Published online Jun 7, 2012. doi: 10.3748/wjg.v18.i21.2619
Revised: March 25, 2012
Accepted: April 9, 2012
Published online: June 7, 2012
AIM: To assess whether juvenile chronic ferric iron ingestion limit colitis and dysbiosis at adulthood in rats and mice.
METHODS: Two sets of experiments were designed. In the first set, recently weaned mice were either orally administered ferrous (Fe2+) iron salt or ferric (Fe3+) microencapsulated iron for 6 wk. The last week of experiments trinitrobenzene sulfonic acid (TNBS) colitis was induced. In the second set, juvenile rats received the microencapsulated ferric iron for 6 wk and were also submitted to TNBS colitis during the last week of experiments. In both sets of experiments, animals were sacrificed 7 d after TNBS instillation. Severity of the inflammation was assessed by scoring macroscopic lesions and quantifying colonic myeloperoxidase (MPO) activity. Alteration of the microflora profile was estimated using quantitative polymerase chain reaction (qPCR) by measuring the evolution of total caecal microflora, Bacteroidetes, Firmicutes and enterobacteria.
RESULTS: Neither ferrous nor ferric iron daily exposures at the juvenile period result in any effect in control animals at adulthood although ferrous iron repeated administration in infancy limited weight gain. Ferrous iron was unable to limit the experimental colitis (1.71 ± 0.27 MPO U/mg protein vs 2.47 ± 0.22 MPO U/mg protein in colitic mice). In contrast, ferric iron significantly prevented the increase of MPO activity (1.64 ± 0.14 MPO U/mg protein) in TNBS-induced colitis. Moreover, this positive effect was observed at both the doses of ferric iron used (75 and 150 mg/kg per day po - 6 wk). In the study we also compared, in both rats and mice, the consequences of chronic repeated low level exposure to ferric iron (75 mg/kg per day po - 6 wk) on TNBS-induced colitis and its related dysbiosis. We confirmed that ferric iron limited the TNBS-induced increase of MPO activity in both the rodent species. Furthermore, we assessed the ferric iron incidence on TNBS-induced intestinal microbiota dysbiosis. At first, we needed to optimize the isolation and quantify DNA copy numbers using standard curves to perform by qPCR this interspecies comparison. Using this approach, we determined that total microflora was similar in control rats and mice and was mainly composed of Firmicutes and Bacteroidetes at a ratio of 10/1. Ferric juvenile administration did not modify the microflora profile in control animals. Total microflora numbers remained unchanged whichever experimental conditions studied. Following TNBS-induced colitis, the Firmicutes/Bacteroidetes ratio was altered resulting in a decrease of the Firmicutes numbers and an increase of the Bacteroidetes numbers typical of a gut inflammatory reaction. In parallel, the subdominant population, the enterobacteria was also increased. However, ferric iron supplementation for the juvenile period prevented the increase of Bacteroidetes and of enterobacteria numbers consecutive to the colitis in both the studied species at adulthood.
CONCLUSION: Rats and mice juvenile chronic ferric iron ingestion prevents colitis and dysbiosis at adulthood as assessed by the first interspecies comparison.
- Citation: Ettreiki C, Gadonna-Widehem P, Mangin I, Coëffier M, Delayre-Orthez C, Anton PM. Juvenile ferric iron prevents microbiota dysbiosis and colitis in adult rodents. World J Gastroenterol 2012; 18(21): 2619-2629
- URL: https://www.wjgnet.com/1007-9327/full/v18/i21/2619.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i21.2619
In humans neonates never carry iron deficiency at birth and needs remain low during the first trimester of life because of a slowdown of red blood cells production as well as physiological hemolysis[1]. From the fourth month of life, iron requirements increase and appropriate diet supply, generally corresponding to food diversification, becomes necessary. Western countries recommendations leaded to iron fortification of food for infants to prevent any risk of anemia. Classical forms of supplementation are ferrous (Fe2+) salts but are associated with frequent gastrointestinal side effects leading to poor compliance. In addition to this, presence of ferrous iron in the colon could select gut microbiota for humans that are unfavorable to the host because iron is known to be essential for the growth and virulence of many pathogenic enterobacteria[2]. To limit the unfavorable effects linked to ferrous iron absorption, food industry manufacturers have the possibility of using ferric (Fe3+) forms mainly as of pyrophosphate. However, this form displays not only a poor solubility in the food matrix but also a low bioavailability. To bypass these constraints, ferric pyrophosphate may be microencapsulated in lecithin beads (Lipofer®). The incidence of the daily ingestion of this form in juvenile individuals has not yet been investigated.
At the gut level, and particularly during the perinatal period, the crosstalk between bacteria, epithelium and lymphoid tissue is establishing. This crosstalk is involved in modelling the memory mechanisms of systemic immunity response[3], when those systems mature to reach the full functionality of the intestinal barrier. In humans, postnatal dominant colonisation by facultative anaerobic bacteria, among which we find enterobacteria, quickly gives way to dominant anaerobic bacteria development within a couple of weeks, in order to reach an equilibrium during the second year of life[4]. Intestinal microbiota is a large and extremely complex ecosystem, constituted by dynamic and diverse bacterial communities. The adult human tract environment harbours different bacterial phylotypes superior to 400 different species[5,6]. In permanent contact with the mucosal surface through symbiotic interactions, it takes a prominent part on the maintenance of health of the host[7]. A 10/1 Firmicutes/Bacteroidetes ratio is considered to be representative of an health status[8] which reflects a stable equilibrium between bacteria. However, some groups, that are generally subdominant, such as enterobacteria may become potentially pathogenic and can affect host homeostasis[9]. Several studies have described a higher level of Bacteroidetes and enterobacteria associated to a reduction of Firmicutes in inflammatory bowel disease (IBD) patients[9-14]. IBD are a group of chronic disorders of the gastrointestinal tract and include Crohn’s disease (CD) and ulcerative colitis. The pathogenesis is not known but involves, at least in part a loss of tolerance towards the commensal colonic microbiota. Therefore, disruption of this crosstalk results in a deregulation of the immune response to the gut microbiota and may lead to development of chronic inflammation such as IBD[9].
Since ferrous oral iron may be at the origin of enterobacteria proliferation[2] and since this development may contribute to intestinal mucosal barrier function deregulation leading to intestinal inflammation, we hypothesized that ferric iron supplementation may modulate microbiota profile settlement when given to juvenile animals and could modify the course of an experimental colitis in young adults. However, as individuals vary in their resistance to pathogenic stimuli and as interspecies comparisons remain difficult to address, particularly when analysing microbiota profile, this study aimed at: (1) comparing the incidence of microencapsulated ferric iron with the ferrous iron on experimental colitis in mice; (2) evaluating the dose-response effect of ferric iron in this model of inflammation in mice; and (3) analyzing the consequences of repeated exposure to ferric iron on a moderate colitis and microbiota dysbiosis on two models of rodents (mice and rats) to allow interspecies comparisons.
Ferric pyrophosphate microencapsulated in lecithin beads (Lipofer®) used in this study was kindly provided as a stable solution by Lipofoods SA (Barcelona, Spain). Anesthetics were obtained from Centravet (Nancy, France). All other chemical molecules were purchased from Sigma-Aldrich SA (St Quentin-Fallavier, France) except if specified.
Experiments were conducted using male BalbC mice (15-17 g) and male Wistar rats (100-125 g) obtained from HARLAN Laboratories, Ganat (France). All animals were housed in stainless steel cages under controlled temperature (21 ± 1 °C) and a 12 h light-dark cycles. They had free access to food (A04, SAFE, Epinay sur Orge, France) and water throughout the study. This study was performed at the Animal House Unit of the Institut Polytechnique LaSalle Beauvais (policy agreement No. A60) and received prior approval from both the animal protocol review committee and of the Picardie Council veterinary office.
Two sets of experiments have been designed. In the first series of experiments, 4 groups of 16 male BalbC mice (10-12 g) were used. Three groups received either a solution of ferrous iron (150 mg/kg per day po) or ferric iron (75 or 150 mg/kg per day po - Lipofer®) daily for 6 wk. The 4th group received water. 2,4,6 Trinitrobenzene sulfonic acid (TNBS) colitis was induced the last week of the experiment on half of each group of animals (n = 8/sub-group). The other half served as control (sham colitis). In the second series of experiments, 16 male Wistar rats (75-100 g) were used. The animals were separated in two groups receiving either ferric iron (Lipofer®) at the dose of 75 mg/kg per day po or water under the same conditions for 6 wk. Colitis was also induced during the last week of the experiment. Half of each group of animals were submitted to TNBS colitis (n = 8), the other half (n = 8) served as a control (sham colitis). Body weight was monitored throughout the experiments.
Among the chemically induced experimental colitis[15], TNBS in 50% ethanol is one of the classical models because this mix induces a barrier break resulting in severe colitis with penetrating ulcers, a reduced colon length and thickening of the colon wall as observed in IBD patients[16]. Mice were fasted overnight prior to induction of colitis but were allowed free access to water. They were anesthetized with a mixture (50% v/v) of ketamine and xylazine (100 mg/mL) diluted in saline (NaCl 0.9%-w/v) at a dose 1 mL/kg ip. TNBS (Sigma Aldrich, France) diluted in 50% of ethanol (v/v) was injected via a polyethylene catheter inserted at 4 cm from the anus at the dose of 100 mg/kg in 25 μL to induce an experimental colitis. Control mice were also anesthetized and received an equal volume of saline. Following instillation mice were maintained in a head-down position for 2 min and received 0.2 mL sc of saline to prevent dehydration. Their awakening was closely monitored. Mice were sacrificed 7 d later. Rats were anesthetized with the same mixture of anesthetics at the dose of 5 mL/kg ip and the solution of TNBS was administered at 7 cm from the anus at the dose of 40 mg/kg in 100 μL. Saline (0.5 mL sc) was administered to prevent dehydration. Rats were sacrificed 7 d later. At sacrifice, macroscopic lesions were evaluated and pieces of proximal colon (1 cm from the caeco-colonic junction) and caecal content were collected, snap frozen and stored at -80 °C until further evaluation in both rat and mice experiments.
Macroscopic damage scores: After sacrifice, the colon was removed immediately and severity of colonic mucosal alteration was determined according to a modified scale of Wallace et al[17]. Briefly, determination of the inflammatory damage was based on the presence of mucosal hyperaemia and bowel wall thickening, presence and extent of ulceration and necrosis, and the event of adhesions and diarrhea. Final quotation was ranging from 0 (normal appearance) to 10 (severe damage).
Myeloperoxidase assay: Myeloperoxidase (MPO) activity, a marker of polynuclear neutrophils, was measured in pieces of colon adjacent to the instillation point as described previously[18]. Briefly, frozen pieces of the proximal colon were homogenised in a phosphate buffer (50 mmol/L, pH = 6) containing hexadecyl trimethyl ammonium bromide (0.5% w/v) with a tissue lyser II (Qiagen, France). The homogenates were submitted to 3 cycles of freezing/thawing (Liquid N2, 1 min/37 °C, 10 min) and then further disrupted with a sonicator (Bioblock scientific, France) and then centrifuged (6000 g at 4 °C for 15 min). Supernatants were collected for measuring MPO activity and total protein contents. Samples were diluted into a reaction buffer containing O- dianisidine dihydrochloride (1 mg/mL) and hydrogen peroxide (3 × 10-4 % v/v). Human MPO from purified neutrophils was used as a standard. The absorbance was measured after 10 min of incubation at 450 nm. Total protein content was assessed from the supernatants according to Lowry’s method (Bio Rad DC Protein Assay, France).
As we planned to assess microflora DNA from caecal content as well as comparing two species to validate the repeatability, we aimed at optimizing the bacterial DNA extraction by improving the extraction procedure of Gram positive bacteria and by determining the appropriate amount of the initial sample to treat.
DNA extraction: Total DNA from caecal samples (25, 50, 100 and 200 mg content) was extracted using the Qiamp DNA stool Mini Kit (Qiagen, France). Before proceeding according to the manufacturer’s recommendations, frozen samples were lysed in an ASL lysis buffer and incubated for 3 consecutive cycles of freezing/thawing (Liquid N2, 1 min/37 °C, 10 min). The lysates were clarified by centrifugation (14 000 g at 4 °C for 3 min). Polymerase chain reaction (PCR) inhibitors and impurities were absorbed by action of an inhibitex tablet (Qiagen). The supernatants were collected following a second centrifugation and DNA was automatically purified in the Qiacube automat (Qiagen, France) using Qiamp Minispin columns. Concentrations were determined on a Hellma TrayCel using a Biophotometer (Eppendorf, France). Absorbance ratios at 260/280 and at 260/230 were determined to quantify and assess the purity of DNA samples.
Plasmids for standard curves: Competent DH10b cells were used for the cloning experiments described previously[19]. The recombinant plasmids containing specific 16S rRNA gene inserts either from Firmicutes, or Bacteroidetes, or enterobacteria or a consensus sequence for total microflora, were purified using a HiSpeed Plasmid Maxi kit (Qiagen, France). DNA concentration was determined following measurement of optical densities both at 260 nm and 280 nm before converting it into 16S rRNA gene copy numbers as described previously[19]. Standard curves were established from serial dilutions of recombinants plasmids performed using real-time quantitative PCR. Copy numbers of the plasmid were calculated following a previously established equation [Copy numbers = 6.02 × 1023 (copy/mol) × DNA amount (g)/DNA length (dp) × 660 (g/mol per dp)][20].
Real time polymerase chain reaction: Two pairs of primers (enterobacteria and Firmicutes) corresponding to specific bacterial regions targets within 16S rRNA gene were designed (Table 1). The two other pairs used (Total Flora and Bacteroidetes) had already been designed[21-23]. qPCR was performed using an ABI Prism 7300 sequence detector system (Applied Biosystems, France) on both plasmids and samples extracted DNA. Reactions were performed in duplicate using the Sybr Green PCR master mix (Qiagen, France) in a final volume of 25 μL with 0.3 μmol/L final concentration of each primer and appropriate dilutions of DNA samples. Amplification was initiated at 95 °C for 3 min to activate Taq plus DNA polymerase followed by 40 cycles at 95 °C for 3 s and 61 °C for 30 s. Two consecutive tenfold series of dilutions were realized to verify the linearity (slope of -3.32). A melting step was added and curves were analysed to look for any unspecific amplification. Standard curves were obtained following amplification under similar conditions of different samples containing different numbers of copies from the respective specific clones of the targeted gene. PCR efficiency (E) was calculated according to the equation from the standard curve: E = 10 -(1/ slope) -1 according to Ibekwe and Grieve[24]. qPCR was realised using the cycle number threshold (Ct) and was based on the calculated standard curves. Each qPCR assay systematically included control reactions performed in parallel to the samples.
| PCR assay | Primers | Primers Sequences 5’→3’ | Accession number (NCBI) | Linear regression curves with coefficient of correlation | Sources of reference |
| Total Bacteria | UnivF | TCCTACGGGAGGCAGCAGTG | - | Y = -2.941 x + 36.580 | Watanabe et al[21], 2001 |
| UnivR | TTACCGCGGCTGCTGGCACG | r² = 0.9997 | Nadkarni et al[22], 2002 | ||
| Bacteroidetes | BacF | CCTWCGATGGATAGGGGTT | - | Y = -2.908 x + 35.839 | Firmesse et al[23], 2008 |
| BactR | TCCCCAGGTGGAATACTTAACG | r² = 0.9995 | |||
| Enterobacteria | EntF | CATTGACGTTACCCGCAGAAGAA | AX110239/AX109631 | Y = -3.192 x + 36.762 | This study |
| EntR | CGCTTGCACCCTCCGTATTA | AF293850/U26176 | r² = 0.9747 | ||
| Firmicutes | FirmF | ACCCGCGTCTGATTAGCTAGTT | M59090/L34627 | Y = -3.298 x + 39.136 | This study |
| FirmR | CCTCTCAGGCCGGCTACTG | Y10584/FJ345661 | r² = 0.9924 |
Results were expressed in mean ± SD error to the mean. Macroscopic lesions scores were compared using the Wilcoxon test for non parametric data followed by the Dunn post-test. For all the others parameters, data were submitted to an ANOVA followed by the Tuckey post-test. A value of P < 0.05 was considered to be significant.
Because of high inter- and intra-species variations, we aimed at improving the DNA extraction steps by determining the best amount of caecal content to use and by adding the preliminary step to improve the extraction of Gram positive bacteria. The best yield of DNA extraction was obtained when using 100 mg of caecal content and by adding a preliminary thermic lysis step (data not shown). Specificity of the 16S rRNA gene targeted primers used for qPCR was tested both in silico and using pure DNA extracts from specific target strains (positive controls) and by confronting it to no target species (negative controls). Selected primers (Table 1) were run and the specificities of the amplification products were confirmed by the analysis of the dissociation curve in both caecal samples and DNA controls. We thus determined that a unique target gene was amplified for each species. Analysis of the regression curve for Ct values obtained from serial dilution of DNA samples showed a linear correlation for all target DNA regions; for all the microbiota species studied, the coefficient of correlation (r²) was higher than 0.97 and amplification efficiencies were between 100 % and 120 % which involves curve slopes ranging from -3.29 to -2.9 (Table 1). All below mentioned analyses have been performed under those conditions.
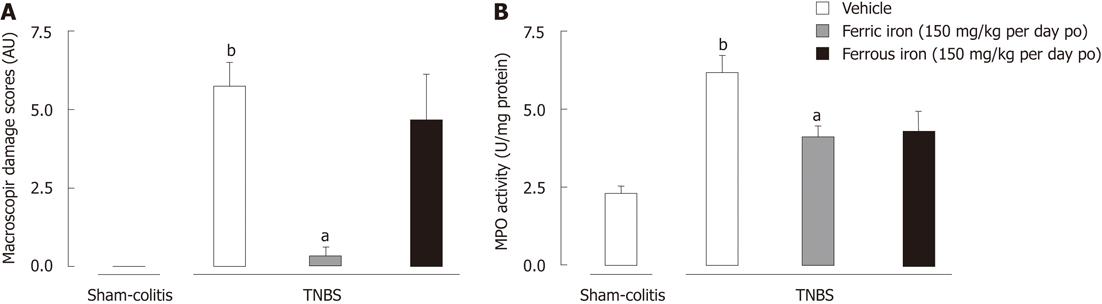
Repeated daily ingestion of ferrous iron (150 mg/kg per day po - 6 wk) by juvenile mice did not prevent the induction of a TNBS-induced moderate colitis. In fact, juvenile ferrous iron daily exposure failed to limit macroscopic lesions (Figure 1A) in TNBS treated mice. In contrast, juvenile ferric iron prevented these lesions (Figure 1A) in inflamed mice. Furthermore, while TNBS enema resulted in an increased MPO activity in controls (2.47 ± 0.22 MPO U/mg protein vs 0.91 ± 0.09 MPO U/mg protein), we did not observe any significant reduction of MPO activity in mice exposed to a repeated ingestion of ferrous iron (1.71 ± 0.27 MPO U/mg protein vs 2.47 ± 0.22 MPO U/mg protein in the TNBS group) (Figure 1B). In contrast, the daily exposure of juvenile mice to the same dose of ferric iron (150 mg/kg per day po - 6 wk) was able to limit the inflammatory response since MPO activity was significantly lower (P < 0.05) (1.64 ± 0.14 MPO U/mg protein vs 2.47 ± 0.22 MPO U/mg protein in the TNBS group) (Figure 1B). Furthermore, we observed that before inducing TNBS colitis (5 wk of treatment), mice treated with ferrous iron put on significantly (P < 0.05) less weight than mice treated with ferric iron and controls (124.6% ± 1.12% and 136.6% ± 1.33 % vs 143.7% ± 2.29 % in control, respectively).
We also aimed at determining the dose-response effect of ferric iron supplementation on the same model of colitis in mice.
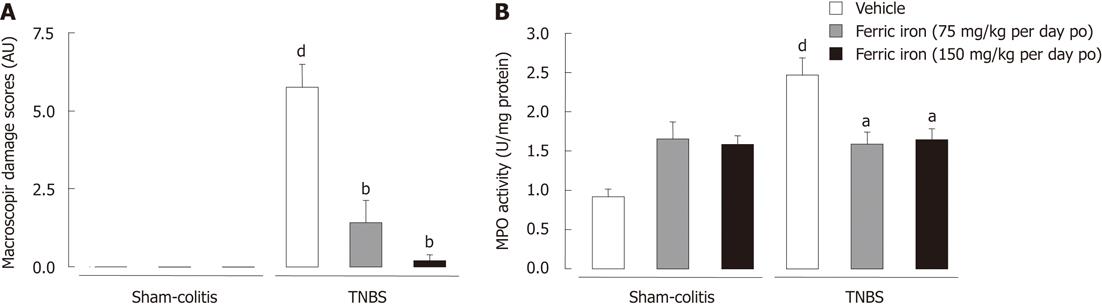
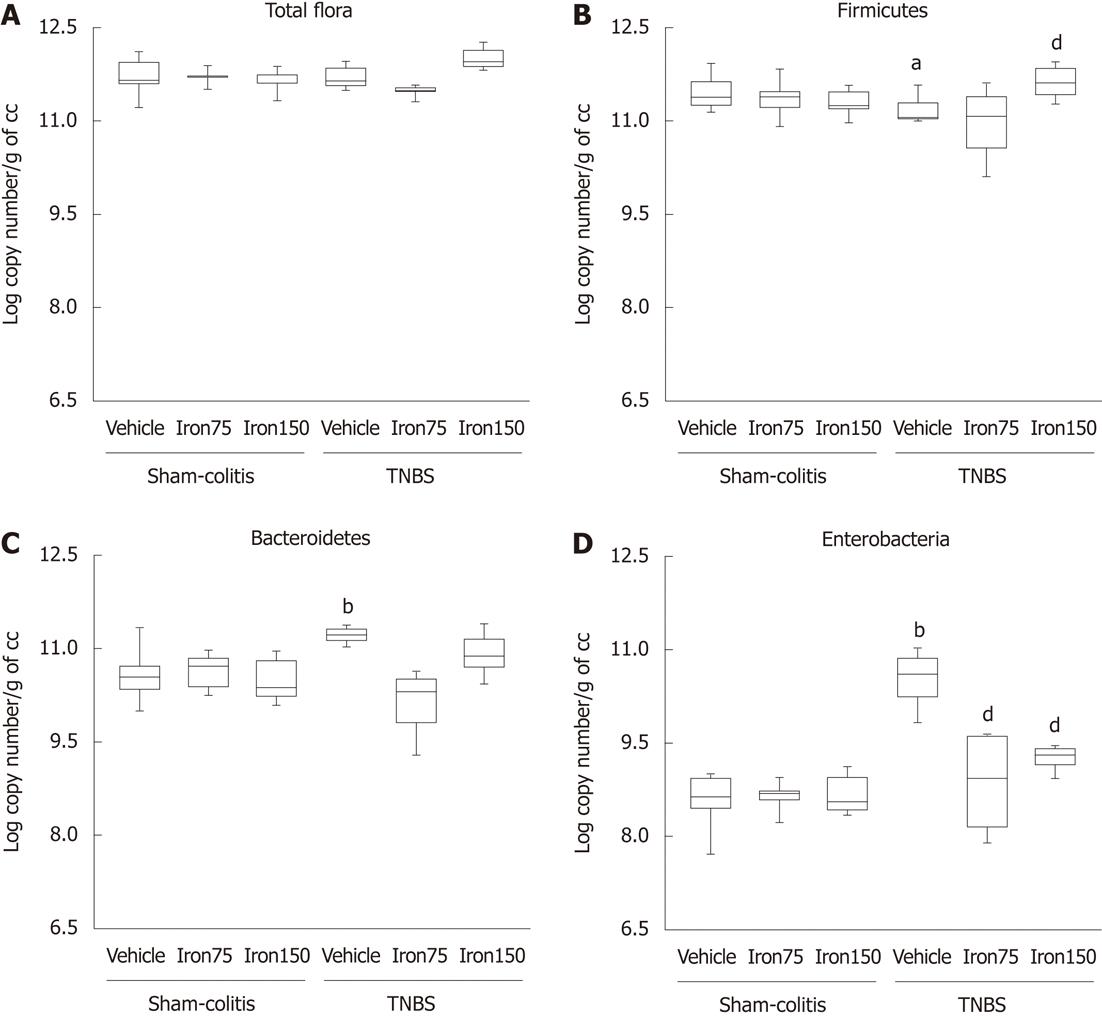
In control animals, the repeated administration of both 75 and 150 mg/kg per day po (6 wk) of ferric iron did not result in any alteration of growth, nor did it result in colonic inflammation or an alteration of the microflora profile. In fact, weight gain under iron treatment remained similar whichever species considered (142.7% ± 2.66% for 75 mg/kg per day and 136.6% ± 1.33 % for 150 mg/kg per day vs 143.7% ± 2.29 % in controls). Similarly, no macroscopic alterations of the gut mucosa have been observed on mice following either the low or the high chronic ferric supplementation (Figure 2A). Those results were correlated to low levels of MPO activity in the colonic mucosa following exposure to both doses of ferric iron (1.65 ± 0.21 MPO U/mg protein and 1.58 ± 0.11 MPO U/mg protein for respectively 75 and 150 mg/kg per day ferric iron po vs 0.91 ± 0.09 MPO U/mg protein in controls) (Figure 2B). Daily consumption of ferric iron by juvenile rodents did not modify the bacteria profile since the total flora number remained at circa 11.8 log copy number/g of caecal content (Figure 3A). The Firmicutes number was estimated to be around 11.4 log copy number/g of caecal content (Figure 3B); The Bacteroidetes number remained around 10.5 log copy number/g caecal content (Figure 3C); and enterobacteria levels at 8.7 ± 0.1 log copy number/g of caecal content (Figure 3D).
Daily administration of both the doses of ferric iron on juvenile rodents prevented the TNBS-induced colitis at adulthood. In fact, exposure to ferric iron supplementation before inducing an experimental colitis limited the onset of macroscopic lesions (1.42 ± 0.72 AU and 0.2 ± 0.2 AU vs 5.75 ± 0.75 AU in TNBS-treated mice) (Figure 2A) and the increase of colonic MPO activity (1.58 ± 0.15 MPO U/mg protein and 1.64 ± 0.14 MPO U/mg protein vs 2.47 ± 0.22 MPO U/mg protein in TNBS-treated mice) (Figure 2B). The limitation of the inflammatory lesions was correlated to the maintenance of a healthy microflora profile. This supplementation also prevented the decrease of the Firmicutes (Figure 3B) and increase of the Bacteroidetes and enterobacteria populations (Figure 3C and D). Since efficiency was observed with the dose of 75 mg/kg per day po for 6 wk, we chose to continue with this dose of ferric iron.
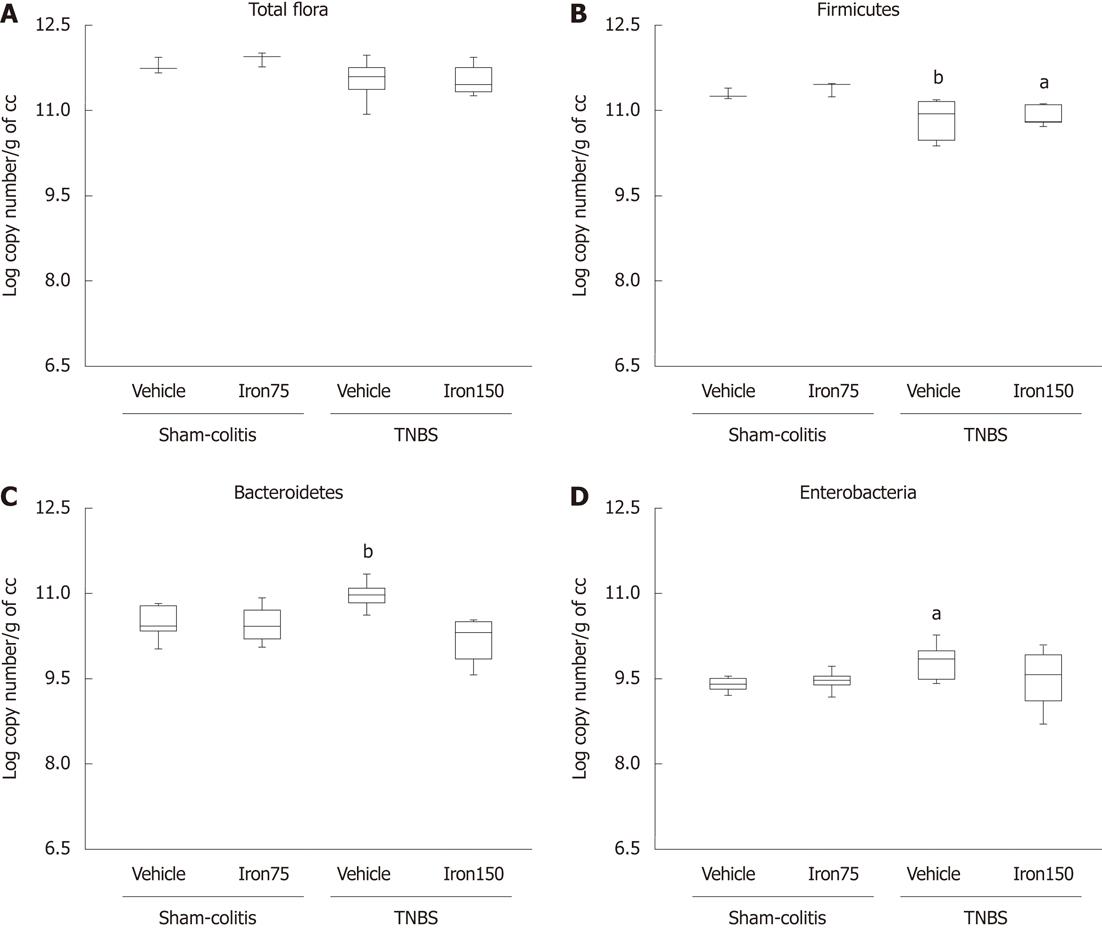
Good comparisons need to be performed under the same conditions necessitating the induction of a comparable inflammatory reaction that is representative of pathophysiological conditions observed in humans. Using the optimised technique of extraction and quantification of DNA, total flora in control rats and mice was estimated to be around 11.8 log copy number/g of caecal content in control animals (Figures 3A and 4A). We also noticed that it is mainly composed of Firmicutes (around 11.3 log copy number/g of caecal content in both species) (Figures 3B and 4B) and Bacteroidetes (around 10.5 ± log copy number/g caecal content) (Figures 3C and 4C) followed by an enterobacteria population ranging from 8.6 ± 0.1 log copy number/g of caecal content in mice to 9.4 ± 0.1 log copy number/g of caecal content in rats (Figures 3D and 4D).
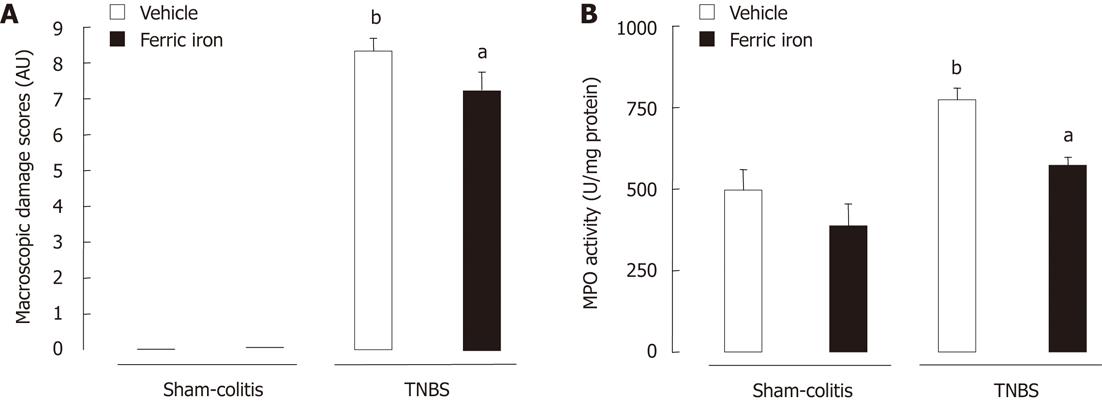
Instillation of TNBS resulted in a significant (P < 0.05) increase of macroscopic damage scores in rats (8.33 ± 0.35 AU) and mice (5.75 ± 0.75 AU) (Figures 2A and 5A) as well as a significant (P < 0.05) increase of MPO activity in rats (773.7 ± 36.62 MPO U/mg protein vs 496.1 ± 63.94 MPO U/mg protein) and mice (2.47 ± 0.22 MPO U/mg protein vs 0.91 ± 0.09 MPO U/mg protein) (Figures 2B and 5B). However, we did not observe any significant modification of total microflora neither in rats nor in mice 7 d after TNBS instillation (circa 11.7 log copy number/g of caecal content in both rodent species) (Figures 3A and 4A). However, the balance between the two major phyla of the bacterial population observed was altered. A significant reduction (P < 0.05) of Firmicutes was registered in both colitic rats (-0.4 Δ log copy number) and mice (-0.3 Δ log copy number) (Figures 3B and 4B) as compared to controls. Conversely, significant higher levels of Bacteroidetes (P < 0.05) were detected in both the colitic groups; it increased by 0.5 Δ log copy number in rats and by 0.7 Δ log copy number in mice (Figures 3C and 4C). Enterobacteria numbers were also found to be significantly higher (P < 0.05) in colitic rats and mice with increases of respectively 0.4 and 2 Δ log copy number/g of caecal content as compared to controls (Figures 3D and 4D).
Juvenile ferric iron supplementation for 6 wk, before inducing an experimental colitis, significantly limited the onset of macroscopic lesions not only in TNBS-treated mice (1.42 ± 0.72 AU vs 5.75 ± 0.75 AU) but also in TNBS-treated rats (7.25 ± 0.49 AU vs 8.33 ± 0.35 AU). Juvenile iron administration also significantly (P < 0.05) prevented the increase of colonic MPO activity in TNBS-treated mice (1.59 ± 0.5 MPO U/mg protein vs 2.47 ± 0.22 MPO U/mg protein) as well as in TNBS-treated rats (572.5 ± 26.39 MPO U/mg protein vs 773.7 ± 36.63 MPO U/mg protein) (Figures 2B and 5B). This juvenile ferric administration (75 mg/kg per day po - 6 wk) did not modify total bacteria number even when inducing colitis (Figure 3A and 4A). Juvenile ferric iron repeated administration before inducing the colitis not only prevented the increase of the Bacteroidetes population (Figures 3B and 4B) but also the enterobacteria population (Figures 3D and 4D) in both mice and rats.
When using a microencapsulated ferric pyrophosphate form (Lipofer®), we were the first to evidence a beneficial effect of ferric iron oral supplementation in juvenile animals to prevent the induction of colitis at adulthood. Ferric pyrophosphate is a water insoluble compound, marketed as a food additive in Europe to fortify infant cereals and chocolate powder drinks[25]. Because of its chemical composition, it has been microencapsulated to improve both its bioavailability and its incorporation in food to be fortified[26], rendering it a good alternative to the ferrous forms without having any of the side effects.
First, we provided evidence of the benefits of ferric iron supplementation during the juvenile period. Iron oral administration is recommended in young infants to prevent anemia and its consecutive neurologic and developmental deficits. Iron also has benefits, as a trace element, in supporting immune function by strengthening not only epithelial barriers but also cellular and humoral immune responses. However, ferrous iron may be at the origin of deleterious effects such as lipid peroxydation[27] or enterobacteria growth facilitation[2]. Here, with ferric iron, we did not observe any negative effect of a repeated administration for 6 wk. In fact, in non-inflamed mice, juvenile ferric iron ingestion did not result in either an inflammatory reaction or did it modify the microflora profile since both total bacteria and enterobacteria numbers remained unchanged as compared to non supplemented sham-colitic mice. This absence of action on gut microbiota profile is in favour of the ferric form instead of the ferrous form for infant food fortification.
Secondly, we aimed at evaluating the dose response effect of ferric iron supplementation in the juvenile period the induction of an experimental colitis at adulthood. Environmental factors such as smoking, luminal enteric bacteria and trace elements such as iron are involved in the pathogenesis of IBD in a genetically susceptible host[28]. In fact, anemia is often described during chronic gut inflammatory disorders such as IBD[29]. This anemia is caused by two factors, the first being impaired proliferation and differentiation of erythroid progenitor cells, and the second being consecutive to iron retention within monocytes and macrophages both activated under the inflammatory conditions. Classically, patients receive either oral or systemic iron supplementation generally as ferrous sulfate or fumarate. However, ferrous iron oral administration often aggravates the inflammatory reaction because of its accumulation in the intestinal lumen and its participation in the Haber-Weiss reaction[27]. Compared to non supplemented mice, ferric iron juvenile supplementation proved to be efficient against this experimental colitis, even at the lowest dose used since their MPO activity levels were similar to those of control mice. The lowest dose of iron used corresponded to an overall daily intake of 1.2 mg iron per day, in other words to 15 μg iron/g mouse/day which is two times lower than the dose used by Werner et al[30] but it is however efficient in our model too.
The positive effect of iron supplementation in mice was corroborated by the results obtained in rats. In our study, we were able to demonstrate that ferric iron oral administration did not induce any alteration but rather prevented the course of an experimental colitis in both rodent species. In fact, both rats and mice submitted to iron supplementation did not develop any sign of inflammation. In both those rodent species, we also induced an experimental inflammatory reaction of comparative intensity. In fact, in comparison to the literature[31], they developed a moderate but homogenous colitis characterised by tissular lesions, ulcerations of the distal colon and neutrophil infiltration. These results are in agreement with a previous report indicating that the TNBS-induced colitis model is associated to an increase of inflammatory response including granulomas and tissular MPO activity[31-33].
Finally, we aimed at evaluating the reproducibility of colonic microflora evaluation under control conditions and its alteration during a moderate experimental colitis in rats and mice. This study is the first to evidence a comparative alteration of gut microbiota during an experimental colitis in two rodent species. Following the qPCR optimisation process described above, we observed no difference in total microbiota numbers between control rats and mice groups. In this work, we also found that caecal microflora of rodents is composed predominantly of Firmicutes followed by Bacteroidetes which profile correlates to the observations realised on humans. In fact, human Firmicutes population is 10 times higher than their Bacteroidetes population; which is considered to be a good indicator of health status[34,35]. In this study, we observed the same ratio profile not only in mice but also in rats. In non supplemented animals and under inflammatory conditions, we did not observe any alteration of total bacteria numbers in both the rodent species. This is in agreement with literature which describes no principal differences in the composition of the total mucosal flora in IBD patients compared to controls[36]. Furthermore, while tissular healing might have already started in the animals, in both murine models we observed a net increase of Bacteroidetes and a reduction of the Firmicutes population indicating a clear reversion of this profile. Similar trends were also observed in clinical studies, showing a lower representation of Firmicutes phyla during inflammatory acute phases[34,37]. As already evidenced, the diminution of the Firmicutes phylum promotes the development and the invasion of tissues by opportunistic bacteria species and the gut becomes very susceptible to invading pathogens among which enterobacteria[38,39]. We also noted, under colitic conditions, a higher level of enterobacteria which is concordant with literature reporting a higher incidence of Escherichia coli in IBD patient compared to healthy subjects[40,41]. We chose to work in comparing rat and mice microbiota alteration during this moderate experimental colitis to ensure that not only could we obtain comparative results to human dysbiosis but also to make sure that the results obtained were linked to the intensity of the inflammatory response rather that to species sensitivity. Such comparisons are of great interest in testing the reproducibility of the method set up. Overall, our results are correlated to clinical observations realised on IBD patients such as fever, diarrhea, weight loss, rectal bleeding[42], and severe alteration of the microbiota equilibrium, especially with Bacteroidetes and enterobacteria increases in opposition to Firmicutes reduction[9,10]. In both rats and mice receiving ferric iron in the juvenile period for 6 wk the microbiota profile was not altered. In addition, rats and mice submitted to an experimental colitis during the last week of iron treatment did not display any modification of either total number bacteria or enterobacteria numbers as compared to non supplemented animals. This is in favour of a positive role of ferric iron onto gut microbiota equilibrium which will limit the onset of the inflammatory reaction as compared to classical ferrous forms. It reinforces the idea that iron is one of the key markers for limiting the onset of the inflammatory response in genetically susceptible patients[30]. Furthermore, we may suggest that ferric iron in contrast to ferrous iron contributes to the settlement of an appropriate microflora during the post natal period and that this profile is less sensitive to inflammatory stimuli. One cannot ascertain the mechanisms producing such results, but we may suggest that the lower incidence of ferric iron on colitis might be linked to, either a lower susceptibility to stimulate oxidative stress reactions as compared to ferrous iron, or to its interactions with the immune system which is partly driving an appropriate microbiota implantation during the perinatal period. Rather than working with animals whose microflora profile had been modified because they display a modified immune pattern[43,44], we decided to work on two rodent species of the same age, gender and submitted to a similar level of an inflammatory stimulus Since this microbiota/immune system interaction is said to be species, gender and age specific.
In conclusion, this study shows comparative rodent dysbiosis to human IBD dysbiosis following a moderate TNBS colitis. It also shows the benefits of ferric iron oral ingestion during the juvenile period in the prevention of an experimental colitis induced at adulthood in healthy animals. These interesting results would necessitate checking how anemic juvenile animals would react to such a treatment and especially to an induced colitis at adulthood. To further understand this incidence of ferric iron on overall intestinal functionalisation, one point we did not address is the role of the immune system and particularly its fine orientation modulation by iron fortification during the perinatal period in regards to the observed effects. We may hypothesize that iron could participate in reinforcing the immune system orientation, thus contributing to the limitation of the inflammatory response due to TNBS. This question will be addressed in another set of experiments. If these results are confirmed, this form of microencapsulated ferric iron could thus be clinically assessed as an interesting alternative to iron sulfate in young individuals at risk of anemia but also in subjects at risk of chronic gut inflammation such as CD.
The authors would like to thank Mr. David Marier for his technical help and Mr Laurent Depelley for kindly providing us with Lipofer®.
Juvenile iron supplementation is now widely accepted to limit the risk of anemia. However, oral iron ingestion may have side effects especially at this period when the crosstalk between bacteria, epithelium and the immune system is being set up. In fact, an impaired settlement of this crosstalk may predispose to chronic diseases such as inflammatory bowel disease (IBD). No one has so far evaluated the consequences of food matrix composition during the postnatal period on the predisposition to develop chronic diseases at adulthood. This study was thus aimed at evaluating the consequences of juvenile chronic iron exposure on the onset of experimental colitis and its related dysbiosis in both rats and mice at adulthood.
In these recent years, research on the role of commensal microbiota has evidenced its strong impact on digestive physiology and particularly its modulating role in IBD. Moreover, recent data evidenced that microbiota implantation in the perinatal period conditions, the maturation of the gut mucosa and the immune system renders the host tolerant to commensal bacteria. Since oral ferrous iron may promote potential pathogenic bacteria growth, which may condition the development of chronic gut inflammation, the authors evaluated in this study the modulation by repeated juvenile administration of ferric iron on the onset of an experimental colitis at adulthood in two rodent species.
Literature largely describes the necessity to maintain a good equilibrium of the microbiota to stay healthy. Food matrix also conditions the diversity and stability of the microbiota especially during the postnatal period crucial for these aspects. This study is the first to evidence the preventive effects of oral ferric iron administration at the juvenile period on the inflammatory response and dysbiosis related to an experimental colitis at adulthood in two rodent species.
This study proposes to evaluate the consequences of oral supplementation at infancy on risk of developing gut inflammation at adulthood. These results necessitate being completed by the evaluation of the consequences of ferric iron on the immune system maturation and to be clinically proven but they could represent a good opportunity for families at risk of developing IBD.
IBD comprises chronic inflammations of the gut and more especially Crohn’s Diseases and Ulcerative Colitis. Microbiota designates the pool of microorganisms harboured in our gut under physiological conditions. They divide into dominant and subdominant and control each other’s growth to reach dynamic equilibrium. Dysmicrobism is an alteration of the microbiota equilibrium. Iron is a metal. In aqueous solution, it exists in two oxidation states: ferrous (Fe2+) and ferric (Fe3+).
The study aimed to study rodent dysbiosis and compare it to human IBD dysbiosis following a moderate trinitrobenzene sulfonic acid colitis. It shows the benefits of ferric iron oral ingestion during the juvenile period in the prevention of an experimental colitis induced at adulthood. The paper is well written and has potential application in food supplemental.
Peer reviewers: Yuan-Ping Han, Assistant Professor of Surgery and Pathology, Keck School of Medicine, University of Southern California, Los Angeles, 91780, CA 90033, United States; Kostas Pantopoulos, Associate Professor, Department of Medicine, McGill University, Lady Davis Institute for Medical Research, 3755 Cote Ste-Catherine Road, Montreal, Quebec H3T 1E2, Canada; Dr. Giuseppe Chiarioni, Gastroenterological Rehabilitation Division of the University of Verona, Valeggio sul Mincio Hospital, Azienda Ospedale di Valeggio s/M, 37067 Valeggio s/M, Italy; Islam Khan, PhD, Professor, Departmenet of Biochemistry, Faculty of Medicine, Kuwait University, PO Box 24923, Safat 13110, Kuwait
S- Editor Lv S L- Editor A E- Editor Xiong L
| 1. | Ziegler EE, Nelson SE, Jeter JM. Iron supplementation of breastfed infants from an early age. Am J Clin Nutr. 2009;89:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Zimmermann MB, Chassard C, Rohner F, N'goran EK, Nindjin C, Dostal A, Utzinger J, Ghattas H, Lacroix C, Hurrell RF. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d'Ivoire. Am J Clin Nutr. 2010;92:1406-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 397] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 3. | Choi YJ, Im E, Chung HK, Pothoulakis C, Rhee SH. TRIF mediates Toll-like receptor 5-induced signaling in intestinal epithelial cells. J Biol Chem. 2010;285:37570-37578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2501] [Cited by in RCA: 2806] [Article Influence: 175.4] [Reference Citation Analysis (2)] |
| 5. | Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5700] [Cited by in RCA: 5676] [Article Influence: 270.3] [Reference Citation Analysis (3)] |
| 6. | Rajilić-Stojanović M, Smidt H, de Vos WM. Diversity of the human gastrointestinal tract microbiota revisited. Environ Microbiol. 2007;9:2125-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Cani PD, Delzenne NM, Amar J, Burcelin R. Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathol Biol (Paris). 2008;56:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Blaut M, Collins MD, Welling GW, Doré J, van Loo J, de Vos W. Molecular biological methods for studying the gut microbiota: the EU human gut flora project. Br J Nutr. 2002;87 Suppl 2:S203-S211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, Jian R, Doré J. Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut. 2003;52:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Rehman A, Lepage P, Nolte A, Hellmig S, Schreiber S, Ott SJ. Transcriptional activity of the dominant gut mucosal microbiota in chronic inflammatory bowel disease patients. J Med Microbiol. 2010;59:1114-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Willing B, Halfvarson J, Dicksved J, Rosenquist M, Järnerot G, Engstrand L, Tysk C, Jansson JK. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn's disease. Inflamm Bowel Dis. 2009;15:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 369] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 12. | Scarpa M, Grillo A, Faggian D, Ruffolo C, Bonello E, D'Incà R, Scarpa M, Castagliuolo I, Angriman I. Relationship between mucosa-associated microbiota and inflammatory parameters in the ileal pouch after restorative proctocolectomy for ulcerative colitis. Surgery. 2011;150:56-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1633] [Cited by in RCA: 1701] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 14. | Conte MP, Schippa S, Zamboni I, Penta M, Chiarini F, Seganti L, Osborn J, Falconieri P, Borrelli O, Cucchiara S. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut. 2006;55:1760-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 280] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 15. | Jurjus AR, Khoury NN, Reimund JM. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods. 2004;50:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 202] [Article Influence: 9.2] [Reference Citation Analysis (2)] |
| 16. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. [PubMed] |
| 17. | Wallace JL, Braquet P, Ibbotson GC, MacNaughton WK, Cirino G. Assessment of the role of platelet-activating factor in an animal model of inflammatory bowel disease. J Lipid Mediat. 1989;1:13-23. [PubMed] |
| 18. | Bradley PP, Christensen RD, Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982;60:618-622. [PubMed] |
| 19. | Vasquez N, Suau A, Magne F, Pochart P, Pélissier MA. Differential effects of Bifidobacterium pseudolongum strain Patronus and metronidazole in the rat gut. Appl Environ Microbiol. 2009;75:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Whelan JA, Russell NB, Whelan MA. A method for the absolute quantification of cDNA using real-time PCR. J Immunol Methods. 2003;278:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 551] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 21. | Watanabe K, Kodama Y, Harayama S. Design and evaluation of PCR primers to amplify bacterial 16S ribosomal DNA fragments used for community fingerprinting. J Microbiol Methods. 2001;44:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 211] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology. 2002;148:257-266. [PubMed] |
| 23. | Firmesse O, Mogenet A, Bresson JL, Corthier G, Furet JP. Lactobacillus rhamnosus R11 consumed in a food supplement survived human digestive transit without modifying microbiota equilibrium as assessed by real-time polymerase chain reaction. J Mol Microbiol Biotechnol. 2008;14:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Ibekwe AM, Grieve CM. Detection and quantification of Escherichia coli O157: H7 in environmental samples by real-time PCR. J Appl Microbiol. 2003;94:421-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Blanco-Rojo R, Pérez-Granados AM, Toxqui L, González-Vizcayno C, Delgado MA, Vaquero MP. Efficacy of a microencapsulated iron pyrophosphate-fortified fruit juice: a randomised, double-blind, placebo-controlled study in Spanish iron-deficient women. Br J Nutr. 2011;105:1652-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Fidler MC, Walczyk T, Davidsson L, Zeder C, Sakaguchi N, Juneja LR, Hurrell RF. A micronised, dispersible ferric pyrophosphate with high relative bioavailability in man. Br J Nutr. 2004;91:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Buffinton GD, Doe WF. Depleted mucosal antioxidant defences in inflammatory bowel disease. Free Radic Biol Med. 1995;19:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 179] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Perl DP, Fogarty U, Harpaz N, Sachar DB. Bacterial-metal interactions: the potential role of aluminum and other trace elements in the etiology of Crohn's disease. Inflamm Bowel Dis. 2004;10:881-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn's disease. Aliment Pharmacol Ther. 2006;24:1507-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Werner T, Wagner SJ, Martínez I, Walter J, Chang JS, Clavel T, Kisling S, Schuemann K, Haller D. Depletion of luminal iron alters the gut microbiota and prevents Crohn's disease-like ileitis. Gut. 2011;60:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 240] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 31. | Punkkinen J, Konkka I, Punkkinen O, Korppi-Tommola T, Färkkilä M, Koskenpato J. Measuring gastric emptying: comparison of 13C-octanoic acid breath test and scintigraphy. Dig Dis Sci. 2006;51:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 846] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 33. | Qiu W, Wu B, Wang X, Buchanan ME, Regueiro MD, Hartman DJ, Schoen RE, Yu J, Zhang L. PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. J Clin Invest. 2011;121:1722-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Doré J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183-1189. [PubMed] |
| 35. | Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J, Corthier G, Furet JP. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. [PubMed] |
| 36. | Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, Weber J, Hoffmann U, Schreiber S, Dietel M. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122:44-54. [PubMed] |
| 37. | Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731-16736. [PubMed] |
| 38. | Tlaskalová-Hogenová H, Stepánková R, Hudcovic T, Tucková L, Cukrowska B, Lodinová-Zádníková R, Kozáková H, Rossmann P, Bártová J, Sokol D. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett. 2004;93:97-108. [PubMed] |
| 39. | Stelter C, Käppeli R, König C, Krah A, Hardt WD, Stecher B, Bumann D. Salmonella-induced mucosal lectin RegIIIβ kills competing gut microbiota. PLoS One. 2011;6:e20749. [PubMed] |
| 40. | Curová K, Kmetová M, Sabol M, Gombosová L, Lazúrová I, Siegfried L. Enterovirulent E. coli in inflammatory and noninflammatory bowel diseases. Folia Microbiol (Praha). 2009;54:81-86. [PubMed] |
| 41. | Thomazini CM, Samegima DA, Rodrigues MA, Victoria CR, Rodrigues J. High prevalence of aggregative adherent Escherichia coli strains in the mucosa-associated microbiota of patients with inflammatory bowel diseases. Int J Med Microbiol. 2011;301:475-479. [PubMed] |
| 42. | Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514-521. [PubMed] |
| 43. | Tlaskalova-Hogenova H, Sterzl J, Stepankova R, Dlabac V, Veticka V, Rossmann P, Mandel L, Rejnek J. Development of immunological capacity under germfree and conventional conditions. Ann N Y Acad Sci. 1983;409:96-113. [PubMed] |
| 44. | Tlaskalová-Hogenová H, Stĕpánková R, Farré M, Funda DP, Reháková Z, Sinkora J, Tucková L, Horak I, Horáková D, Cukrowska B. Autoimmune reactions induced by gliadin feeding in germ-free AVN rats and athymic nude mice. Animal models for celiac disease. Ann N Y Acad Sci. 1997;815:503-505. [PubMed] |