tully O, Kearney K, Lazowick D, Mercogliano G. Esomeprazole induces upper gastrointestinal tract transmucosal permeability increase. Aliment Pharmacol Ther. 2008;28:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Gabello M, Valenzano MC, Barr M, Zurbach P, Mullin JM. Omeprazole induces gastric permeability to digoxin. Dig Dis Sci. 2010;55:1255-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Gabello M, Valenzano MC, Zurbach EP, Mullin JM. Omeprazole induces gastric transmucosal permeability to the peptide bradykinin. World J Gastroenterol. 2010;16:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 6. | Murray LJ, Gabello M, Rudolph DS, Farrell CP, Morgan M, Martin AP, Underwood JC, Valenzano MC, Mullin JM. Transmucosal gastric leak induced by proton pump inhibitors. Dig Dis Sci. 2009;54:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Hopkins AM, McDonnell C, Breslin NP, O'Morain CA, Baird AW. Omeprazole increases permeability across isolated rat gastric mucosa pre-treated with an acid secretagogue. J Pharm Pharmacol. 2002;54:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Reims A, Strandvik B, Sjövall H. Epithelial electrical resistance as a measure of permeability changes in pediatric duodenal biopsies. J Pediatr Gastroenterol Nutr. 2006;43:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Stockmann M, Gitter AH, Sorgenfrei D, Fromm M, Schulzke JD. Low edge damage container insert that adjusts intestinal forceps biopsies into Ussing chamber systems. Pflugers Arch. 1999;438:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Wallon C, Braaf Y, Wolving M, Olaison G, Söderholm JD. Endoscopic biopsies in Ussing chambers evaluated for studies of macromolecular permeability in the human colon. Scand J Gastroenterol. 2005;40:586-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Lambert GP, Broussard LJ, Mason BL, Mauermann WJ, Gisolfi CV. Gastrointestinal permeability during exercise: effects of aspirin and energy-containing beverages. J Appl Physiol. 2001;90:2075-2080. [PubMed] |
| 12. | Meddings JB, Swain MG. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology. 2000;119:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 188] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Parry DM, Duerksen DR. Assessment of intestinal permeability with lactulose/mannitol: gum chewing is a potential confounding factor. Am J Gastroenterol. 2001;96:2515-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Bixquert M. Maintenance therapy in gastro-oesophageal reflux disease. Drugs. 2005;65 Suppl 1:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
Peer reviewers: Dr. Ahmet Tekin, Department of General Surgery, IMC Hospital, Istiklal Cad no:198, Mersin 33100, Turkey; Julian Abrams, MD, MS, Assistant Professor of Clinical Medicine, Division of Digestive and Liver Diseases, Columbia University Medical Center, 622 W 168th Street, PH 20-303, New York, NY 10032, United States
S- Editor Cheng JX L- Editor O’Neill M E- Editor Zhang DN