Published online Jun 7, 2012. doi: 10.3748/wjg.v18.i21.2597
Revised: May 18, 2012
Accepted: May 23, 2012
Published online: June 7, 2012
Motor vehicle accidents (MVAs) are serious social issues worldwide and driver illness is an important cause of MVAs. Minimal hepatic encephalopathy (MHE) is a complex cognitive dysfunction with attention deficit, which frequently occurs in cirrhotic patients independent of severity of liver disease. Although MHE is known as a risk factor for MVAs, the impact of diagnosis and treatment of MHE on MVA-related societal costs is largely unknown. Recently, Bajaj et al demonstrated valuable findings that the diagnosis of MHE by rapid screening using the inhibitory control test (ICT), and subsequent treatment with lactulose could substantially reduce the societal costs by preventing MVAs. Besides the ICT and lactulose, there are various diagnostic tools and therapeutic strategies for MHE. In this commentary, we discussed a current issue of diagnostic tools for MHE, including neuropsychological tests. We also discussed the advantages of the other therapeutic strategies for MHE, such as intake of a regular breakfast and coffee, and supplementation with zinc and branched chain amino acids, on the MVA-related societal costs.
- Citation: Kawaguchi T, Taniguchi E, Sata M. Motor vehicle accidents: How should cirrhotic patients be managed? World J Gastroenterol 2012; 18(21): 2597-2599
- URL: https://www.wjgnet.com/1007-9327/full/v18/i21/2597.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i21.2597
We have read with great interest the recent article by Bajaj et al[1] describing the diagnosis and treatment of minimal hepatic encephalopathy (MHE) to prevent motor vehicle accidents (MVAs), and would strongly recommend it to the readers.
MVAs are serious social issues worldwide[2]. Various factors are intricately involved in the occurrence of MVAs, and driver illness is an important cause[2]. Besides acute myocardial infarction, epileptic seizure, and hypoglycemia related to the use of anti-diabetic agents, liver cirrhosis with MHE has been reported to increase the risk of MVAs[3-6]. Although MHE occurs in up to 80% of patients with chronic liver disease[3] and diagnostic tools and therapeutic strategies for MHE exist[7-11], little information is available about the management of patients with liver cirrhosis with regard to preventing MVAs and subsequently reducing the associated societal costs.
In their study, Bajaj et al[1] performed a cost-effectiveness analysis to identify management strategies for the diagnosis and treatment of MHE in patients with liver cirrhosis to reduce MVA-related societal costs. They found that the diagnosis of MHE by rapid screening using the inhibitory control test (ICT), and subsequent treatment with lactulose could substantially reduce societal costs by preventing MVAs[1]. This is a significant study, and we agree with the authors about the benefits of the use of the diagnostic test and therapeutic management. However, we suggest that the management strategy should be modified to some extent for use in general medical institutions to prevent MVAs on a larger scale.
The ICT is a computerized test of attention and response inhibition that has been used to characterize attention deficit disorders[12]. The ICT consists of the presentation of several letters at 0.5 s intervals, while the subject is instructed to respond or inhibit their response to the specific letter[13]. The ICT is considered reliable and sensitive for the diagnosis of MHE[5,13]. In addition, unlike standard neuropsychological tests, ICT results are significantly associated with the future occurrence of MVAs[3]. However, the test takes approximately 30 min to complete and patients need to be familiar with computer operation. Furthermore, validation and standardization are required for each population, and therefore, so far, the ICT is not universally accessible. Similarly, other diagnostic tools for MHE also require trained personnel and specialized equipment[11]. In fact, an American Association for the Study of Liver Disease (AASLD) survey showed that the majority of AASLD members are not able to test for MHE because of a lack of time, resources, and suitable personnel[14]. Along with Bajaj et al[14], we propose that rapid and simple tools for the diagnosis MHE should be developed urgently, such as biochemical tests or virtual reality driving simulations.
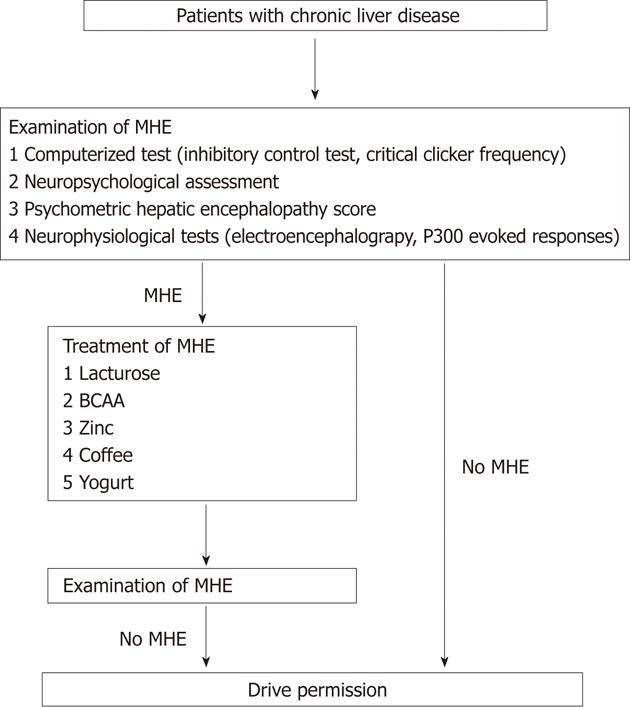
Lactulose has been used to treat hepatic encephalopathy since 1966[15]. Lactulose reduces blood ammonia levels and improves overt hepatic encephalopathy as well as MHE[7]. In their study, Bajaj et al[1] have demonstrated the benefits of lactulose therapy on the occurrence of MVAs and MVA-related societal costs. However, compliance with lactulose treatment is generally poor, primarily because of its side-effects such as abdominal discomfort[16,17]. Recently, other therapeutic strategies for MHE have been reported. First, as prolonged periods of fasting are linked to the development of MHE, having a regular breakfast improves the attention and executive functions of cirrhotic patients with MHE[18]. Second, coffee intake improves cognitive function in elderly people as well as in patients with type 2 diabetes mellitus[19,20]. Although the beneficial effects of coffee on cognitive function have never been investigated in cirrhotic patients, insulin resistance is frequently seen in patients with chronic liver disease[21-23]. In addition, coffee consumption is known to improve hepatic inflammation and fibrosis in patients with chronic liver disease[24]. Third, the blood ammonia level is regulated by the activity of ornithine transcarbamoylase and zinc is a coenzyme required for its up-regulation[25]. Oral zinc supplementation improves hyperammonemia as well as hepatic encephalopathy, as seen in a double-blind randomized controlled trial[26]. Finally, a decrease in serum branched chain amino acids (BCAA) levels is a feature of chronic liver disease[27]. BCAA is a source of glutamate, which detoxifies ammonia by glutamine synthesis in the skeletal muscle and brain[28]. Therefore, BCAA enhances the detoxification of blood ammonia by incorporating ammonia in the process of glutamine production and is currently used for treating patients with hepatic encephalopathy[29]. Thus, a therapeutic approach comprising the intake of a regular breakfast and coffee, and supplementation with zinc and BCAA may improve the cost-effectiveness of MVA-related events in cirrhotic patients with MHE (Figure 1).
Prevention of MVA by the diagnosis and treatment of MHE is an important component in the management of patients with liver cirrhosis. Collaborative researches among medical institutions, automobile companies, and governmental sectors may help further prevent MVAs and subsequently reduce MVA-related societal costs.
Peer reviewers: Ferruccio Bonino, MD, PhD, Professor of Gastroenterology, Director of Liver and Digestive Disease Division, Director of General Medicine 2 Unit, Department of Internal Medicine, University Hospital of Pisa, Via Roma 67, 56124 Pisa, Italy; Andrzej S Tarnawski, MD, PhD, DSc (Med), Professor of Medicine, Chief Gastroenterology, VA Long Beach Health Care System, University of California, Irvine, CA, 5901 E. Seventh Str., Long Beach, CA 90822, United States
S- Editor Cheng JX L- Editor Ma JY E- Editor Xiong L
| 1. | Bajaj JS, Pinkerton SD, Sanyal AJ, Heuman DM. Diagnosis and treatment of minimal hepatic encephalopathy to prevent motor vehicle accidents: a cost-effectiveness analysis. Hepatology. 2012;55:1164-1171. [PubMed] |
| 2. | Huebner WW, Wojcik NC, Jorgensen G, Marcella SP, Nicolich MJ. Mortality patterns and trends among 49,705 U.S.-based women in a petroleum company: update 1979-2000. J Occup Environ Med. 2010;52:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Bajaj JS, Saeian K, Schubert CM, Hafeezullah M, Franco J, Varma RR, Gibson DP, Hoffmann RG, Stravitz RT, Heuman DM. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology. 2009;50:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 241] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 4. | Bajaj JS. Minimal hepatic encephalopathy matters in daily life. World J Gastroenterol. 2008;14:3609-3615. [PubMed] |
| 5. | Bajaj JS, Hafeezullah M, Hoffmann RG, Saeian K. Minimal hepatic encephalopathy: a vehicle for accidents and traffic violations. Am J Gastroenterol. 2007;102:1903-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Wein C, Koch H, Popp B, Oehler G, Schauder P. Minimal hepatic encephalopathy impairs fitness to drive. Hepatology. 2004;39:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 212] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Watanabe A, Sakai T, Sato S, Imai F, Ohto M, Arakawa Y, Toda G, Kobayashi K, Muto Y, Tsujii T. Clinical efficacy of lactulose in cirrhotic patients with and without subclinical hepatic encephalopathy. Hepatology. 1997;26:1410-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Kato A, Suzuki K, Kaneta H, Obara H, Fujishima Y, Sato S. Regional differences in cerebral glucose metabolism in cirrhotic patients with subclinical hepatic encephalopathy using positron emission tomography. Hepatol Res. 2000;17:237-245. [PubMed] |
| 9. | Kato A, Kato M, Ishii H, Ichimiya Y, Suzuki K, Kawasaki H, Yamamoto SI, Kumashiro R, Yamamoto K, Kawamura N. Development of quantitative neuropsychological tests for diagnosis of subclinical hepatic encephalopathy in liver cirrhosis patients and establishment of diagnostic criteria-multicenter collaborative study in Japanese. Hepatol Res. 2004;30:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Sugimoto R, Iwasa M, Maeda M, Urawa N, Tanaka H, Fujita N, Kobayashi Y, Takeda K, Kaito M, Takei Y. Value of the apparent diffusion coefficient for quantification of low-grade hepatic encephalopathy. Am J Gastroenterol. 2008;103:1413-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Dhiman RK, Saraswat VA, Sharma BK, Sarin SK, Chawla YK, Butterworth R, Duseja A, Aggarwal R, Amarapurkar D, Sharma P. Minimal hepatic encephalopathy: consensus statement of a working party of the Indian National Association for Study of the Liver. J Gastroenterol Hepatol. 2010;25:1029-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Crosbie J, Pérusse D, Barr CL, Schachar RJ. Validating psychiatric endophenotypes: inhibitory control and attention deficit hyperactivity disorder. Neurosci Biobehav Rev. 2008;32:40-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Bajaj JS, Hafeezullah M, Franco J, Varma RR, Hoffmann RG, Knox JF, Hischke D, Hammeke TA, Pinkerton SD, Saeian K. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology. 2008;135:1591-1600.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Bajaj JS, Etemadian A, Hafeezullah M, Saeian K. Testing for minimal hepatic encephalopathy in the United States: An AASLD survey. Hepatology. 2007;45:833-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Bircher J, Müller J, Guggenheim P, Haemmerli UP. Treatment of chronic portal-systemic encephalopathy with lactulose. Lancet. 1966;1:890-892. [PubMed] |
| 16. | Kalaitzakis E, Björnsson E. Lactulose treatment for hepatic encephalopathy, gastrointestinal symptoms, and health-related quality of life. Hepatology. 2007;46:949-50; author reply 951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Horsmans Y, Solbreux PM, Daenens C, Desager JP, Geubel AP. Lactulose improves psychometric testing in cirrhotic patients with subclinical encephalopathy. Aliment Pharmacol Ther. 1997;11:165-170. [PubMed] |
| 18. | Vaisman N, Katzman H, Carmiel-Haggai M, Lusthaus M, Niv E. Breakfast improves cognitive function in cirrhotic patients with cognitive impairment. Am J Clin Nutr. 2010;92:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Cropley V, Croft R, Silber B, Neale C, Scholey A, Stough C, Schmitt J. Does coffee enriched with chlorogenic acids improve mood and cognition after acute administration in healthy elderly? A pilot study. Psychopharmacology (Berl). 2012;219:737-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Biessels GJ. Caffeine, diabetes, cognition, and dementia. J Alzheimers Dis. 2010;20 Suppl 1:S143-S150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 432] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 22. | Kawaguchi T, Ide T, Taniguchi E, Hirano E, Itou M, Sumie S, Nagao Y, Yanagimoto C, Hanada S, Koga H. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Eslam M, Aparcero R, Kawaguchi T, Del Campo JA, Sata M, Khattab MA, Romero-Gomez M. Meta-analysis: insulin resistance and sustained virological response in hepatitis C. Aliment Pharmacol Ther. 2011;34:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Kawaguchi T, Sata M. Importance of hepatitis C virus-associated insulin resistance: therapeutic strategies for insulin sensitization. World J Gastroenterol. 2010;16:1943-1952. [PubMed] |
| 25. | Reding P, Duchateau J, Bataille C. Oral zinc supplementation improves hepatic encephalopathy. Results of a randomised controlled trial. Lancet. 1984;2:493-495. [PubMed] |
| 26. | Katayama K. Ammonia metabolism and hepatic encephalopathy. Hepatol Res. 2004;30S:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Reding P, Duchateau J, Bataille C. Oral zinc supplementation improves hepatic encephalopathy. Results of a randomised controlled trial. Lancet. 1984;2:493-495. [PubMed] |
| 28. | Platell C, Kong SE, McCauley R, Hall JC. Branched-chain amino acids. J Gastroenterol Hepatol. 2000;15:706-717. [PubMed] |
| 29. | Moriwaki H, Shiraki M, Fukushima H, Shimizu M, Iwasa J, Naiki T, Nagaki M. Long-term outcome of branched-chain amino acid treatment in patients with liver cirrhosis. Hepatol Res. 2008;38:S102-S106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |