Published online Jun 7, 2012. doi: 10.3748/wjg.v18.i21.2689
Revised: February 6, 2012
Accepted: February 16, 2012
Published online: June 7, 2012
AIM: To investigate and evaluate the change in health-related quality of life (HRQoL) by tumor node metastasis (TNM) staging system in patients with hepatocellular carcinoma (HCC).
METHODS: A total of 140 patients diagnosed with HCC between June 2008 and April 2009 in our department were enrolled to this study. One hundred and thirty-five (96.5%) patients had liver cirrhosis secondary to hepatitis B virus (HBV) infection, 73 (54.07%) of them being HBV DNA positive; the other etiologies of liver cirrhosis were alcoholic liver disease (1.4%), hepatitis C (1.4%) or cryptogenic (0.7%). All subjects were fully aware of their diagnosis and provided informed consent. HRQoL was assessed before treatment using the functional assessment of cancer therapy-hepatobiliary (FACT-Hep) questionnaire. Descriptive statistics were used to evaluate demographics and disease-specific characteristics of the patients. One-way analysis of variance and independent samples t tests were used to compare the overall FACT-Hep scores and clinically distinct TNM stages. Scores for all FACT-Hep items were analyzed by frequency analyses. The mean scores obtained from the FACT-Hep in different Child-Pugh classes were also evaluated.
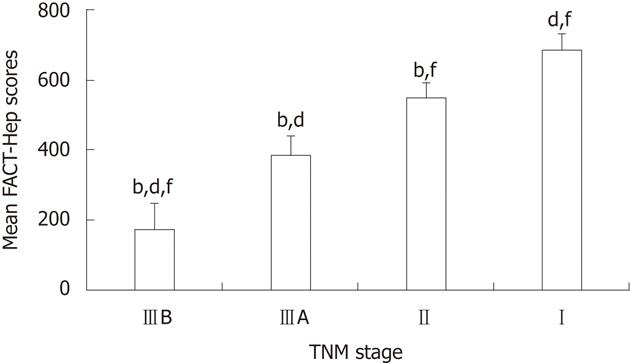
RESULTS: The mean FACT-Hep scores were reduced significantly from TNM Stage I to Stage II, Stage IIIA, Stage IIIB group (687 ± 39.69 vs 547 ± 42.57 vs 387 ± 51.24 vs 177 ± 71.44, P = 0.001). Regarding the physical and emotional well-being subscales, scores decreased gradually from Stage I to Stage IIIB (P = 0.002 vs Stage I; P = 0.032 vs Stage II; P = 0.033 vs Stage IIIA). Mean FACT-Hep scores varied by Child-Pugh class, especially in the subscales of physical well-being, functional well-being and the hepatobiliary cancer (P = 0.001 vs Stage I; P = 0.036 vs Stage II; P = 0.032 vs Stage IIIA). For the social and family well-being subscale, only Stage IIIB scores were significantly lower as compared with Stage I scores (P = 0.035). For the subscales of functional well-being and hepatobiliary cancer, there were significant differences for Stages IIΙ, IIIA and IIIB (P = 0.002 vs Stage I).
CONCLUSION: HRQoL of patients with HCC worsens gradually with progression of TNM stages. The most impaired subscales of HRQoL, as measured by FACT-Hep, were physical and emotional well-being.
- Citation: Qiao CX, Zhai XF, Ling CQ, Lang QB, Dong HJ, Liu Q, Li MD. Health-related quality of life evaluated by tumor node metastasis staging system in patients with hepatocellular carcinoma. World J Gastroenterol 2012; 18(21): 2689-2694
- URL: https://www.wjgnet.com/1007-9327/full/v18/i21/2689.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i21.2689
In recent years, there has been increased interest in quality of life as it pertains to patients’ health status. Many different questionnaires, such as the functional assessment of cancer therapy-hepatobiliary (FACT-Hep), the Health-Related Quality of Life (HRQoL), and the Short Form (36) Health Survey are becoming key instruments in the evaluation of patients’ health status. HRQoL results may be more relevant than length of life, as patients are frequently more concerned about life quality than longevity.
The incidence of hepatocellular carcinoma (HCC) in China is increasing. HCC is now the second leading cause of death of cancers[1]; about 15%-40% of the hepatitis B virus carriers may develop cirrhosis and HCC[2]. Approximately 80% of the patients diagnosed with HCC are unable to undergo surgical resection or transplantation[3]. Non-surgical treatment, such as transcatheter chemo-embolization or chemotherapy may improve the patients’ prognosis to varying degrees[4-8]. Symptoms can be extremely variable in advanced HCC; the compensated patients may be symptomatic for months or decades. The impact is significant on patients’ functioning and well-being. Patients may experience anxiety because of emotional concerns associated with the disease and treatment. Complications and extra-hepatic manifestations of advanced disease may reduce the quality of life, as therapeutic interventions may restrain outdoor activities. These challenges may negatively affect the quality of life, including physical, emotional, and functional well-beings[9].
Assessment of HRQoL with cancer has become an important outcome indicator during the last two decades[10]. The FACT-Hep is the most widely used evaluation tool focusing on hepatobiliary cancer such as HCC, pancreatic cancer and cancers of the gallbladder[11]. In clinical studies, the FACT-Hep performs well in assessing the quality of life of patients with HCC, and is considered to be of utmost importance for improving survival rates and quality of life[11].
The FACT-Hep[12] is a new and important index for evaluation of prognosis of the patients and the results of clinical trials, complementing the traditional end-points assessment, such as tumor response rate and survival time[13-15].
As HCC worsens, quality of life generally decreases, but two unanswered questions remain. First, FACT-Hep has not been used to assess prognosis with a large series of HCC patients at different tumor node metastasis (TNM) stages. Second, quality of life is a broad concept, and no data are available to examine the impact of disease on patients’ self-perception and the factors which are associated with poor HRQoL on the FACT-Hep.
The aim of this study was to determine the HRQoL of patients at different TNM tumor stages of HCC by FACT-Hep, and to determine the factors associated with impaired HRQoL. The TNM Classification of Malignant Tumors was based on physical exam findings, imaging studies (ultrasound, computed tomography or magnetic resonance imaging) and other tests[16]. For solid tumors, such as HCC, TNM is the most commonly used staging system.
Most patients admitted to our department had primary liver cancers. On admission, patients’ quality of life was evaluated using the Chinese version of the FACT-Hep. During an 11-mo period from June 2008 to April 2009, we studied 140 patients with HCC (133 males, 7 females; aged 28-75 years). Patients completed the study questionnaire following the diagnosis of HCC, and prior to therapeutic intervention. All subjects were fully aware of their diagnosis and provided informed consent to participate. The ethical committees of the participating centers approved the study. The TNM staging system[17] (edition 6 published in 2002; and instituted in 2003) was used in this study. Patients were selected based on the demographic characteristics or clinical status.
(1) Patients aged ≥ 18 years; (2) diagnosis of HCC was established by imaging examinations (ultrasound or computed tomography) and confirmed by α1-variable fetoprotein levels exceeding 10 times the normal values, or liver biopsy; (3) patients who had no prior history of malignancy with encephalopathy and no cognitive impairment (as judged by the attending clinician); (4) patients who should speak, read, understand and write Chinese; (5) Karnofsky ≥ 60. The expected survival was at least six months; (6) patients who voluntarily agreed and were able to make the decision to participate in the study; and (7) patients who provided written informed consent.
(1) Illiteracy; (2) current psychosis or homicidal ideation; (3) serious visual or auditory disease; (4) evidence of cognitive impairment or psychiatric disturbance that would prevent informed consent; (5) physical condition too poor to complete the required the 20-25 min of questionnaires; and (6) patient’s family requests that the patient’s condition should be kept a secret from the patients.
The FACT-Hep is a 45-item, self-report instrument designed to measure HRQoL in patients with HCC. The FACT-Hep consists of 27-item FACT-General (FACT-G), which assesses generic HRQoL concerns using five subscales, and the newly validated 18-item hepatobiliary subscale, which assesses specific symptoms of hepatobiliary cancer and side effects of treatment. The five FACT-Hep subscales are: (1) physical well-being (PWB, 7 questions); (2) social and family well-being (SFWB, 7 questions); (3) emotional well-being (EWB, 6 questions); and (4) functional well-being (FWB, 7 questions); and (5) hepatobiliary cancer subscale (HepCS, 20 questions).
The FACT-Hep shows a high internal consistency at initial assessment (Cronbach’s alpha range: 0.72-0.94) and retesting (Cronbach’s alpha range: 0.81-0.94)[11]. Measurement stability is also high for all aggregated scales (test-retest correlation range: 0.84-0.91; interclass correlation coefficient range: 0.82-0.90)[11]. The FACT-Hep can be used independently as a brief measure of disease-related symptoms and functioning in assessing HRQoL of patients with HCC[11]. In this study, the FACT-Hep was translated into Chinese and adjusted appropriately based on the local cultural background. Early research using the Chinese version of the FACT-Hep showed a better reliability and validity (a reliability > 0.5 and a validity > 0.73)[11]. The present study aimed to assess the relationship between the HRQoL in HCC patients as measured by the Chinese version of the FACT-Hep and TNM tumor stage.
All FACT items were rated on 5-point scales ranging from 1 to 5. Converse items should be unified before analysis. The PWB, FWB, SFWB and EWB were summed to get the FACT-G total score. The FACT-G and HepCS scores were summed to obtain the FACT-Hep total score. Higher scores on all subscales of the FACT-Hep reflect higher functioning and fewer symptoms.
Patients completed the FACT-Hep after receiving uniform written instructions from a medical doctor (Li MD) or nurse (Dong HJ). Following FACT-Hep, another doctor (Lang QB) reviewed the forms for missing items. If there were more than three missing items, we requested that the patient complete the FACT-Hep again.
SPSS 13.0 software was used to process and analyze the data. Descriptive statistics were used to evaluate demographic and disease-specific characteristics. One-way analysis of variance and independent samples t tests (P < 0.05) were used to compare FACT-Hep scores between clinically distinct groups.
FACT-Hep questionnaires were issued to 145 patients. Two patients dropped out of the study due to disease exacerbation and three questionnaires were omitted from analysis because of missing data (> 5 questions). In total, 140 questionnaires were completed (with a completion rate of 98.19%). The average time for finishing a FACT-Hep was 13.50 ± 2 min.
Of the 140 patients, 133 were male (95.0%) and the mean age at diagnosis was 52.34 ± 9.73 years (range: 28-75). The TNM tumor stages were as follows: Stage I: 49 cases; Stage II: 35 cases; Stage IIIA: 29 cases; and Stage IIIB: 27 cases. All patients had cirrhosis. One hundred and thirty-five (96.5%) subjects had liver cirrhosis secondary to hepatitis B virus (HBV) infection, with 73 (54.07%) of them being HBV DNA positive; the other etiologies of liver cirrhosis included alcoholic liver disease (2), hepatitis C (2) or cryptogenic (1). Demographic and clinical characteristics are shown in Table 1.
| Factors | P value | |
| Age (yr), median (range) | 52 (28–75) | 0.3509 |
| Sex | < 0.001 | |
| Male | 133 (95.0) | |
| Female | 7 (5.0) | |
| Level of education | < 0.0001 | |
| Primary school | 20 (14.0) | |
| Secondary school | 56 (40.0) | |
| Commercial or vocational school | 64 (46.0) | |
| Tumor, node, metastasis stage | ||
| I | 49 (35.0) | 0.0023 |
| II | 35 (25.0) | |
| IIIA | 29 (20.7) | |
| IIIB | 27(19.3) | |
| IIIC | 0 | |
| IV | 0 | |
| Etiology | ||
| Hepatitis B | 135 (96.5) | |
| Hepatitis C | 2 (1.4) | |
| Alcoholic | 2 (1.4) | |
| Cryptogenic | 1 (0.7) | |
| Child-Pugh class | ||
| A | 84 (60.0) | |
| B | 29 (20.7) | |
| C | 27 (19.3) |
Median FACT-Hep scores decreased as TNM stage advanced, and FACT-Hep scores were strongly associated with TNM stage (Figure 1).
Mean FACT-Hep subscale scores were: PWB 16.47 ± 4.272; SFWB 14.52 ± 3.351; EWB 12.74 ± 3.394; FWB 17.90 ± 4.06; and HepCS 41.46 ± 9.52. The overall mean differences are presented in Table 2.
| FACT-Hep subscales | mean ± SD | Sum of scores | F | P value |
| PWB | 16.47 ± 4.27 | 276.86 | 16.99 | 0.000 |
| SFWB | 14.52 ± 3.35 | 37.08 | 1.71 | 0.184 |
| EWB | 12.74 ± 3.39 | 131.33 | 7.11 | 0.001 |
| FWB | 17.57 ± 3.93 | 165.32 | 5.84 | 0.004 |
| HepCS | 41.46 ± 9.52 | 754.79 | 5.72 | 0.004 |
Scores for each FACT-Hep item worsened with increasing severity of hepatic cirrhosis, based on the Child-Pugh classification (Table 3). Further analyses of factors impacting specific HRQoL were performed using percent analysis.
| n | PWB | SFWB | EWB | FWB | HepCS | |
| TNM stage | ||||||
| I | 49 | 21 ± 2.15df | 15.81 ± 3.68 | 15.11 ± 2.79df | 19.96 ± 3.20df | 48.52 ± 8.36df |
| II | 35 | 19.07 ± 3.41bf | 14.69 ± 2.82 | 13.38 ± 3.01b | 19.24 ± 2.21b | 45.45 ± 10.01b |
| IIIA | 29 | 16.29 ± 2.24bd | 14.66 ± 3.3 | 13.06 ± 3.08b | 18.37 ± 3.98b | 41.29 ± 7.05b |
| IIIB | 27 | 12.41 ± 2.88bdf | 13.61 ± 3.32a | 10.84 ± 3.15bdf | 14.69 ± 3.39bd | 35.35 ± 7.44bd |
| Child-Pugh class | ||||||
| A | 73 | 19.56 ± 2.79 | 15.29 ± 3.34 | 14.12 ± 2.89 | 19.58 ± 3.26j | 46.12 ± 8.79j |
| B | 40 | 16.65 ± 2.79h | 14.25 ± 3.08 | 11.8 ± 3.05 | 16.48 ± 2.98h | 38.88 ± 6.61h |
| C | 27 | 10.52 ± 2.41h | 12.85 ± 3.21h | 10.41 ± 3.49hj | 13.78 ± 3.48hj | 32.7 ± 7.38hj |
Physical well-being: Lack of physical strength was reported by 86% of patients; nausea by 51%; illness affecting the role at home by 83%; pain by 66%; recent uncomfortable feelings by 92%; and frequent bed rest by 55%. Patients had variable decreases in daily exercise capacity.
Social and family well-being: Patients kept close contact with friends (92%); received emotional support at home (59%); had support from friends (69%); had family who understood the patient’s condition (49%); communicated about their condition with family (69%); and had close ties with a lover (43%). Only five patients answered questions regarding their sex lives (3.5%).
Emotional well-being: Sorrow and sad emotions were reported by 83% of patients; fear of death by 79%; and concern about disease progression by 74%. Only 53% of patients completed the item regarding current treatment and losing confidence in overcoming the current disease.
Functional well-being: Working ability was reported by 41%; sense of satisfaction with work by 51%; enjoyment of life by 55%; acceptance of the disease by 59%; insomnia by 95%; enjoyment from hobbies by 63%; and quality of life satisfaction by 52%.
Hepatobiliary cancer subscale: Patients reported stomach distension (65%); weight loss (57%); abnormal intestinal function (87%); digestive dysfunction (87%); diarrhea (64%); good appetite (36%); concern regarding appearance (61%); shoulder and back pain (71%); constipation (54%); fatigue (84%); ability to independently accomplish daily affairs (47%); jaundice 60 cases (43%); fever (36%); itching (27%); food taste changes (48%); cold sensitivity (61%); dry mouth (76%); stomach pain (63%); and swollen ankles (20%). No patients had bile drainage tubes.
Our study showed that patients with HCC had a perceived health status which varies by TNM stage for most FACT-Hep items. This conclusion was based on questionnaires widely used in Chinese clinical studies which indicate that the quality of life is an important prognostic factor and predicts survival time in patients with HCC[18].
HCC, an end-stage complication of liver disease, is expected to affect quality of life, but limited results have been reported previously[19]. Both disease-related and treatment-related symptomatic relief has been the primary goal in advanced HCC management because of the low survival rate of the patients. Alleviating clinical symptoms and improving quality of life have become targets for HCC treatment[20]. Quality of life and related factors in patients with liver cancer have been reported in some studies as indicators of treatment efficacy[21-23]. The FACT-Hep has been widely used in clinical studies to assess HRQoL[11]. Although traditional clinical diagnostic indicators (survival time and tumor response rates) and patients’ subjective feelings are the primary components of quality of life for patients with HCC, the FACT-Hep can provide more comprehensive clinical evaluations[24,25].
There are several staging systems for HCC, such as the Japan Integrated Staging score, the new barcelona clinic liver cancer (BCLC) staging classification, and the Tokyo score. These proposed staging systems consider both tumor size and liver function for HCC evaluation[26].
We classified patients’ disease status using the BCLC and found that advancing BCLC stage was associated with a decreasing trend for FACT-Hep scores, because BCLC staging is related to liver function. Patients with cirrhosis had lower FACT-Hep scores (lower scores represent lower quality of life, Table 3). Previous studies have assessed the relationship between liver function and HRQoL deterioration and fatigue in patients with quantified inflammatory activity and degree of fibrosis[27]. Additionally, quality of life in patients with cirrhosis secondary to primary biliary cirrhosis or chronic hepatitis C has been reported previously[28-30].
The FACT-HepG mean scores showed that HRQoL in HCC patients significantly declined from TNM Stage I to Stage II to Stage IIIA to Stage IIIB. Thus, FACT-Hep scores could reflect varying levels of HCC disease severity.
Our results demonstrate that HCC has a significant and potentially adverse impact on physical health and psychological well-being, causing disruptions to patients’ normal lives. Because the SFWB status of patients with HCC was impaired, family and friends’ emotional support becomes particularly important. Although these symptoms may appear minor in the clinical setting, these factors may significantly predict the poor quality of life. Unfortunately, relevant information about sex life on the FACT-Hep questionnaire was limited and controversial; only five patients completed these questions. This limited response may be due to Chinese cultural norms regarding discussion of sexual activity. Throughout the five FACT-Hep items, we found that EWB was variably impaired. Thus, it appears that patients who have an established diagnosis of HCC experience a wide range of negative emotional symptoms such as sorrow and fear of death. Patients reported various levels of FWB based on the disease stage. On the HepCS, patients most commonly reported abnormal intestinal function, digestive dysfunction and fatigue.
Some limitations must be considered in evaluating these results. To avoid the variability of different therapeutic effects, we chose a simple analysis of untreated patients on admission. However, we believe that this approach may more accurately reflect the relationship between FACT-Hep results and TNM stage. Additionally, it may be more significant, in both clinical work and clinical perspective studies, to use the FACT-Hep as a tool to evaluate the patients’ quality of life prior to treatment. Careful consideration of digestive dysfunction and emotional support is needed, as symptoms greatly impact HRQoL. Only 3.5% of patients responded to questions regarding sexual activity on the FACT-Hep scale. Cultural influences may preclude the use of these questions and it may be more instructive to focus on HCC patients’ psychological states in tumor remission and long-term quality of life, which are other important elements of FACT-Hep. Regarding sleep, the sleep disorders in patients with advanced HCC may be due to the increased burden of physical and psychological factors.
Frequency percents of depression, anxiety and other psychiatric symptoms were significantly higher than other factors. This may be related to the disease itself, which is often associated with a range of negative emotional responses at the time of diagnosis. Furthermore, the poor prognosis, short survival time, need for repeated treatment, and high treatment cost may directly affect patients’ mental health status, as demonstrated by the FACT-Hep results for stress, anxiety, fear of death and disease progression.
In conclusion, this study demonstrated that advancing TNM tumor stages were associated with the declining patient quality of life. These results may be useful for physicians to adjust HCC management according to patients’ physical condition, extent of disease, and quality of life. Results of this study could be used to provide improved health services to meet the needs of HCC patients at different TNM tumor stages prior to initiating the treatment. In addition, more attention should be paid to the sad emotions, digestive dysfunction, fatigue and insomnia. Despite these findings, more definite evidence of the benefits of FACT-Hep questionnaire is required to justify its use. We plan to perform a long-term follow-up of these HCC patients to closely monitor the quality of life changes and to explore and predict their trends.
The incidence of hepatocellular carcinoma (HCC) in China is increasing, and HCC is now the second leading cause of death of cancers. Although many therapies have improved survival rates, symptoms become extremely variable in advanced HCC disease; compensated patients may be symptomatic for months or decades. In recent years, there has been increased interest in quality of life as it pertains to patients’ health status. Many different questionnaires, such as the functional assessment of cancer therapy-hepatobiliary (FACT-Hep), the health-related quality of life (HRQoL), and the short form (36) health survey are becoming key components in the evaluation of patients’ health status. HRQoL results may be more relevant than length of life, as patients are frequently more concerned about life quality than longevity.
The Chinese version of the FACT-Hep is a new and important tool for the evaluation of prognosis and clinical trials, complementing the traditional end-point methods such as tumor response rate and survival time. This study demonstrated that advancing tumor node metastasis (TNM) stages were associated with the declining patient quality of life. These results may be useful for physicians to adjust HCC management according to patients’ physical condition, extent of disease, and quality of life.
As HCC disease worsens, quality of life generally decreases, but two unanswered questions remain. First, FACT-Hep has not been used to assess prognosis with a large series of HCC patients at different TNM tumor stages. Second, quality of life is a broad concept, and no data are available to examine the impact of disease on patients’ self-perception and the factors associated with poor HRQoL on the FACT-Hep.
This study demonstrated that advancing TNM tumor stages were associated with the declining patient quality of life. These results may be useful for physicians to adjust HCC management according to patients’ physical condition, extent of disease, and quality of life. Results of this study could be used to provide improved health services to meet the needs of HCC patients at different TNM tumor stages prior to initiating the treatment
The FACT-Hep is a 45-item, self-report instrument designed to measure HRQoL in patients with HCC. The FACT-Hep consists of 27-item FACT-G, which assesses generic HRQoL concerns using five subscales, and the newly validated 18-item hepatobiliary subscale, which assesses specific symptoms of hepatobiliary cancer and side effects of treatment. The five FACT- Hep subscales are: (1) physical well-being (7 questions); (2) social and family well-being (7 questions); (3) emotional well-being (6 questions); and (4) functional well-being (7 questions); and (5) hepatobiliary cancer subscale (20 questions).
The paper quantifies the quality of life of 140 consecutive and unselected patients with HCC. They have used the FACT-Hep questionnaire and classified their patients according to TNM staging system.
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA. Cancer J Clin. 2005;55:74-108. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13562] [Article Influence: 645.8] [Reference Citation Analysis (3)] |
| 2. | Liu J, Fan D. Hepatitis B in China. Lancet. 2007;369:1582-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, Schrag D, Jamison PM, Jemal A, Wu XC. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 692] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 4. | Geschwind JF, Ramsey DE, Choti MA, Thuluvath PJ, Huncharek MS. Chemoembolization of hepatocellular carcinoma: results of a metaanalysis. Am J Clin Oncol. 2003;26:344-349. [PubMed] |
| 5. | Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int. 2008;7:237-257. [PubMed] |
| 6. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2303] [Article Influence: 100.1] [Reference Citation Analysis (1)] |
| 7. | El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752-1763. [PubMed] |
| 8. | Cahill BA, Braccia D. Current treatment for hepatocellular carcinoma. Clin J Oncol Nurs. 2004;8:393-399. [PubMed] |
| 9. | Tuinman MA, Hoekstra HJ, Sleijfer DT, Fleer J, Vidrine DJ, Gritz ER, Hoekstra-Weebers JE. Testicular cancer: a longitudinal pilot study on stress response symptoms and quality of life in couples before and after chemotherapy. Support Care Cancer. 2007;15:279-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Ohara-Hirano Y, Kaku T, Hirakawa T, Noguchi Y, Hirata N, Shinkoda H, Kitahara E, Saito T, Amada S, Ohki M. Uterine cervical cancer: a holistic approach to mental health and it's socio-psychological implications. Fukuoka Igaku Zasshi. 2004;95:183-194. [PubMed] |
| 11. | Heffernan N, Cella D, Webster K, Odom L, Martone M, Passik S, Bookbinder M, Fong Y, Jarnagin W, Blumgart L. Measuring health-related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer therapy-hepatobiliary questionnaire. J Clin Oncol. 2002;20:2229-2239. [PubMed] |
| 12. | Zhu ZC, Lang QB, Chen Z, Li DT, Ling CQ. [Evaluation of Chinese version of the Functional Assessment of Cancer Therapy-Hepatobiliary questionnaire]. Zhongxiyi Jiehe Xuebao. 2008;6:341-345. [PubMed] |
| 13. | Que HF, Chen HF, Xu JN, Liu S, Lu DM, Tang HJ. [Discussion of relationship between quality of life and clinical effect assessment of malignant tumor treated with traditional Chinese medicine]. Zhongxiyi Jiehe Xuebao. 2005;3:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Steel J, Baum A, Carr B. Quality of life in patients diagnosed with primary hepatocellular carcinoma: hepatic arterial infusion of Cisplatin versus 90-Yttrium microspheres (Therasphere). Psychooncology. 2004;13:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | You J. [Significance and necessity of developing quality of life questionnaire for cancer patients adapting to traditional Chinese medicine]. Zhongxiyi Jiehe Xuebao. 2006;4:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Huang YH, Chen CH, Chang TT, Chen SC, Wang SY, Lee HS, Lin PW, Huang GT, Sheu JC, Tsai HM. Evaluation of predictive value of CLIP, Okuda, TNM and JIS staging systems for hepatocellular carcinoma patients undergoing surgery. J Gastroenterol Hepatol. 2005;20:765-771. [PubMed] [DOI] [Full Text] |
| 17. | Sobin LH, Gospodarowicz MK, Wittekind C. Editors. TNM Classification of Malignant Tumors. 7th ed. Oxford: John Wiley and Sons 2009; . |
| 18. | Poon RT, Fan ST, Yu WC, Lam BK, Chan FY, Wong J. A prospective longitudinal study of quality of life after resection of hepatocellular carcinoma. Arch Surg. 2001;136:693-699. [PubMed] |
| 19. | Steel JL, Chopra K, Olek MC, Carr BI. Health-related quality of life: Hepatocellular carcinoma, chronic liver disease, and the general population. Qual Life Res. 2007;16:203-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 20. | Sun V, Ferrell B, Juarez G, Wagman LD, Yen Y, Chung V. Symptom concerns and quality of life in hepatobiliary cancers. Oncol Nurs Forum. 2008;35:E45-E52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Fielding R, Wong WS. Quality of life as a predictor of cancer survival among Chinese liver and lung cancer patients. Eur J Cancer. 2007;43:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Steel JL, Geller DA, Carr BI. Proxy ratings of health related quality of life in patients with hepatocellular carcinoma. Qual Life Res. 2005;14:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Lai HL, Lin SY, Yeh SH. [Exploring uncertainty, quality of life and related factors in patients with liver cancer]. Huli Zazhi. 2007;54:41-52. [PubMed] |
| 24. | Wang YB, Chen MH, Yan K, Yang W, Dai Y, Yin SS. Quality of life after radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinoma: comparison with transcatheter arterial chemoembolization alone. Qual Life Res. 2007;16:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Steel JL, Eton DT, Cella D, Olek MC, Carr BI. Clinically meaningful changes in health-related quality of life in patients diagnosed with hepatobiliary carcinoma. Ann Oncol. 2006;17:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Chung H, Kudo M, Takahashi S, Hagiwara S, Sakaguchi Y, Inoue T, Minami Y, Ueshima K, Fukunaga T, Matsunaga T. Comparison of three current staging systems for hepatocellular carcinoma: Japan integrated staging score, new Barcelona Clinic Liver Cancer staging classification, and Tokyo score. J Gastroenterol Hepatol. 2008;23:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Teuber G, Schäfer A, Rimpel J, Paul K, Keicher C, Scheurlen M, Zeuzem S, Kraus MR. Deterioration of health-related quality of life and fatigue in patients with chronic hepatitis C: Association with demographic factors, inflammatory activity, and degree of fibrosis. J Hepatol. 2008;49:923-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Bonkovsky HL, Snow KK, Malet PF, Back-Madruga C, Fontana RJ, Sterling RK, Kulig CC, Di Bisceglie AM, Morgan TR, Dienstag JL. Health-related quality of life in patients with chronic hepatitis C and advanced fibrosis. J Hepatol. 2007;46:420-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Poupon RE, Chrétien Y, Chazouillères O, Poupon R, Chwalow J. Quality of life in patients with primary biliary cirrhosis. Hepatology. 2004;40:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Montali L, Tanaka A, Riva P, Takahashi H, Cocchi C, Ueno Y, Miglioretti M, Takikawa H, Vecchio L, Frigerio A. A short version of a HRQoL questionnaire for Italian and Japanese patients with Primary Biliary Cirrhosis. Dig Liver Dis. 2010;42:718-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Peer reviewer: Dr. Nimer Assy, Liver Unit, Ziv Medical Center, PO Box 1008, Safed 13100, Israel
S- Editor Gou SX L- Editor Ma JY E- Editor Xiong L