Published online Jun 14, 2012. doi: 10.3748/wjg.v18.i22.2756
Revised: September 3, 2011
Accepted: April 12, 2012
Published online: June 14, 2012
The liver is the main organ responsible for the metabolism of drugs and toxic chemicals, and so is the primary target organ for many organic solvents. Work activities with hepatotoxins exposures are numerous and, moreover, organic solvents are used in various industrial processes. Organic solvents used in different industrial processes may be associated with hepatotoxicity. Several factors contribute to liver toxicity; among these are: species differences, nutritional condition, genetic factors, interaction with medications in use, alcohol abuse and interaction, and age. This review addresses the mechanisms of hepatotoxicity. The main pathogenic mechanisms responsible for functional and organic damage caused by solvents are: inflammation, dysfunction of cytochrome P450, mitochondrial dysfunction and oxidative stress. The health impact of exposure to solvents in the workplace remains an interesting and worrying question for professional health work.
- Citation: Malaguarnera G, Cataudella E, Giordano M, Nunnari G, Chisari G, Malaguarnera M. Toxic hepatitis in occupational exposure to solvents. World J Gastroenterol 2012; 18(22): 2756-2766
- URL: https://www.wjgnet.com/1007-9327/full/v18/i22/2756.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i22.2756
Some studies have suggested that exposure to organic solvents may induce liver toxicity[1,2] because most chemicals are metabolized in the liver and toxic metabolites generated through the metabolism are the main cause of liver damage.
Work activities with hepatotoxin exposure are numerous and include chemists, dry cleaners, farm workers, painters, health care workers, nurses, and printers. Organic solvents are used in various industrial processes such as spray painting, paint manufacturing, degreasing, metal processing, aeronautical and auto manufacturing maintenance and manufacturing, as well as various chemical storage facilities. Exposure to hepatotoxins can occur through intentional or accidental ingestion in food or absorption of toxic contaminants through the skin. Contamination includes the ingestion of water, skin absorption via water baths, and volatilization of solvents, and heated bathrooms with a shower of water.
Although a number of industrial chemicals are known to be hepatotoxins, liver disease from occupational exposure is rarely suspected or diagnosed[3].
Three conditions must be fulfilled for the diagnosis of professional toxic hepatitis: (1) Liver damage should take place after occupational exposure to a substance; patient occupational history and the workplace in question is necessary; (2) Liver enzymes must increase to at least double the upper limit of normal levels; and (3) Tertiary conditions, such as other causes of liver disease, must be excluded[4,5].
The most important factors contributing to toxicity liver are protein binding, species differences, points of binding inside the liver intracellular, nutritional condition, genetic factors, interaction with medications in use, alcohol abuse and interaction, and age. For the age factor, it has been shown that age susceptibility clearly plays a role. For instance, neonatal rats are less susceptible to carbon tetrachloride and bromobenzene toxicity as compared to adult animals[6].
The hepatotoxic effects of some of the solvents were recognized as early as 1887. Very little is known about the frequency of occupational liver injury by solvents. It is still difficult to assess the damage from exposure due to difficult controls in the workplace[7-9]. Clinical presentation of occupational liver disease may be acute/subacute or chronic, but is often insidious.
Occupational toxic hepatitis can be divided into three types: hepatocellular, cholestatic and mixed (Table 1).
| Type of disease | ALT | ALP | γ-GT | Bilirubin | Bile acids |
| Hepatocellular | > 2 ULN | N | > 2 | Elevated levels | Elevated levels |
| Cholestatic | N | > 2 ULN | > 4 | Normal or moderate level | Elevated levels |
| Mixed | > 2 ULN | ≥ ULN | > 2 | Normal or moderate level | Elevated Levels |
Liver damage is likely to be more severe in the hepatocellular type than in the cholestatic or mixed type; a patient with elevated bilirubin levels in hepatocellular liver injury indicates serious liver disease.
Patients with the cholestatic or mixed type are likely to develop chronic disease more frequently than those with the hepatocellular type.
The solvents suspected to be responsible for liver occupational disease are: dimethylformamide (DMF), dimethylacetamide (DMA), trichloroethylene (TCE), tetrachloroethylene, carbon tetrachloride, xylene, toluene, and chloroform, whose organoleptic properties and main uses are schematically presented in Table 2.
| Solvents | Organoleptic properties | Main uses | Main mode of absorption |
| Dimethylformamide | Water-soluble liquid | Production of organic chemicals, resins, fibers, paints, inks and adhesives | Inhalation |
| Colorless | Vinyl resins, adhesives and epoxy formulations | Skin | |
| Odorless | Purification and/or separation of acetylene | ||
| Polar polymer | Production of polyacrylic or cellulose triacetate fibres and pharmaceuticals | ||
| Industrial paint | |||
| Protective coatings, films, printing inks and adhesives | |||
| Pharmaceutical industry | |||
| Formulation of pesticides | |||
| Dimethylacetamide | Colorless | Organic chemistry | Inhalation |
| Water miscible | Vinyl resins | Skin | |
| High boiling point | Cellulose derivatives | Gastric | |
| Polar | Polyacrylonitrile | ||
| Greasy | Linear polyesters and styrene | ||
| Production process of antibiotics like cephalosporins | |||
| Production of X-ray contrast media | |||
| Manufacture of polyimide resins, polysulfones and cellophane | |||
| Trichloroethylene | Non-flammable liquid | Volatile anesthetic (in the past) | Inhalation |
| Clear | Food industry (e.g., the decaffeination of coffee and the | Skin | |
| Pleasant smell | preparation of flavoring extracts from hops and spices) | Gastric | |
| Volatile | |||
| Organic compound | |||
| Tetrachloroethylene | Colorless liquid | Dry cleaning and metal cleaning, | Inhalation |
| Volatile | veterinary anthelmintic, textile industry, | Skin | |
| High stabile | automotive and other metalworking industries, | Gastric | |
| Non-flammable | dry-cleaning industry | ||
| Carbon tetrachloride | Liquid | Refrigerant | Inhalation |
| Easily evaporates | Pesticide | Skin | |
| Sweet smell | Gastric | ||
| Unpleasant smell (> 10 ppm) | |||
| Xylene | Flammable liquid | Resins, gums, rubber cleaners, degrease paints, lacquers, varnishes, | Inhalation |
| Light-colored or colorless, strong odor | adhesives, cements, inks, gasoline | Skin | |
| Toluene | Refractive liquid | Paints, coatings, synthetic fragrances, adhesives, inks, cleaning | Inhalation |
| Colorless | agents, pharmaceuticals, dyes, cosmetic nail products, and | Skin | |
| Flammable soluble in water | synthesis of organic chemicals | ||
| Pungent odor | |||
| Chloroform | Colorless | Pharmaceutical industry | Inhalation |
| Sweet-smelling | Dyes and pesticides | ||
| Dense liquid | Reagent | ||
| Anesthetic |
The solvents that follow are the most extensively used in the chemical industry.
DMF is an organic compound with the formula (CH3)2NC(O)H that takes the form of a colorless, water-soluble liquid. Pure dimethylformamide is odorless, whereas technical grade or degraded dimethylformamide often has a fishy smell due to the impurity of dimethylamine. Its name is derived from the fact that it is a derivative of formamide, the amide of formic acid.
Dimethylformamide has been termed the universal solvent and is used commercially as a solvent for vinyl resins, adhesives and epoxy formulations (the latter for use in laminated printed circuit boards); for purification and/or separation of acetylene, acid gases and aliphatic hydrocarbons; and in the production of polyacrylic or cellulose triacetate fibres and pharmaceuticals. It is also used as a catalyst in carboxylation reactions; in organic synthesis; as an industrial paint; as a carrier for gases, and in inks and dyes in printing and fibre-dyeing applications[10-12].
It is widely used for resins and polar polymers and in applications such as protective coatings, films, printing inks and adhesives. It is also used in the pharmaceutical industry in the formulation of pesticides, and in the manufacture of synthetic leathers[13,14].
Occupational exposure to dimethylformamide may occur in the production of organic chemicals, resins, fibers, paints, inks and adhesives. Exposure can also occur during the use of ink coatings, adhesives, in the synthetic leather industry, and in the repair of aircraft.
In 100 workers occupationally exposed to this solvent for at least one year (mean exposure of 5 years; range = 1-15 years), a statistically significant incidence of hepatic impairment was found, as indicated by elevated gamma-glutamyl transpeptidase levels and digestive disturbances[15].
Symptoms of irritation occurring during work with DMF include watery eyes, dry throat, and coughing. The exposed workers also reported a reduced sense of smell and dry coughs. Workers exposed to DMF also reported facial flushing and palpitations after ingesting alcohol. This condition is related to alcohol intolerance, characterized by a disulfiram-type reaction.
DMA is an organic compound with the formula CH3C(O)N(CH3)2 that is widely used in the synthetic fiber and resin industries[16].
It is colorless, water miscible, has a high boiling point, and is commonly used as a polar solvent in organic chemistry, as a solvent for vinyl resins, cellulose derivatives, polyacrylonitrile, linear polyesters and styrene. It is also used as in catalyst and solvent elimination, cyclization, alkylation reactions and halogenations.
DMA is a widely used solvent in acrylic and elasthane fiber spinning, and is also used as: a solvent in the production of X-ray contrast media; a solvent in the production process of antibiotics like cephalosporins (such as cefadroxil, cefalexin and cefradine); and a solvent and reaction medium in the manufacture of polyimide resins, polysulfones and cellophane.
The hepatic toxicity of DMA is well known in animals, with reports of an increase in liver weight, steatosis, hepatic focal cystic degeneration, transaminasemia, biliary hyperplasia and centrilobular single cell necrosis[17].
It was discovered that a worker in a polyurethane plastic producing plant who was accidentally exposed to DMA mixed with ethylenediamine developed chemical induced hepatitis with several toxic features[18].
There was another report of a male worker in a factory of synthetic stretch fabric who was exposed to mixed solvents, including DMA, in a confined space continuously for 4-6 h/d for three days developing hepatic injury with other clinical manifestations of acute DMA intoxication. Toxic hepatitis following excessive skin exposure to DMA was reported among workers from a new production line of acrylic fiber[19,20].
TCE is a volatile organic compound with the chemical formula C2HCl3. A chlorinated hydrocarbon, it is used as an industrial solvent, and takes the form of a clear non-flammable liquid with a sweetish smell resembling chloroform.
Until 1975, it was used as a volatile anesthetic (however, it produced depression of the central nervous system) and inhaled obstetrical analgesic in millions of patients, as well as an extractant in food-processing. It is now used for vapor degreasing and as a solvent[21].
TCE is a solvent for a wide variety of organic materials, but is also used in the food industry for the decaffeination of coffee and the preparation of flavoring extracts from hops and spices.
Higher concentrations can cause tachypnea, and many types of cardiac arrhythmias which are exacerbated by epinephrine (adrenaline).
TCE has also been used as an inhaled patient controlled analgesic agent, mainly for the treatment of trigeminal neuralgia.
It was found that 10% of workers exposed to TCE became jaundiced with massive hepatic necrosis[22].
The data in humans, although limited, clearly suggests a toxic effect on human livers. Case reports describe TCE as inducing hepatitis and liver necrosis[23].
Also, known under the name tetrachloroethene, tetrachloroethene is a chlorocarbon with the formula Cl2C=CCl2. It is a colorless liquid that is volatile, highly stable, and nonflammable, and mainly used as a solvent in dry cleaning and metal cleaning. It is also used for veterinary anthelmintic, processing and finishing in the textile industry, as an extraction solvent, grain fumigant, heat-exchange fluid, and in the manufacture of fluorocarbons.
Tetrachloroethylene is an excellent solvent for organic materials. It is also used to degrease metal parts in the automotive and other metalworking industries and appears in certain consumer products including spot removers, paint strippers, silicone lubricants, and food[24,25]. Its toxicity presents itself as different effects in the central nervous system, kidneys and liver. Symptoms of toxicity from exposure include fatigue, dizziness, headache, vomiting and nausea, signs of hepatic or renal failure, and pulmonary edema[26].
Tetrachloroethylene causes irritation of the eyes and nose mucosal. Severe exposure can lead to behavior alteration, coma and death[24,26].
Carbon tetrachloride, also known by numerous other names, is an organic compound with the formula CCl4 that takes the form of a clear liquid that very easily evaporates. Most carbon tetrachloride that finds its way into the environment is therefore found as a gas.
Carbon tetrachloride does not burn easily. It normally has a sweet smell, but this can change to a more unpleasant odor when the concentration of carbon tetrachloride reaches 10 parts per million parts of air (ppm).
In the 20th century, carbon tetrachloride was commonly used as a dry cleaning solvent, as a refrigerant, and in lava lamps[27]. One specialty use of carbon tetrachloride was by stamp collectors to reveal watermarks on the backs of postage stamps without damaging the stamp. However, once it became apparent that carbon tetrachloride exposure had severe adverse health effects, safer alternatives such as tetrachloroethylene were found for these applications, and its use in these roles declined from about 1940 onward.
Carbon tetrachloride was used as a pesticide to kill insects in stored grain but, in 1970, it was banned in consumer products in the United States. Before the Montreal Protocol, large amounts of carbon tetrachloride were used to produce the Freon refrigerants R-11 (trichlorofluoromethane) and R-12 (dichlorodifluoromethane). However, these refrigerants were identified as playing an important role in ozone depletion, and therefore their use was banned. Carbon tetrachloride is still used to manufacture less destructive refrigerants however.
Carbon tetrachloride is one of the most powerful solvents toxic to the liver, and is widely used in scientific research to assess liver damage and hepatoprotective agents[28].
Indeed, carbon tetrachloride has been known for many years to be toxic to the liver. It has been shown to produce hepatic damage including necrosis and fatty degeneration in various experimental animal species[29,30]. Several experiments have also shown that single doses can cause areas of necrosis in the liver within minutes[31,32], as well as liver enzyme abnormalities known to indicate liver damage[33,34].
Xylene is a clear, light-colored or colorless, flammable liquid which evaporates rapidly and is also called “xylol,”“dimethylbenzene,” or “mixed xylenes”. Its odor is strong and sweetish like other aromatic solvents.
Xylene may be found in: solvents for gums, resins, rubber cleaners, degreaser paints, lacquers, varnishes, adhesives, cements, epoxy resins, inks, dyes, and gasoline.
The xylene in commercial use is composed of a mixture of the three isomers ortho-xylene, meta-xylene, and para-xylene; the meta-isomer predominates in these mixtures. O-Xylene and m-xylene are clear, colorless, flammable liquids that have characteristically sweet, balsam-like odors. At low temperatures, the para-isomer occurs in the form of clear, colorless plates[35].
Toluene occurs as a colorless, flammable, refractive liquid that is slightly soluble in water, has a sweet and pungent odor, and has the chemical formula C6H5CH3[36].
It is used to produce benzene and as a solvent in paints, coatings, synthetic fragrances, adhesives, inks, and cleaning agents. Toluene is also used in the production of polymers used to make nylon, plastic soda bottles, polyurethanes, and for pharmaceuticals, dyes, cosmetic nail products, and the synthesis of organic chemicals[36]. Exposed to toluene may occur from breathing ambient or indoor air, from the use of common household products (paints, paint thinners, adhesives, synthetic fragrances and nail polish), and cigarette smoke. The deliberate inhalation of paint or glue may result in high levels of exposure to toluene, as well as other chemicals, in solvent abusers[36].
Toluene exposure may also occur in the workplace, especially in occupations such as printing or painting, where toluene is frequently used as a solvent. Automobile emissions are the principal source of toluene in the ambient air. Toluene may be released to the ambient air during the production, use, and disposal of industrial and consumer products that contain toluene. Levels of toluene measured in rural, urban, and indoor air averaged 1.3, 10.8, and 31.5 micrograms per cubic meter (μg/m3), respectively[36].
Toluene is metabolized by the liver; however, the liver does not appear to be a primary target for toluene toxicity. Indeed, the central nervous system (CNS) is the primary target for toluene toxicity in both humans and animals for acute and chronic exposures. CNS dysfunction (which is often reversible) and narcosis have been frequently observed in humans acutely exposed to low or moderate levels of toluene by inhalation; symptoms include fatigue, sleepiness, headaches, and nausea. CNS depression and death have occurred at higher levels of exposure.
A study of printing factory workers who were exposed to toluene at a concentration of less than 200 ppm showed minimal changes to liver enzymes[37]. A study by Svensson et al[38] looked at 47 rotogravure workers occupationally exposed to toluene and showed elevation of liver enzymes and chemical hepatitis.
Hepatotoxicity has been observed in the literature in individuals exposed to xylene and toluene[39].
Chloroform is a volatile organic compound that takes the form of a colorless liquid with a non-irritating odor and a slightly sweet taste. It will burn only when it reaches very high temperatures. In the past, chloroform was used as an inhaled anesthetic during surgery, but it is not used for that purpose today. Today, chloroform is used to make other chemicals and can also be formed in small amounts when chlorine is added to water. Other names for chloroform are “trichloromethane” and “methyl trichloride”.
It is produced as a byproduct of water chlorination and the bleaching of paper. Chloroform may also be elicited as a vehicle exhaust. In 1923, Meyer and Pessoa showed the toxicity of chloroform to the human liver[40].
The pathophysiologic mechanisms of hepatotoxicity are still being explored, but are characterized by organic and functional damage of the liver. The principal alterations are: (1) Disruption of the hepatocyte; with a decrease in ATP levels. Disassembly of actin fibrils at the surface of the hepatocyte with blistering and rupture of the membrane; (2) Disruption of the transport proteins; toxins may affect transport proteins at the canalicular membrane and can interrupt bile flow. It also detects interruption of transport pumps; (3) Cytolytic T-cell activation: the covalent binding of a toxin to the P-450 enzyme acts as an immunogen, activating T cells and cytokines and stimulating a multifaceted immune response; (4) Apoptosis of hepatocytes; activation of the apoptotic pathways by the tumor necrosis factor-alpha receptor of Fas may trigger the cascade of intercellular caspases, which results in programmed cell death; and (5) Bile duct injury; toxic metabolites excreted in bile may cause injury to the bile duct epithelium[41]. In cultured rat hepatocytes, the hydrophobic bile acid glycochenodeoxycholate at pathophysiologically relevant concentrations (20-100 mmol/L) induces apoptosis, as documented by cell shrinkage, nuclear condensation and lobulation, caspase activation, DNA fragmentation, and phosphatidylserine externalization. Thus, bile acids provide a valuable model to dissect the mechanisms of liver cell apoptosis and the role of apoptosis in liver injury from endogenous toxicants. Apoptosis occurs by one of two pathways: (1) a death receptor pathway; and (2) the mitochondrial pathway.
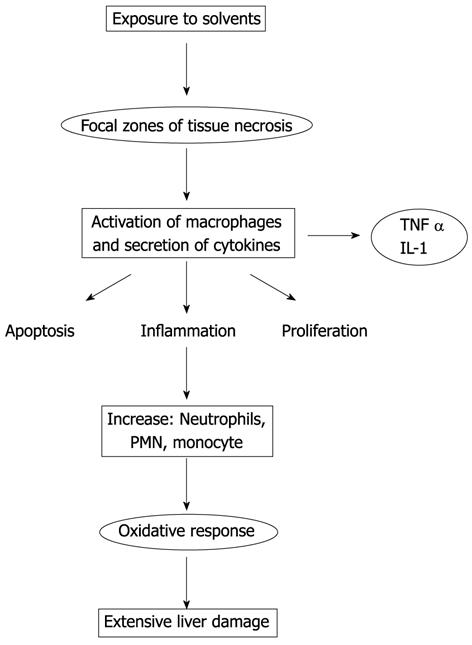
The main pathogenic mechanisms responsible for functional and organic damage caused by solvents are: inflammation, dysfunction of cytochrome P450, mitochondrial dysfunction and oxidative stress.
Inflammation plays an important role in classical chemical toxicities[42,43]. Hepatic non-parenchymal cells, the Kupffer, sinusoidal endothelial, and fat-storing or Ito (stellate) cells, and recruited leukocytes, i.e., monocytes and neutrophils, contribute to the pathogenesis of hepatic toxicity.
Proinflammatory cytokines, chemokines, reactive oxygen and nitrogen species, that promote oxidative stress in the damage induced by toxic substances, are produced by Kupffer cells and neutrophils.
In response to a direct action of the chemical, the Kupffer cells are activated resulting in the production of proinflammatory cytokines such as interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF) α, and chemokine receptor chemokines.
Each of these factors can upregulate expression of β-2 integrin [cluster of differentiation (CD)11b/CD18] and prime neutrophils for reactive oxygen species (ROS) formation. C5a also stimulates Kupffer cells to release ROS.
The aforementioned cytokines can regulate genes for the production of factors that induce and/or promote apoptosis, or that stimulate the proliferation of hepatocytes.
These cytokines can mediate many pathological effects, including inflammatory cell infiltrates, lipogenesis, fibrogenesis and cholestasis[44].
For example, TNF α/IL-6 induction occurs within minutes following CCl4 exposure and is responsible for the activation of nuclear transcription factors including AP-1, NFκB, and STAT 3[45,46], which regulate genes involved in cell growth. Liver toxicity induced by solvents is presented schematically in Figure 1.
In addition, cytokines activate the expression of adhesion molecules on endothelial cells and hepatocytes. If primed neutrophils receive a chemotactic signal from the parenchyma, they will transmigrate and adhere to hepatocytes. This leads to the final activation of neutrophil with degranulation (protease release) and adherence-dependent oxidant stress, which causes cell necrosis. Mediators generated during cell injury, such as lipid peroxidation products (LPO) and chemokines, become chemotactic signals for further neutrophil activation and transmigration[47].
Therefore, both neutrophils and Kupffer cells, directly or through activation of a complement, are activated by solvents and drugs toxicity, tissue trauma, ischemia-reperfusion, sepsis, and other pathophysiological events. In particular, Kupffer cells release cytotoxic mediators, such as proinflammatory mediators, and reactive oxygen species, such as cytokines and chemokines. Functionally, complement factors (e.g., C5a) and cytokines prime and activate neutrophils to promote their recruitment into the hepatic vasculature. Chemotactically-stimulated neutrophils extravasate and adhere to parenchymal cells. Through the release of reactive oxygen and proteases, they induce necrotic cell death adhesion molecules on neutrophils (β-2 integrins, especially CD11b/CD18) and ICAM-1 on endothelial cells and hepatocytes, which are are essential for neutrophil margination, extravasation, and oxidant production.
Cytokines can induce hepatic adhesion molecule and chemokine formation, which in turn is modulated by oxidant stress[48].
Cytochrome P450 (P450 or CYP) plays an important role in the biotransformation of many endogenous compounds and xenobiotics. The most common enzyme system for oxidation of xenobiotics is cytochrome P450, also known as CYP450 monooxygenase, hydroxylase or oxidase[49,50].
The liver is the major source of P450, although it is expressed in various extrahepatic tissues like the kidney[51].
P450 isoform CYP2E1 is the most abundant isoform in the human liver and therefore is responsible for the metabolism of a wide variety of exogenous and endogenous substrates[52,53]. CYP2E1 dependent ethanol metabolism produces oxidative stress through generation of ROS, a possible mechanism by which solvents are hepatotoxic[54,55].
The cyt p450 is a membrane protein for the disposal of xenobiotics. It is mainly associated with the smooth membranes of the ER of liver cells, while the enzymes for changes to endogenous substances (prostaglandins, cholesterol, etc.) are mainly associated with mitochondria.
For example, toxic substances that are known to be substrates of cytochrome P450 enzymes include acetaminophen, cyclophosphamide, doxorubicin and diclofenac.
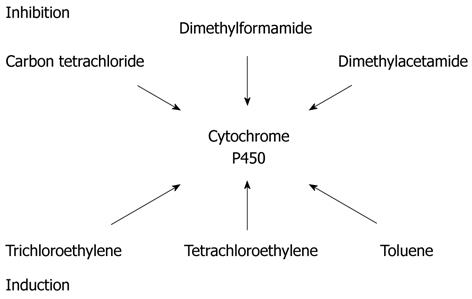
The detoxification process via enzymatic systems, principally the glutathione and cytochrome P450, is different genetically and therefore plays a significant role in the way some individuals detoxify solvents differently from others. It has been shown that all conditions resulting in reduced activity of cytochrome P450, reduced the ability to detoxify solvents and, alternatively, increase the percentage of fat in the liver (e.g., malnutrition). In the study of toxic hepatitis there is a need to evaluate the potential inhibition and inductions of some of the substances involved, and assess the potential interaction with the myriad of drugs that are substrates of CYP[56,57] (Figure 2).
The metabolite responsible for the liver damaging effect of carbon tetrachloride is a C Chloride III which is formed from carbon tetrachloride[31-58].
The studies by Brady et al[59] and Lindros et al[60] have shown that the enzymes involved in the bioactivation of carbon tetrachloride are cytochrome P-450, localized in the liver endoplasmic reticulum.
Various endogenous and exogenous substances impair mitochondrial β-oxidation to cause micro-vesicular steatosis through oxidative stress and damage to mitochondrial proteins, lipids, and DNA. In humans, these oxidative lesions cause mitochondrial DNA (mtDNA) deletions[61].
In normal mitochondria, enzymes involved in the import and β-oxidation of fatty acids or the tricarboxylic acid cycle are encoded by nuclear DNA[62]. Import polypeptides and enzymes involved in the β-oxidation of long-chain fatty acids are in the inner membrane, while those involved in the β-oxidation of medium- and short-chain chain fatty acids or the tricarboxylic acid cycle are in the matrix, together with mtDNA[62].
mtDNA is a circular, double-stranded molecule. Each cell contains many copies of this DNA, as there are several mtDNA copies in a single mitochondrion and many mitochondria per cell[62,63]. MtDNA is extremely sensitive to oxidative damage owing to its proximity to the inner membrane (the main cellular source of ROS), the absence of protective histones, and incomplete repair mechanisms in mitochondria[62-64].
Many solvents (cationic and amphiphilic) are able to concentrate in mitochondria as a result of the mitochondrial membrane potential[65]. Accumulation of these solvents within liver mitochondria inhibits fatty acid β-oxidation (causing steatosis) and electron transfer along the respiratory chain[65]. Overly reduced respiratory chain intermediates react with oxygen to form the superoxide anion. ROS oxidize fat deposits[65]. Similarly, in alcohol abuse, increased ROS formation causes extensive peroxidation of fat deposits and frequent steatohepatitis[66].
The oxidative damage caused by free radicals is thought to be a basic mechanism underlying many pathological conditions, including hepatotoxicity by solvents.
Oxidative stress develops when there is an imbalance between the pro-oxidant and antioxidant ratio, leading to the generation of ROS. Environmental contaminants such as solvents, herbicides, and insecticides are known to modulate antioxidant defensive systems and cause oxidative damage in organisms by ROS production[67,68].
Oxidative damage accumulates more in mitochondria than in the rest of the cells because electrons continually leak from the respiratory chain to form damaging ROS (Smith, R., 1999).
ROS, such as hydrogen peroxide (H2O2), superoxide anion O2-, and hydroxyl radical (OH•) at supranormal levels, can react with biological macromolecules potentially leading to enzyme inactivation, LPO, DNA damage and cell death, but at low concentrations their effects are less pronounced[69].
These free radicals are capable of damaging many cellular components such as DNA, proteins and lipids[70].
Toxic effects on the liver have been studied as early as 1887 and it was determined that there must be a change in the rate of the metabolism of these compound in order to create toxic products, otherwise toxicity will not occur. This chain of events is obligatory from a pharmacokinetic point of view for the majority of solvents[7-9].
There are a few clinical features associated with occupational liver disease including fatigue, appetite loss, arthralgia, hypertransaminasemia, hypergamma glutamyl transferase (GT), and splenomegaly. As there are many causes of liver injury, it is essential to exclude other aetiologies. Other aetiologies include viral hepatitis, biliary diseases, alcohol abuse, and non-alcoholic fatty liver disease.
Clinical presentation of occupational liver disease may be acute/subacute or chronic but is often insidious. Since some of the solvents may cause chronic health effects, it may take decades to study and document such events. Some hepatotoxins are capable of causing malignancy. The most famous example is vinyl chloride which was once thought to be safe and was used for many years until it was found to cause liver tumors.
Signs and symptoms of toxic hepatitis occurring may include: jaundice, itching, and abdominal pain in the upper right portion of the abdomen, fatigue, loss of appetite, nausea and vomiting, rash, weight loss, and dark or tea-color urine.
In acute toxic hepatitis the patient’s condition is similar to viral hepatitis and rapidly deteriorates, resulting in marked liver dysfunction, encephalopathy and coagulopathy. The features of toxic hepatitis are: apoptosis of hepatocytes, ischemic liver injury, sepsis, and cholestasis. Hepatocyte apoptosis and necrosis, when massive, result in fulminant hepatic failure[71].
Acute exposure and toxicity has been associated with liver necrosis, and liver steatosis, and chronic exposure has been associated with liver cirrhosis. The mechanism of injury is most likely the result of metabolic changes by the liver.
Liver damage in the form of hepatomegaly, jaundice, and elevation in levels of several hepatic transaminases and bilirubin has also been reported.
Orthotropic liver transplantation (OLT) has improved the survival of these patients (49% undergo OLT), yet 37% die while awaiting OLT.
Steatosis (fat accumulation in the liver) is important in order to look at the effect of solvents, which are known to be toxic to the liver. Steatosis is the result of anomalous transport of lipids and, as a consequence, accumulation of lipids in the liver. For that reason, exposure to hepatotoxic solvents is clinically associated with liver steatosis, among others, and is a good clinical marker of solvent hepatotoxicity (having ruled out other factors).
After steatosis, necrosis is the second most common effect of liver damage as a result of hepatotoxic solvents. It is the result of destruction of the cell architecture, as well as damage of the biochemical pathways.
Chronic effects on the liver in long-term occupational exposure to low levels of organic solvents remain undetermined.
Indeed, epidemiological studies in this field were difficult for several reasons.
These include: (1) the cause of chronic liver injury can be attributed to a number of factors and not only isolated in occupational exposure; and (2) failure to control chronic liver damage among workers because of vague symptoms and signs, and lack of specificity and sensitivity of conventional liver enzyme tests[72].
Many cases of liver cirrhosis with no known aetiology raise the suspicion that some may be of occupational origin.
Liver damage can be of two types: hepatocellular damage (death of liver cells), in which alanine aminotransferase and aspartate aminotransferase are altered; and cholestatic damage (bile stasis) with an increase of parameters such as alkaline phosphatase and γ-GT. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities are routinely used as clinical endpoints indicative of hepatotoxicity. Toxic hepatic damage includes necrosis and fatty degeneration.
The liver is one of the target organs for toxins, thus biological effects monitoring is required medical surveillance of workers exposed to hepatotoxin. It is also important to evaluate platelet count abnormalities and serum bilirubin in patients.
The evaluation of plasma enzyme showed the advantage of being in the past been well tested in clinical practice, but the main disadvantage is that the enzymes are not organ specific and this can cause occasional diagnostic problems in clinical practice[73,74].
Alanine aminotransferase is considered to be liver-specific in the rat[75]. Aspartate aminotransferase activity is high in the rat liver, along with the kidney, pancreas and erythrocytes[76], which means that elevated serum AST is indicative of tissue and cellular damage, but is not specific for hepatotoxicity.
As a general rule, clinically significant liver injury is often defined as ALT > 3 times the upper limit of normal (ULN)[77].
A factor that may portend a worse prognosis than the elevation of transaminases litter alone is associated with the increase with aminotransferase[78,79].
Serum levels of γ-GT have been recognized as a marker of hepatobiliary disease. Conditions that could cause increases in γ-GT levels are different; hepatotoxic agents and other non-hepatic factors such as renal, pulmonary, and myogenic (including cardiac) disorders. Indeed γ-GT is not merely a sensitive marker for liver and bile disorders, but it may also serve as a risk marker for a multiplicity of other chronic diseases; the metabolic syndrome (namely, obesity, hypertension, lipid metabolism, and in particular type 2 diabetes) for example[80,81].
The measurement of total bile acids in serum may be a more sensitive indicator of hepatic function and has the advantage of being organ specific. Measurement of bile acids has not been standard clinical practice, however, and interpretation of high results might present difficulties.
Bile acids are synthesized from cholesterol in the liver, and secreted to the duodenum via the bile duct[82].
Secreted bile acids are reabsorbed almost completely into the intestine and returned to the liver (Hofman, 2007). Thanks to efficient enterohepatic circulation of bile acids, less than 10% of intestinal bile acids are eliminated in the feces. The secretion of bile acids into the systemic circulation is small. The blood concentration of bile acids is less than 10 μmol/L under normal conditions, but is increased in the case of a disorder of the liver or biliary tract[82]. Hepatotoxicity implicated from occupational and drug exposure has also been associated with elevations in total bile acid concentrations. Retention of bile constituents within the hepatocyte during cholestasis is associated with hepatocyte apoptosis.
In cholestatic disease, endogenously generated bile acids produce hepatocellular apoptosis by stimulating Fas translocation from the cytoplasm to the plasma membrane, where self-aggregation occurs, to trigger apoptosis. In cholestasis, secretion is impaired, resulting in elevated concentrations[83] of toxic bile acids (TBA) within hepatocytes. At pathophysiologic concentrations, TBA trigger translocation of intracellular Fas bearing vesicles to the plasma membrane where they self-aggregate in the absence of ligand. Activated Fas receptor complexes on the plasma membrane then cause caspase 8 activation and an apoptotic cascade.
Evaluation of the concentration of bile acids is not widely used as a routine screening test. Recently, a simpler and more rapid method is the direct enzymatic assay of urine sulfated bile acids (USBA)[84].
Mild forms of toxic hepatitis may not cause any symptoms and may be detected only by blood tests. Histological samples obtained from workers exposed to solvents that had only mild biochemical abnormalities have shown prominent fatty change, or steatosis, with degrees of inflammation and fibrosis, which suggest that parenchymal changes may be an early feature of solvent induced liver injury.
The diagnosis of toxic hepatitis improves with the use of imaging techniques (ultrasound, contrast enhanced ultrasonography, computed tomography, magnetic resonance imaging). In most cases the findings are not characteristic, but history and laboratory investigations allow us to make a diagnostic hypothesis. Indeed, they are essential in determining the clinical-pathological example for the evaluation of steatosis[85].
Routine evaluation includes ultrasound (US) and contrast enhanced ultrasound (CEUS), as reliable methods of first instance. Assessment may include, where necessary, computed tomography (CT) scans, magnetic resonance imaging (MRI) and liver biopsy.
Presently, the most widely used method for assessment of fatty liver is abdominal ultrasound, as it has several advantages. It is not invasive, is readily available, and provides reliable information.
CT and MRI techniques are sensitive for the evaluation of steatosis, but are more expensive and less readily available[86].
However, it is necessary to clarify that recent studies have shown that abdominal ultrasound is not accurate in cases of chronic liver disease with fibrosis. In the latter case, it is important to confirm the steatosis and hepatic fibrosis assessment CT[87].
Toxic hepatitis is characterized by different degrees of steatosis and fibrosis, which can lead to cirrhosis.
Various degrees of steatosis can be defined as: (1) “light steatosis”, presence of slight “bright liver” and no deep attenuation; (2) “moderate steatosis”, presence of mild “bright liver” and with deep attenuation; and (3) “severe steatosis”, presence of diffusely severe “bright liver” and deep attenuation without visibility of the diaphragm.
Chronic toxic hepatitis can progress to cirrhosis and liver failure. Early diagnosis of cirrhosis is important to prevent the development of severe liver failure. The diagnosis of cirrhosis of the liver requires a histological demonstration for the evaluation of abnormal nodules of regeneration and fibrosis. Therefore, liver biopsy is still considered the gold standard instrument. However, liver biopsy is limited by a number of disadvantages, such as availability of expert practitioners, cost, invasiveness of the procedure, and risk of complications (bleeding, pneumothorax, pain perforation and bile peritonitis)[88,89].
Many occupational activities can cause abnormalities in liver function tests without any symptoms suggestive of liver disease.
In patients with abnormalities in liver function tests without an obvious cause, a careful history including not only drugs, but also herbal remedies, should first be obtained.
History taking should also include occupational activity, exposition time, alcohol consumption and underlying chronic liver disease.
| 1. | Franco G, Fonte R, Candura F. Hepatotoxicity of organic solvents. Br J Ind Med. 1986;43:139. [PubMed] |
| 2. | Franco G. New perspectives in biomonitoring liver function by means of serum bile acids: experimental and hypothetical biochemical basis. Br J Ind Med. 1991;48:557-561. [PubMed] |
| 3. | Døssing M. Occupational toxic liver damage. J Hepatol. 1986;3:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs--II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46:1331-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 416] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 5. | Maria VA, Victorino RM. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology. 1997;26:664-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 294] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Mitchell JR, Jollow DJ, Gillette JR, Brodie BB. Drug metabolism as a cause of drug toxicity. Drug Metab Dispos. 1973;1:418-423. [PubMed] |
| 7. | Brautbar N, Williams J. Industrial solvents and liver toxicity: risk assessment, risk factors and mechanisms. Int J Hyg Environ Health. 2002;205:479-491. [PubMed] |
| 8. | Ostertag R. Die toedliche Nachwirkung des Chloroforms. Virchows Arch. 1889;118:250. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 11] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Stiles HJ, McDonald S. Delayed chloroform poisoning. Scott Med Surg J. 1904;15:97. |
| 10. | American Conference of Governmental Industrial Hygienists. Documentation of the Threshold Limit Values and Biological Exposure Indices. 6th ed. Cincinnati: American Conference of Governmental Industrial Hygienists 1991; 488-490. |
| 11. | Lewis RJ. Jr Hawley’s Condensed Chemical Dictionary. 12th ed. New York: Van Nostrand Reinhold 1993; 416. |
| 12. | Marsella J. Formic acid and derivatives. Kirk-Othmer Encyclopedia of Chemical Technology. 4th Ed. New York: John Wiley 1994; 967-976. |
| 13. | Gescher A. Metabolism of N,N-dimethylformamide: key to the understanding of its toxicity. Chem Res Toxicol. 1993;6:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 66] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Redlich CA, Beckett WS, Sparer J, Barwick KW, Riely CA, Miller H, Sigal SL, Shalat SL, Cullen MR. Liver disease associated with occupational exposure to the solvent dimethylformamide. Ann Intern Med. 1988;108:680-686. [PubMed] |
| 15. | Cirla AM, Pisati G, Invernizzi E, Torricelli P. Epidemiological study on workers exposed to low dimethylformamide concentrations. G Ital Med Lav. 1984;6:149-156. [PubMed] |
| 16. | Perbellini L, Princivalle A, Caivano M, Montagnani R. Biological monitoring of occupational exposure to N,N-dimethylacetamide with identification of a new metabolite. Occup Environ Med. 2003;60:746-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Kennedy GL. Biological effects of acetamide, formamide, and their mono and dimethyl derivatives: an update. Crit Rev Toxicol. 2001;31:139-222. [PubMed] |
| 18. | Marino G, Anastopoulos H, Woolf AD. Toxicity associated with severe inhalational and dermal exposure to dimethylacetamide and 1,2-ethanediamine. J Occup Med. 1994;36:637-641. [PubMed] |
| 19. | Su TC, Lin PH, Chiu MJ, Chu TS, Chang MJ, Wang JD, Cheng TJ. Dimethylacetamide, ethylenediamine, and diphenylmethane diisocyanate poisoning manifest as acute psychosis and pulmonary edema: treatment with hemoperfusion. J Toxicol Clin Toxicol. 2000;38:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Baum SL, Suruda AJ. Toxic Hepatitis from Dimethylacetamide. Int J Occup Environ Health. 1997;3:1-4. [PubMed] |
| 21. | American Conference of Governmental Industrial Hygienists. Trichloroethylene. 2005;based on the documentation of the threshold limit values for chemical substances and physical agents and biological exposure indices. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 2005: 1-6. |
| 22. | Klockars M. Solvents and the liver. Safety and health aspects of organic solvents. New York: Alan R Liss 1986; 139-154. |
| 23. | Bond GR. Hepatitis, rash and eosinophilia following trichloroethylene exposure: a case report and speculation on mechanistic similarity to halothane induced hepatitis. J Toxicol Clin Toxicol. 1996;34:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Gold LS, De Roos AJ, Waters M, Stewart P. Systematic literature review of uses and levels of occupational exposure to tetrachloroethylene. J Occup Environ Hyg. 2008;5:807-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for tetrachloroethylene. Atlanta: US Department of Health and Human Services, Public Health Services 1997; . |
| 26. | Garnier R, Bédouin J, Pépin G, Gaillard Y. Coin-operated dry cleaning machines may be responsible for acute tetrachloroethylene poisoning: report of 26 cases including one death. J Toxicol Clin Toxicol. 1996;34:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Doherty RE. A History of the Production and Use of Carbon Tetrachloride, Tetrachloroethylene, Trichloroethylene and 1,1,1-Trichloroethane in the United States: Part 1-Historical Background; Carbon Tetrachloride and Tetrachloroethylene. Environ Forensics. 2000;1:69-81. [DOI] [Full Text] |
| 28. | Seifert WF, Bosma A, Brouwer A, Hendriks HF, Roholl PJ, van Leeuwen RE, van Thiel-de Ruiter GC, Seifert-Bock I, Knook DL. Vitamin A deficiency potentiates carbon tetrachloride-induced liver fibrosis in rats. Hepatology. 1994;19:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Adams EM, Spencer HC, Rowe VK, Mccollister DD, Irish DD. Vapor toxicity of carbon tetrachloride determined by experiments on laboratory animals. AMA Arch Ind Hyg Occup Med. 1952;6:50-66. [PubMed] |
| 30. | Prendergast JA, Jones RA, Jenkins LJ, Siegel J. Effects on experimental animals of long-term inhalation of trichloroethylene, carbon tetrachloride, 1,1,1-trichloroethane, dichlorodifluoromethane, and 1,1-dichloroethylene. Toxicol Appl Pharmacol. 1967;10:270-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 53] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Rechnagel RO, Glende EA. Carbon tetrachloride hepatotoxicity: an example of lethal cleavage. CRC Crit Rev Toxicol. 1973;2:263-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 491] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 32. | Reynolds ES. Comparison of early injury to liver endoplasmic reticulum by halomethanes, hexachloroethane, benzene, toluene, bromobenzene, ethionine, thioacetamide and dimethylnitrosamine. Biochem Pharmacol. 1972;21:2555-2561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | von Oettingen WF. The Toxicity and Potential Dangers of Aliphatic and Aromatic Hydrocarbons. Yale J Biol Med. 1942;15:167-184. [PubMed] |
| 35. | American Conference of Governmental Industrial Hygienists (ACGIH). Documentation of the thresholds limit values and biological exposure indices. 7th ed. Cincinnati: American Conference of Governmental Industrial Hygienists 2001; . |
| 36. | Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Toluene (Update). Atlanta, GA: US Department of Health and Human Services, Public Health Service 1994; . |
| 37. | Guzelian P, Mills S, Fallon HJ. Liver structure and function in print workers exposed to toluene. J Occup Med. 1988;30:791-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Svensson BG, Nise G, Erfurth EM, Nilsson A, Skerfving S. Hormone status in occupational toluene exposure. Am J Ind Med. 1992;22:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Chen JD, Wang JD, Jang JP, Chen YY. Exposure to mixtures of solvents among paint workers and biochemical alterations of liver function. Br J Ind Med. 1991;48:696-701. [PubMed] |
| 40. | Meyer J, Pessoa SB. A study of the toxicity of carbon tetrachloride. Am J Trop Med. 1923;3:177. |
| 41. | Mehta N, Murthy UK, Kaul V, Alpert S, Abruzzese G, Teitelbaum C. Outcome of retinopathy in chronic hepatitis C patients treated with peginterferon and ribavirin. Dig Dis Sci. 2010;55:452-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 42. | Luster MI, Simeonova PP, Gallucci R, Matheson J. Tumor necrosis factor alpha and toxicology. Crit Rev Toxicol. 1999;29:491-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Schook LB, Laskin DL. Xenobiotics and Inflammation. San Diego: Academic Press 1994; . |
| 44. | Tilg H. The role of cytokines in the pathophysiology of chronic liver diseases. Int J Clin Lab Res. 1993;23:179-185. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Bruccoleri A, Gallucci R, Germolec DR, Blackshear P, Simeonova P, Thurman RG, Luster MI. Induction of early-immediate genes by tumor necrosis factor alpha contribute to liver repair following chemical-induced hepatotoxicity. Hepatology. 1997;25:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 114] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Kovalovich K, DeAngelis RA, Li W, Furth EE, Ciliberto G, Taub R. Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology. 2000;31:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 244] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 47. | Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury. J Gastroenterol Hepatol. 2000;15:718-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 251] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 48. | Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166-176. [PubMed] |
| 49. | Porter TD, Coon MJ. Cytochrome P-450. Multiplicity of isoforms, substrates, and catalytic and regulatory mechanisms. J Biol Chem. 1991;266:13469-13472. [PubMed] |
| 50. | Recknagel RO, Glende EA, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139-154. [PubMed] |
| 51. | Ding X, Kaminsky LS. Human extrahepatic cytochromes P450: function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu Rev Pharmacol Toxicol. 2003;43:149-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 569] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 52. | Guengerich FP. Cytochrome P450s and other enzymes in drug metabolism and toxicity. AAPS J. 2006;8:E101-E111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 409] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 53. | Zhou SF. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab. 2008;9:310-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 514] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 54. | Bondy SC. Ethanol toxicity and oxidative stress. Toxicol Lett. 1992;63:231-241. [RCA] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 144] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 55. | Dianzani MU. Lipid peroxidation in ethanol poisoning: a critical reconsideration. Alcohol Alcohol. 1985;20:161-173. [PubMed] |
| 56. | Lin JH, Lu AY. Inhibition and induction of cytochrome P450 and the clinical implications. Clin Pharmacokinet. 1998;35:361-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 552] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 57. | Li AP. Screening for human ADME/Tox drug properties in drug discovery. Drug Discov Today. 2001;6:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 329] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 59. | Brady JF, Li D, Ishizaki H, Lee M, Ning SM, Xiao F, Yang CS. Induction of cytochromes P450IIE1 and P450IIB1 by secondary ketones and the role of P450IIE1 in chloroform metabolism. Toxicol Appl Pharmacol. 1989;100:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Lindros KO, Cai YA, Penttilä KE. Role of ethanol-inducible cytochrome P-450 IIE1 in carbon tetrachloride-induced damage to centrilobular hepatocytes from ethanol-treated rats. Hepatology. 1990;12:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Mansouri A, Fromenty B, Berson A, Robin MA, Grimbert S, Beaugrand M, Erlinger S, Pessayre D. Multiple hepatic mitochondrial DNA deletions suggest premature oxidative aging in alcoholic patients. J Hepatol. 1997;27:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Fromenty B, Pessayre D. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol Ther. 1995;67:101-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 459] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 63. | Schon EA, Bonilla E, DiMauro S. Mitochondrial DNA mutations and pathogenesis. J Bioenerg Biomembr. 1997;29:131-149. [PubMed] |
| 64. | Bogenhagen DF. Repair of mtDNA in vertebrates. Am J Hum Genet. 1999;64:1276-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 65. | Berson A, De Beco V, Lettéron P, Robin MA, Moreau C, El Kahwaji J, Verthier N, Feldmann G, Fromenty B, Pessayre D. Steatohepatitis-inducing drugs cause mitochondrial dysfunction and lipid peroxidation in rat hepatocytes. Gastroenterology. 1998;114:764-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 262] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 66. | Malaguarnera M, Vacante M, Avitabile T, Malaguarnera M, Cammalleri L, Motta M. L-Carnitine supplementation reduces oxidized LDL cholesterol in patients with diabetes. Am J Clin Nutr. 2009;89:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 67. | Risso-de Faverney C, Devaux A, Lafaurie M, Girard JP, Bailly B, Rahmani R. Cadmium induces apoptosis and genotoxicity in rainbow trout hepatocytes through generation of reactive oxygene species. Aquat Toxicol. 2001;53:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 123] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 68. | Monteiro DA, de Almeida JA, Rantin FT, Kalinin AL. Oxidative stress biomarkers in the freshwater characid fish, Brycon cephalus, exposed to organophosphorus insecticide Folisuper 600 (methyl parathion). Comp Biochem Physiol C Toxicol Pharmacol. 2006;143:141-149. [PubMed] |
| 69. | Peña-Llopis S, Ferrando MD, Peña JB. Fish tolerance to organophosphate-induced oxidative stress is dependent on the glutathione metabolism and enhanced by N-acetylcysteine. Aquat Toxicol. 2003;65:337-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 70. | Malaguarnera L, Madeddu R, Palio E, Arena N, Malaguarnera M. Heme oxygenase-1 levels and oxidative stress-related parameters in non-alcoholic fatty liver disease patients. J Hepatol. 2005;42:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 71. | Lee WM. Acute liver failure. N Engl J Med. 1993;329:1862-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 443] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 72. | Warnes TW, Jain SK, Smith A. Hepatotoxic effects of workplace exposures. Hunter’s diseases of occupations. 9th ed. London: Arnold 2000; 881-900. |
| 73. | Misslbeck NG, Campbell TC, Roe DA. Increase in hepatic gamma-glutamyltransferase (GGT) activity following chronic ethanol intake in combination with a high fat diet. Biochem Pharmacol. 1986;35:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 74. | Teschke R, Rauen J, Neuefeind M, Petrides AS, Strohmeyer G. Alcoholic liver disease associated with increased gamma-glutamyltransferase activities in serum and liver. Adv Exp Med Biol. 1980;132:647-654. [PubMed] |
| 75. | Boyd JW. The mechanisms relating to increases in plasma enzymes and isoenzymes in diseases of animals. Vet Clin Pathol. 1983;12:9-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 102] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 76. | Tennant B. Hepatic function. Clinical Biochemistry of Domestic Animals. 5th ed. Toronto: Academic Press 1997; 327-352. [DOI] [Full Text] |
| 77. | Watkins PB, Seeff LB. Drug-induced liver injury: summary of a single topic clinical research conference. Hepatology. 2006;43:618-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 78. | Reuben A. Hy's law. Hepatology. 2004;39:574-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 79. | Björnsson E. Drug-induced liver injury: Hy's rule revisited. Clin Pharmacol Ther. 2006;79:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 80. | Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 835] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 81. | Loew M, Boeing H, Stürmer T, Brenner H. Relation among alcohol dehydrogenase 2 polymorphism, alcohol consumption, and levels of gamma-glutamyltransferase. Alcohol. 2003;29:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Chiang JY. Regulation of bile acid synthesis. Front Biosci. 1998;3:d176-d193. [PubMed] |
| 83. | Dawson PA. Bile secretion and the enterohepatic circulation of bile acids. Gastrointestinal and Liver Disease. St Louis: WB Saunders Co 2002; 1051-1064. |
| 84. | Obatake M, Muraji T, Satoh S, Nishijima E, Tsugawa C. Urinary sulfated bile acids: a new simple urine test for cholestasis in infants and children. J Pediatr Surg. 2002;37:1707-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Ba-Ssalamah A, Schima W, Schmook MT, Linnau KF, Schibany N, Helbich T, Reimer P, Laengle F, Wrba F, Kurtaran A. Atypical focal nodular hyperplasia of the liver: imaging features of nonspecific and liver-specific MR contrast agents. AJR Am J Roentgenol. 2002;179:1447-1456. [PubMed] |
| 86. | Charatcharoenwitthaya P, Lindor KD. Role of radiologic modalities in the management of non-alcoholic steatohepatitis. Clin Liver Dis. 2007;11:37-54, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 87. | Perez NE, Siddiqui FA, Mutchnick MG, Dhar R, Tobi M, Ullah N, Saksouk FA, Wheeler DE, Ehrinpreis MN. Ultrasound diagnosis of fatty liver in patients with chronic liver disease: a retrospective observational study. J Clin Gastroenterol. 2007;41:624-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 88. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1761] [Article Influence: 70.4] [Reference Citation Analysis (1)] |
| 89. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [PubMed] |
Peer reviewers: Bruno Stieger, Professor, Division of Clinical Pharmacology and Toxicology, Department of Medicine, University Hospital, Zurich 8091, Switzerland; Yukihiro Shimizu, MD, PhD, Kyoto Katsura Hospital, 17 Yamada-Hirao, Nishikyo, Kyoto 615-8256, Japan
S- Editor Cheng JX L- Editor Rutherford A E- Editor Zhang DN