Published online May 28, 2012. doi: 10.3748/wjg.v18.i20.2569
Revised: September 27, 2011
Accepted: October 27, 2011
Published online: May 28, 2012
AIM: To investigate the role of expressions of Ki-67, p53, epidermal growth factor receptor (EGFR) and cyclooxygenase-2 (COX-2) in gastrointestinal stromal tumor (GIST) grading and prognosis.
METHODS: Tumor tissue was collected retrospectively from 96 patients with GIST. Antibodies against Ki-67, p53, EGFR and COX-2 were used for immunohistochemical staining. Tumor grading was designated according to a consensus system and the staining was quantified in 3 categories for each antibody in the statistical analysis.
RESULTS: The Ki-67 expression in GISTs was significantly associated with the size of the tumors, mitotic rate and the risk of malignancy (χ2 = 15.51, P = 0.02; χ2 = 22.27, P < 0.001; χ2 = 20.05; P < 0.001). The p53 expression was also significantly correlated with mitotic rate and the risk of malignancy (χ2 = 9.92, P = 0.04; χ2 = 9.97; P = 0.04). Over-expression of Ki-67 was strongly correlated with poor survival (χ2 = 10.44, P = 0.006), but no correlation was found between the expression of p53, EGFR or COX-2 and survival. Multivariate analysis further demonstrated that Ki-67 expression (relative risk = 15.78, 95% CI: 4.25-59.37) could be used as an independent prognostic value for GIST patients. Adjuvant imatinib therapy could improve clinical outcomes in the patients with high risk and intermediate risk of recurrence after complete tumor resections (median survival time: 52 mo vs 37 mo, χ2 = 7.618, P = 0.006).
CONCLUSION: Our results indicated that the expression of Ki-67 could be used as an independent prognostic factor for GIST patients.
- Citation: Jiang J, Jin MS, Suo J, Wang YP, He L, Cao XY. Evaluation of malignancy using Ki-67, p53, EGFR and COX-2 expressions in gastrointestinal stromal tumors. World J Gastroenterol 2012; 18(20): 2569-2575
- URL: https://www.wjgnet.com/1007-9327/full/v18/i20/2569.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i20.2569
Gastrointestinal stromal tumor (GIST) is one of the most frequent mesenchymal neoplasms of the gastrointestinal tract. In the elderly, micro-GIST (the tumor size smaller than 1 cm) is detected in 20%-30% of individuals over 60 years old[1,2]. GIST occurs along the gastrointestinal tract and commonly invades in the stomach and small intestine. The tumors rarely arise from extragastrointestinal sites, such as omentum or mesentery[3]. Most GISTs express c-kit. Monoclonal antibodies against c-kit, DOG1 and protein kinase C theta have been developed as helpful diagnostic adjuncts in pathology[4-6].
GISTs have a wide clinical spectrum, ranging from virtually benign to highly aggressive tumors. Up to 30% of GISTs recur and progress to metastatic disease even after the complete excision of tumors. Despite a remarkable progress in the understanding of GISTs, it is still difficult to make a prognosis due to the variability of disease[7]. According to the National Institutes of Health (NIH) classification system, GISTs are classified into four categories: very low, low, intermediate and high risk[8]. The prognosis of patients is commonly stratified based on tumor size and mitotic counts in the NIH system. Previous studies have demonstrated that nuclear atypia and tumor necrosis all contribute to prognostic outcomes of GIST patients. Further, some studies showed that gastric GISTs had lower risks of recurrence than nongastric tumors with the same size and same mitotic count[9]. The four-point classification only distinguishes GISTs with high-risk from those with low-risk[10]. The system using multiple histopathological parameters for GIST prognosis is subjective and lacks reproducibility[11].
The proliferation marker Ki-67, tumor suppressor gene p53, cyclooxygenase-2 (COX-2) and epidermal growth factor receptor (EGFR) have been identified as prognostic biomarkers in tumors of epithelial origin. However, there has been no study analyzing these markers systematically in a large cohort of mesenchymal tumors, especially in GISTs[12,13]. In this study, Ki-67, p53, EGFR and COX-2 expressions were fully investigated in the GIST tumor specimens from 96 patients and the grade of the tumor was established based on the immunohistochemical staining of each protein. The grades were then compared with patients’ clinical features and roles of prognostic values for GISTs were evaluated. The study indicated that determination of these tumorigenetic and cell proliferative proteins provides alternative measurements for follow-up and prognosis.
From January 2005 through December 2009, 134 patients were initially diagnosed as having mesenchymal gastrointestinal tumors at Jilin University First Hospital. Thirty-three patients were excluded from the study due to recurred tumors or the tumors being partially resected. Thus, 101 patients underwent successful surgical operations for complete resection of tumors. Following the surgery, patients with high-risk and intermediate risk were treated with imatinib (Glivec®, Novartis Pharmaceuticals, Basel, Switzerland) at a dose of 400 mg/d for 3 years. No imatinib treatment was given before the surgery. Five cases were lost to follow-up. Ninety-six patients were retrospectively evaluated in the study. Informed consents were obtained from all patients and the study was approved by the local human ethical committee of Jilin University First Hospital. Original hematoxylin and eosin-stained sections were reviewed in each case by two pathologists (Jin MS and Wang YP) according to GIST characteristics described by Miettinen[14]. All tumors from 96 patients were confirmed to be GISTs based on a combination of histological evaluations (highly cellular spindles/epithelioids/mixed cell tumors), and c-kit, DOG1, CD34 positive staining. The clinical information regarding the patients is summarized in Table 1.
| n | % of 96 GISTs | |
| Age (yr, median age = 55 yr) | ||
| < 40 | 12 | 12.5 |
| 40-60 | 52 | 54.2 |
| > 60 | 32 | 33.3 |
| Sex | ||
| Male | 57 | 59.4 |
| Female | 39 | 40.6 |
| Site | ||
| Esophagus | 3 | 3.1 |
| Gastric | 45 | 46.9 |
| Intestine | 37 | 38.5 |
| EGIST | 11 | 11.5 |
| Tumor size (cm, median size = 7.0) | ||
| ≤ 2 | 7 | 7.3 |
| > 2 to ≤ 5 | 29 | 30.2 |
| > 5 to ≤ 10 | 34 | 35.4 |
| > 10 | 26 | 27.1 |
| Mitotic rate (per 50 HPFs) | ||
| ≤ 5 | 54 | 56.3 |
| 6 to 10 | 28 | 29.2 |
| > 10 | 14 | 14.6 |
| Risk of malignancy | ||
| High risk | 45 | 46.9 |
| Intermediate risk | 24 | 25.0 |
| Low risk | 24 | 25.0 |
| Very low risk | 3 | 3.1 |
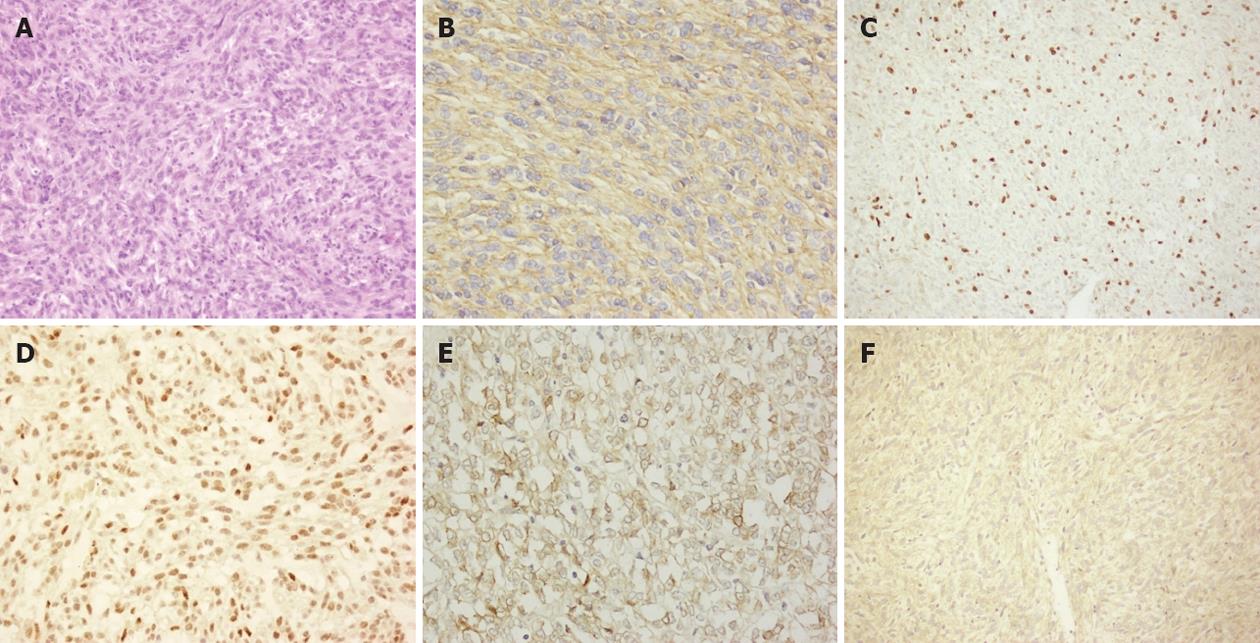
Histological sections (4 μm) of 10% formalin-fixed, paraffin-embedded material were used for immunohistochemical staining. Prior to a primary antibody staining, the slide was pretreated with citric acid or ethylenediaminetetraacetic acid buffer in a pressure cooker for antigen retrieval. Endogenous peroxidase activity was quenched by 3% H2O2 blocking reagent for 10 min. The slide was incubated with a primary antibody at 4 °C overnight, and then immunostained with the avidin-biotin peroxidase complex (DAKO, CA). Finally, the slide was stained with diaminobenzidine according to the manufacturer’s protocol (DAKO, CA). The slide was rinsed three times with phosphate buffered saline after each step of staining. The sections were stained with primary antibodies against c-kit (Clone: YR145, dilution: 1/50, Cell Marque Corporation, CA), CD34 (QBEnd/10, dilution: 1/100, Neomarkers, CA), Ki-67 (MIB-1, dilution: 1/100, DAKO, Carpinteria, CA), DOG1 (SP31, dilution: 1/100, Spring Bioscience, Pleasanton), SMA (IA4, dilution: 1/200, Cell Marque Corporation, CA), p27 (1B4, dilution: 1/20, Novocastra), p53 (SP5, dilution: 1/100, Zymed Laboratories, San Francisco), S-100 (6E6, dilution: 1/100, Neomarkers, CA), Desmin (D33, dilution:1/50, Cell Marque Corporation, CA), EGFR (EGFR.113, dilution: 1/200, Novocastra Laboratories Ltd, Newcastle, United Kingdom), and COX-2 (SP21, dilution: 1/50, Neomarkers, CA, United States), respectively. All primary antibodies used in the study were biotinylated monoclonal antibodies. The stained slides were evaluated quantitatively or semi-quantitatively by two independent pathologists who were blinded from clinical data. Percentages of positive cells stained with a special antibody observed by two pathologists were consistent and the mean values were determined.
The nuclear staining for Ki-67 and p53, and cytoplasmic immunostaining for EGFR and COX-2, were considered as positive cells of the reaction. According to previous studies[15,16], the following scoring assessments for Ki-67 and p53 were used. The score 0 was assigned for < 5%, 1 for > 5% and < 10%, 2 for > 10% of Ki-67 staining positive cells. The p53 scoring system was 0 assigned for < 5%, 1 for > 5% and < 25%, 2 for > 25% of p53 staining positive cells. EGFR scoring system was 0 assigned for < 10%, 1 for > 10% and < 60%, 2 for > 60% of EGFR positive cells based on the systems described by Nakagawa et al[17] and Gumurdulu et al[18]. The COX-2 scoring system was 0 assigned for no positive cells; 1 for < 25% and score 2 for > 25% of COX-2 staining positive cells according to Fux et al[19].
The Chi-square test and Fisher’s exact test were used to analyze relationships between clinicopathological features and expression levels of biomarkers. Kaplan-Meier test was applied with a log-rank test to study associations between categorical variables and the mean values of survival among groups. Cox proportional hazards regression analysis was used to estimate a hazard risk for survival and 95% CI was applied. The SPSS program (version 18.0) was used for statistical analysis. A P value of < 0.05 was considered statistically significant.
Clinicopathological features of the patients are summarized in Table 1. The median age of 96 patients was 55 years (range, 26-82 years). Histomorphology showed that the neoplastic cells were predominantly spindle-shaped (83/96, 86.5%). Based on the modified NIH risk consensus system, 45 (46.9%), 24 (25.0%), 24 (25.0%) and 3 (3.1%) cases were classified as high-risk, intermediate-risk, low risk and very low risk categories, respectively. Fifty-three cases (55.2%) had mild nuclear atypia; 32 cases (33.4%) showed severe nuclear atypia, but 11 patients (11.4%) had no nuclear atypia. Tumor necrosis was found in 39 cases of the patients (40.6%).
At the time of study, the mean or the median duration of the follow-up period was 31 mo or 29 mo, respectively. Medical charts were available for 96 of 101 patients (95%). Sixty-nine patients (54.2%) received the imatinib treatment at a dose of 400 mg/d for 13 mo to 36 mo (median, 26 mo). Thirty-seven patients (82%) from the high risk group and 15 patients (62.5%) from the intermediate group required the imatinib treatment. Disease specific 1, 2, 3 and 4 year survival probabilities were 0.97, 0.89, 0.79, and 0.77 (0.65-0.87), respectively. Of the 96 cases, 19 patients (19.8%) died from GISTs and 6 patients (6.3%) died from unrelated causes.
Eighty-eight (91.3%) tumor specimens were stained positive for c-kit. The tumors isolated from 8 patients (8.7%) were negative for c-kit but positive for DOG1 and/or CD34 staining. Reactivity with Desmin was found in 3 (3.1%) cases. Positive SMA and S-100 staining were also noted in 46 (47.9%) and 12 (12.5%) cases, respectively. Based on the Ki-67 index, 53.1% of tumors (n = 51) scored 0; 34.4% (n = 33) scored 1; and 12.5% (n = 12) scored 2. 34.4% of tumor specimens (n = 33) were p53 staining positive in the nuclei of over 25% of the cells. EGFR staining was found in most cases. Forty-two (43.8%) cases scored 2, and 27 (28.1%) cases scored 1 for EGFR staining. COX-2 overexpressed in 36 (37.5%) cases (Table 2 and Figure 1).
| Variable | Ki-67 | P53 | EGFR | COX-2 | ||||||||||||
| 0+ | 1+ | 2+ | P value | 0+ | 1+ | 2+ | P value | 0+ | 1+ | 2+ | P value | 0+ | 1+ | 2+ | P value | |
| Tumor size (cm) | ||||||||||||||||
| ≤ 2 | 6 | 1 | 0 | 0.02 | 3 | 3 | 1 | 0.47 | 1 | 3 | 3 | 0.41 | 2 | 2 | 3 | 0.07 |
| > 2 to ≤ 5 | 21 | 7 | 1 | 8 | 14 | 7 | 6 | 12 | 11 | 17 | 7 | 5 | ||||
| > 5 to ≤ 10 | 15 | 15 | 4 | 9 | 10 | 15 | 11 | 8 | 15 | 20 | 9 | 5 | ||||
| > 10 | 9 | 10 | 7 | 5 | 11 | 10 | 9 | 4 | 13 | 21 | 1 | 4 | ||||
| Total | 51 | 33 | 12 | 25 | 38 | 33 | 27 | 27 | 42 | 60 | 19 | 17 | ||||
| Mitotic rate (per 50 HPFs) | ||||||||||||||||
| ≤ 5 | 39 | 12 | 3 | < 0.001 | 17 | 20 | 17 | 0.04 | 16 | 16 | 22 | 0.77 | 38 | 8 | 8 | 0.41 |
| > 5 to ≤ 10 | 10 | 14 | 4 | 8 | 13 | 7 | 7 | 6 | 15 | 14 | 8 | 6 | ||||
| > 10 | 2 | 7 | 5 | 0 | 5 | 9 | 4 | 5 | 5 | 8 | 3 | 3 | ||||
| Risk of malignancy | ||||||||||||||||
| Very low + low risk | 23 | 4 | 0 | < 0.001 | 9 | 11 | 7 | 0.04 | 6 | 11 | 10 | 0.56 | 17 | 6 | 4 | 0.50 |
| Intermediate risk | 13 | 9 | 2 | 9 | 11 | 4 | 7 | 5 | 12 | 12 | 5 | 7 | ||||
| High risk | 15 | 20 | 10 | 7 | 16 | 22 | 14 | 11 | 20 | 31 | 8 | 6 | ||||
Clinicopathological features and GIST grades categorized by the staining of Ki-67, p53, EGFR or COX-2 are established in Table 3. The expression of Ki-67 was significantly associated with tumor size (P = 0.02), mitotic rate (P < 0.001) and the risk of malignancy (P < 0.001). The p53 expression was also correlated with mitotic rate (P = 0.04), tumor site (P = 0.02) and the risk of malignancy (P = 0.04). The levels of COX-2 protein were significantly higher in gastric tumors and spindle cell-like tumors (P < 0.001 and P = 0.05, respectively). In contrast, no correlation was found between the EGFR expression and clinicopathological factors or the risk of malignancy.
| Variable | RR (95% CI) | P value |
| Risk of malignancy | ||
| Very low/low risk | 1.00 (reference) | < 0.001 |
| Intermediate risk | 1.32 (0.81-21.2) | |
| High risk | 12.23 (1.61-92.81) | |
| Ki-67 expression | ||
| 0+ | 1.00 (reference) | < 0.001 |
| 1+ | 3.75 (0.97-14.54) | |
| 2+ | 15.78 (4.25-59.37) | |
| P53 expression | ||
| 0+ | 1.00 (reference) | 0.20 |
| 1+ | 2.11 (0.24-18.37) | |
| 2+ | 4.49 (0.54-37.11) | |
| EGFR expression | ||
| 0+ | 1.00 (reference) | 0.50 |
| 1+ | 1.97 (0.60-6.50) | |
| 2+ | 1.17 (0.36-3.77) | |
| COX-2 expression | ||
| 0+ | 1.00 (reference) | 0.19 |
| 1+ | 0.84 (0.42-4.36) | |
| 2+ | 1.99 (0.55-7.21) |
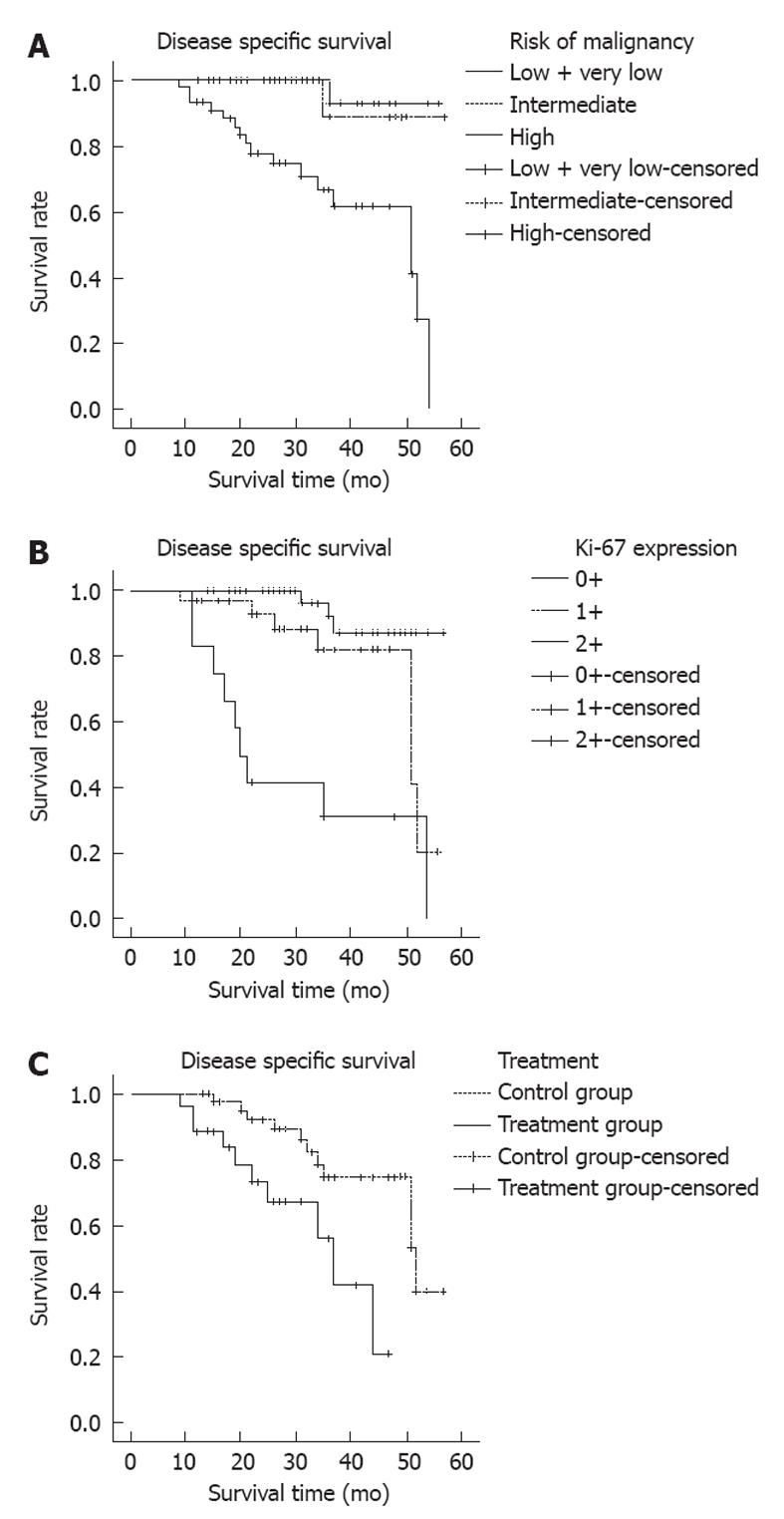
The 3-year survival rates for disease specific survival (DSS) were 100%, 89%, 79% and 67% for groups at very low-risk, low-risk, intermediate-risk and high risk by the modified NIH risk categories, respectively. Associations between DSS and different protein biomarkers were analyzed using a multivariate analysis (Table 3 and Figure 2). The survival rates were strongly associated with tumor size (P = 0.004), mitotic count (P = 0.001), tumor location (P = 0.018), the NIH modified risk criteria (P < 0.001, Figure 2A), Ki-67 amplification (P < 0.001, Figure 2B), and adjuvant imatinib therapy (median survival period 52 mo vs 37 mo, χ2 = 7.618, P = 0.006, Figure 2C). No significance was found when comparing survival rates with the EGFR expression, or the COX-2 expression. In the high-risk group, Ki-67 overexpression was significantly associated with poor survival (χ2 = 10.44, P = 0.006), but no statistical significance was found between the p53 expression and survival (χ2 = 4.744, P = 0.089). Using a multivariate analysis, a poor survival was observed in the high risk category [relative risk (RR) = 12.23; 95% CI: 1.61-92.81] graded using the modified NIH risk consensus system or in category 2 scored by the Ki-67 expression (RR = 15.78; 95% CI: 4.25-59.37) (Table 3).
In the absence of reliable genetic and immunohistochemical biomarkers in GISTs, the tumor size and mitotic rate are often used to assess risk probabilities in GIST patients. Large retrospective cohort studies have shown that the NIH classification carries substantial prognostic value[20]. Using classical morphological parameters, our results were consistent with previous studies on the prognosis of GIST patients.
Ki-67, a nuclear protein associated with cell proliferation, expresses in all cell cycle phases except for G0. A recent study demonstrated that the automated assessments of Ki-67 staining with computing image analysis can be used for prognostic assessments of patients with breast cancer[21]. However, the prognostic value of Ki-67 as a potential biomarker has not been fully investigated in GISTs[22,23]. The present study shows that the expression of Ki-67 or p53 is significantly associated with many clinicopathological features in GISTs; higher score for Ki-67 staining was directly correlated with poor survival; Ki-67 was superior to other protein markers tested in survival assessments, particularly in the high risk group, suggesting that Ki-67 immunostaining is a reliable and independent marker for the prediction of clinical outcomes in patients with GISTs.
The tumor suppressor p53 plays an important role in the regulation of cell cycle, DNA repair and programmed cell death. The functional loss of p53 disrupts these pathways and results in the selection of tumor cells with growth advantage[24]. p53 has been reported as a prognostic marker in a wide variety of carcinomas, as well as in GISTs[25]. A study showed that impaired p53 expression was often found in advanced GISTs and a strong effect of p53 on the progression-free survival was also observed[18]. The accumulation of p53 protein was significantly associated with mitotic rate and the risk of malignancy in the present study.
The activation of EGFR is associated with cell growth and transformation. There are few reports analyzing the EGFR expression in GISTs. A study has suggested that a transforming growth factor alpha (TGF-α)/EGFR autocrine loop is present in GISTs, in which TGF-α promotes the proliferation of GIST tumor cells through an interaction of EGFR with HER-1[26]. Co-expressions of EGFR and several EGFR ligands were observed with the upregulation of ADAM17 in GISTs. The authors suggested that the EGFR activation was through shedding of EGFR ligands by ADAM17 and consequently resulted in GIST progression and growth[17]. To our knowledge, there has been no study assessing prognostic values of EGFR in a large cohort of GISTs. However, no significant association was found between the EGFR expression and prognostic analysis of GISTs in our study.
Increased COX-2 expression has been observed in colorectal adenoma and carcinoma[27]. The induction of COX-2 has been shown to promote cell growth, inhibit apoptosis and enhance cell motility and adhesion[28]. Over-expression of COX-2 has tumorigenic effects in animal models[29]. Expression levels of COX-2 and vascular endothelial growth factor were found to be significantly higher in malignant GISTs than those in benign and intermediate GISTs[30]. A study reported a correlation between the COX-2 expression and tumor cell proliferation in GISTs, but no association was found between COX-2 expression and mortality, metastasis, tumor size, the risk of stages, the dose of tyrosine kinase inhibitors, or the rate of complete resection[31]. Our results demonstrated that levels of COX-2 expression were significantly different between gastric tumors and nongastric tumors (P < 0.001), but no significant relationship was found between the COX-2 expression and risk factors, or survival.
Imatinib therapy reduces rates of recurrence in GISTs. Nevertheless, it remains unclear how to screen patients who would be more likely to benefit from the adjuvant therapy. In our study, we found that imatinib treatment could significantly improve 3-year DSS rates in the intermediate and high risk categories of patients after a complete tumor resection.
In conclusion, Ki-67 expression is significantly associated with GIST malignancy and can be used as a putative prognostic marker in GISTs. p53 and COX-2 also provide additional valuable information in the evaluation of malignancy and types of GISTs.
Gastrointestinal stromal tumors (GISTs) are known to have a wide variability in malignancy and prognosis. Risk grading based on tumor size, location and mitotic counts has been proposed to predict adverse outcomes of GIST in the literature.
Recent molecular studies have found that the deregulations of Ki-67, cyclin A, B1, D1, E, cdc2 and other cell-cycle regulators were significantly associated with a shorter disease-free survival in GISTs.
In this study, expressions of Ki-67, p53, epidermal growth factor receptor (EGFR) and COX-2 were investigated in a large cohort of GIST patients and their roles as prognostic values for GISTs were also evaluated. To our knowledge, this is the first assessment of the prognostic value of EGFR in patients with GISTs.
The immunohistochemical staining of these tumorigenetic and cell proliferative proteins provides an alternative approach for follow-up and clinical decisions regarding the treatment for GISTs.
This paper describes a retrospective clinicopathological study of GIST.
| 1. | Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Rossi S, Gasparotto D, Toffolatti L, Pastrello C, Gallina G, Marzotto A, Sartor C, Barbareschi M, Cantaloni C, Messerini L. Molecular and clinicopathologic characterization of gastrointestinal stromal tumors (GISTs) of small size. Am J Surg Pathol. 2010;34:1480-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Nilsson B, Bümming P, Meis-Kindblom JM, Odén A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103:821-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 884] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 4. | Andersson J, Bümming P, Meis-Kindblom JM, Sihto H, Nupponen N, Joensuu H, Odén A, Gustavsson B, Kindblom LG, Nilsson B. Gastrointestinal stromal tumors with KIT exon 11 deletions are associated with poor prognosis. Gastroenterology. 2006;130:1573-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Liegl B, Hornick JL, Corless CL, Fletcher CD. Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am J Surg Pathol. 2009;33:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | Miettinen M, Wang ZF, Lasota J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am J Surg Pathol. 2009;33:1401-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Kang YN, Jung HR, Hwang I. Clinicopathological and immunohistochemical features of gastointestinal stromal tumors. Cancer Res Treat. 2010;42:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Nakamura N, Yamamoto H, Yao T, Oda Y, Nishiyama K, Imamura M, Yamada T, Nawata H, Tsuneyoshi M. Prognostic significance of expressions of cell-cycle regulatory proteins in gastrointestinal stromal tumor and the relevance of the risk grade. Hum Pathol. 2005;36:828-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419. [PubMed] |
| 10. | Dorn J, Spatz H, Schmieder M, Barth TF, Blatz A, Henne-Bruns D, Knippschild U, Kramer K. Cyclin H expression is increased in GIST with very-high risk of malignancy. BMC Cancer. 2010;10:350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC, Joensuu H. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 488] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 12. | Song Z, Wang JL, Pan YL, Tao DY, Gan MF, Huang KE. Survival and prognostic factors analysis in surgically resected gastrointestinal stromal tumor patients. Hepatogastroenterology. 2009;56:149-153. [PubMed] |
| 13. | Ruiz-Tovar J, Diez-Tabernilla M, Housari G, Martinez-Molina E, Sanjuanbenito A. Gastrointestinal stromal tumors: actin expression, a new prognostic factor? Am Surg. 2010;76:1244-1250. [PubMed] |
| 14. | Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 872] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 15. | Di Vizio D, Demichelis F, Simonetti S, Pettinato G, Terracciano L, Tornillo L, Freeman MR, Insabato L. Skp2 expression is associated with high risk and elevated Ki67 expression in gastrointestinal stromal tumours. BMC Cancer. 2008;8:134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Meara RS, Cangiarella J, Simsir A, Horton D, Eltoum I, Chhieng DC. Prediction of aggressiveness of gastrointestinal stromal tumours based on immunostaining with bcl-2, Ki-67 and p53. Cytopathology. 2007;18:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Nakagawa M, Nabeshima K, Asano S, Hamasaki M, Uesugi N, Tani H, Yamashita Y, Iwasaki H. Up-regulated expression of ADAM17 in gastrointestinal stromal tumors: coexpression with EGFR and EGFR ligands. Cancer Sci. 2009;100:654-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Gumurdulu D, Erdogan S, Kayaselcuk F, Seydaoglu G, Parsak CK, Demircan O, Tuncer I. Expression of COX-2, PCNA, Ki-67 and p53 in gastrointestinal stromal tumors and its relationship with histopathological parameters. World J Gastroenterol. 2007;13:426-431. [PubMed] |
| 19. | Fux R, Schwab M, Thon KP, Gleiter CH, Fritz P. Cyclooxygenase-2 expression in human colorectal cancer is unrelated to overall patient survival. Clin Cancer Res. 2005;11:4754-4760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Huang HY, Huang WW, Lin CN, Eng HL, Li SH, Li CF, Lu D, Yu SC, Hsiung CY. Immunohistochemical expression of p16INK4A, Ki-67, and Mcm2 proteins in gastrointestinal stromal tumors: prognostic implications and correlations with risk stratification of NIH consensus criteria. Ann Surg Oncol. 2006;13:1633-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Konsti J, Lundin M, Joensuu H, Lehtimäki T, Sihto H, Holli K, Turpeenniemi-Hujanen T, Kataja V, Sailas L, Isola J. Development and evaluation of a virtual microscopy application for automated assessment of Ki-67 expression in breast cancer. BMC Clin Pathol. 2011;11:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Romeo S, Debiec-Rychter M, Van Glabbeke M, Van Paassen H, Comite P, Van Eijk R, Oosting J, Verweij J, Terrier P, Schneider U. Cell cycle/apoptosis molecule expression correlates with imatinib response in patients with advanced gastrointestinal stromal tumors. Clin Cancer Res. 2009;15:4191-4198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Gunji Y, Nikaidou T, Okazumi S, Matsubara H, Shimada H, Nabeya Y, Aoki T, Makino H, Miyazaki S, Ochiai T. Evaluation of Ki-67 and p53 expression in primary and repeated liver metastases of GISTs. Hepatogastroenterology. 2005;52:829-832. [PubMed] |
| 24. | Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2178] [Cited by in RCA: 2503] [Article Influence: 147.2] [Reference Citation Analysis (0)] |
| 25. | Hata Y, Ishigami S, Natsugoe S, Nakajo A, Okumura H, Miyazono F, Matsumoto M, Hokita S, Aikou T. P53 and MIB-1 expression in gastrointestinal stromal tumor (GIST) of the stomach. Hepatogastroenterology. 2006;53:613-615. [PubMed] |
| 26. | Cai YC, Jiang Z, Vittimberga F, Xu X, Savas L, Woda B, Callery M, Banner B. Expression of transforming growth factor-alpha and epidermal growth factor receptor in gastrointestinal stromal tumours. Virchows Arch. 1999;435:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Chan AT. COX-2 expression in adenoma: an imperfect marker for chemoprevention. Gut. 2010;59:568-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Wu WK, Sung JJ, Lee CW, Yu J, Cho CH. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer Lett. 2010;295:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 29. | Spugnini EP, Porrello A, Citro G, Baldi A. COX-2 overexpression in canine tumors: potential therapeutic targets in oncology. Histol Histopathol. 2005;20:1309-1312. [PubMed] |
| 30. | Miao R, Liu N, Wang Y, Li L, Yu X, Jiang Y, Li J. Coexpression of cyclooxygenase-2 and vascular endothelial growth factor in gastrointestinal stromal tumor: possible relations to pathological parameters and clinical behavior. Hepatogastroenterology. 2008;55:2012-2015. [PubMed] |
| 31. | Sevinc A, Camci C, Sari I, Kalender ME, Er O, Soyuer I, Dikilitas M, Yilmaz U, Sagol O, Alacacioglu A. Cyclooxygenase-2 expression in gastrointestinal stromal tumours. Asian Pac J Cancer Prev. 2010;11:849-853. [PubMed] |
Peer reviewer: Yasuhiro Matsumura, MD, PhD, Investigative Treatment Division, Research Center for Innovative Oncology, National Cancer Center Hospital East, 6-5-1 Kashiwanoha, Kashiwa, Chiba 277-8577, Japan
S- Editor Tian L L- Editor Logan S E- Editor Li JY