Published online May 28, 2012. doi: 10.3748/wjg.v18.i20.2472
Revised: February 3, 2012
Accepted: March 9, 2012
Published online: May 28, 2012
AIM: To determine the anti-Helicobacter property of Lactobacillus plantarum B7 (L. plantarum) B7 supernatants in vitro and the protective effects of L. plantarum B7 on serum tumor necrosis factor-alpha (TNF-α), gastric malondialdehyde (MDA) level, apoptosis, and histopathology in Helicobacter pylori (H. pylori)-induced gastric inflammation in rats.
METHODS: In vitro, the inhibition of H. pylori growth was examined using L. plantarum B7 supernatants at pH 4 and pH 7 and at the concentration of 1×, 5× and 10× on plates inoculated with H. pylori. The inhibitory effect of H. pylori was interpreted by the size of the inhibition zone. In vitro, male Sprague-Dawley rats were randomly divided into four groups including group 1 (control group), group 2 (H. pylori infected group), group 3 (H. pylori infected with L. plantarum B7 106 CFUs/mL treated group) and group 4 (H. pylori infected with L. plantarum B7 1010 CFUs/mL treated group). One week after H. pylori inoculation, L. plantarum B7 106 CFUs/mL or 1010 CFUs/mL were fed once daily to group 3 and group 4, respectively, for one week. Blood and gastric samples were collected at the end of the study.
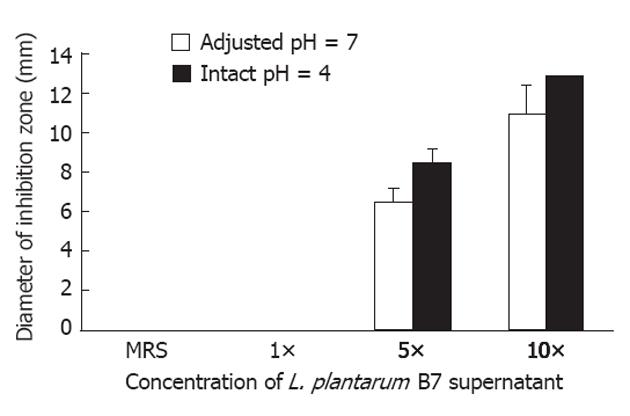
RESULTS: In vitro, at intact pH 4, mean inhibitory zone diameters of 8.5 mm and 13 mm were noted at concentrations of 5× and 10× of L. plantarum B7 supernatant disks, respectively. At adjusted pH 7, L. plantarum B7 supernatants at concentrations of 5× and 10× yielded mean inhibitory zone diameters of 6.5 mm and 11 mm, respectively. In the in vitro study, in group 2, stomach histopathology revealed mild to moderate H. pylori colonization and inflammation. The level of gastric MDA and epithelial cell apoptosis were significantly increased compared with group 1. The serum TNF-α level was significant decreased in group 3 compared with group 2 (P < 0.05). In addition, L. plantarum B7 treatments resulted in a significant improvement in stomach pathology, and decreased gastric MDA level and apoptotic epithelial cells.
CONCLUSION: L. plantarum B7 supernatant inhibits H. pylori growth. This inhibition was dose-dependent and greater at pH 4. Moreover, L. plantarum B7 attenuated H. pylori-induced gastric inflammation.
-
Citation: Sunanliganon C, Thong-Ngam D, Tumwasorn S, Klaikeaw N.
Lactobacillus plantarum B7 inhibitsHelicobacter pylori growth and attenuates gastric inflammation. World J Gastroenterol 2012; 18(20): 2472-2480 - URL: https://www.wjgnet.com/1007-9327/full/v18/i20/2472.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i20.2472
Helicobacter pylori (H. pylori) is a gram-negative, spiral shaped bacterium that has the unique ability of being able to colonize the human gastric mucosa and infects more than half of the world’s population. H. pylori causes chronic gastritis, plays an etiologic role in peptic ulcer disease and is considered a risk factor in the development of gastric cancer and gastric lymphoma[1]. In 1994, H. pylori was classified as a type I carcinogen by the World Health Organization[2].
H. pylori infection is characterized by enhanced production of proinflammatory mediators such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-2, IL-6 and IL-8 and the infiltration of lamina propria with inflammatory cells. H. pylori lipopolysaccharides (LPS) and released surface proteins stimulate lamina propria mononuclear cells and macrophages to produce proinflammatory cytokines such as TNF-α, IL-1β, and the generation of reactive oxygen species (ROS)[3]. TNF-α and IL-1β are potent inducers of IL-8 expression in many cell types. Furthermore, H. pylori is capable of interacting with epithelial cell surfaces to produce IL-8. The release of these inflammatory mediators results in the expression of CD11b/CD18 on leukocytes and intercellular adhesion molecule-1 on endothelial cells, the migration of leukocytes to a site of inflammation, and finally the generation of ROS[4].
ROS can react with the double bonds of polyunsaturated fatty acids (PUFAs), present in the membranes of phospholipids, resulting in lipid peroxidation. One of the major secondary oxidation products of peroxidized PUFAs is malondialdehyde (MDA)[5]. H. pylori also induces gastric epithelial cell apoptosis both in vitro[6] and in vivo[7]. Studies have shown that the H. pylori colonized stomach contains more apoptotic epithelial cells than normal epithelial cells. Moreover, the increased numbers of apoptotic epithelial cells decrease to normal after eradication of H. pylori[7].
H. pylori eradication is suboptimal because current treatment regimens result in adverse side effects, poor compliance, and an increasing prevalence of antibiotic resistance[8]. Therefore, alternative treatments are of interest.
Lactobacilli are probiotics which, when administered in adequate amounts, may confer a benefit to the host[9]. The most commonly used organisms in probiotic products are Lactobacillus sp. and Bifidobacterium sp.[10]. L. plantarumis commonly found in the human gastrointestinal tract (GI-tract). It is important in the production of a variety of fermented foods such as sauerkraut, Korean kimchi, cheese, sausages and stockfish, and is also used as a probiotic. Moreover, there is increasing evidence that L. plantarum has anti-Helicobacter activity and shows modulatory effects on the immune system[11,12]. Importantly, L. plantarum is acid and bile tolerant, survives passage through the GI-tract, and is safe in humans and animals.
The aim of this study was to examine the in vitro anti-Helicobacter activity of L. plantarum B7 supernatants using the disk diffusion method and the effects of L. plantarum B7 on gastric histopathology, serum TNF-α, gastric MDA level, and cell apoptosis in H. pylori infection in vivo.
The disk diffusion method was used to assess the anti-H. pylori activity of L. plantarum B7 supernatants at intact and neutralized pH and various concentrations of 1×, 5× and 10× against H. pylori.
Bacterial strains and culture conditions:H. pylori ATCC 43504 was grown on Columbia agar (Oxoid, Basingstoke, United Kingdom) containing 7% sheep blood and 7% horse serum. Plates were incubated at 37 °C under microaerophilic conditions (10% CO2, 5% O2 and 85% N2) produced by a gas generating system, AnaeroPack (MGC, Japan), for 72 h in an anaerobic jar (Oxoid, Basingstoke, United Kingdom).
L. plantarum B7, isolated from Thai dyspeptic patients, was stored in de Man-Rogosa-Sharpe (MRS) broth (Oxoid, Basingstoke, United Kingdom) with 20% glycerol at -80 °C. This strain was recovered from frozen stock and cultivated twice on MRS agar anaerobically (10% CO2, 10% H2 and 80% N2) at 37 °C in an anaerobic jar for 48 h. A single colony of L. plantarum B7 was then inoculated into 10 mL of MRS broth and grown at 37 °C under anaerobic conditions for 24 h in a 15 mL conical centrifuge tube (Corning, New York, United States). The OD600 of the culture was determined using a spectrophotometer (Bio-Rad Smart SpecTM Plus), adjusted to OD600 with 0.1 in 10 mL of MRS broth and incubated for 48 h. After incubation, the culture supernatant was collected by centrifugation at 1000 ×g for 10 min at 4 °C and then filtered using a 0.22 μm pore size filter unit (Minisart, Germany). The supernatant of Lactobacillus without the cell pellet was called the Lactobacillus condition media (LCM). The concentration and pH of LCM were adjusted to 1×, 5× and 10× by speed-vacuum drying (speed-vacuum, Savant Instruments, United States) and resuspending in an appropriate volume of intact pH 4 and adjusted pH 7 MRS broth. Sterile 6 mm-membrane disks (Whatman, Maidstone, United Kingdom) were then dipped into the resuspended LCM for at least 1 h at room temperature.
Disk diffusion method: The various concentrations of L. plantarum B7 supernatants were evaluated at two pH values, intact pH 4, and adjusted pH 7 with NaOH. H. pylori was spread on Columbia blood agar plates, and L. plantarum B7 (LCM) disks were placed directly on the surface of the agar. The plates were incubated under microaerophilic conditions at 37 °C for 72 h, after which the diameters of the inhibition zones were measured in millimeters. In this study, the MRS broth was used as a negative control. The experiments were carried out in duplicate and mean values of the growth inhibition zones were measured.
Bacteria preparation:H. pylori was subcultured twice on Columbia blood agar. Plates were incubated at 37 °C under microaerophilic conditions for 72 h. L. plantarum B7 was originally obtained from Thai dyspeptic patients who visited King Chulalongkorn Memorial Hospital. This strain was cultivated twice on MRS agar anaerobically at 37 °C for 48 h.
Animal preparation: Thirty-two male Sprague-Dawley rats (Salaya Research Animal Center, Mahidol University, Bangkok, Thailand), weighing about 150-250 g at the beginning of the experiment, were used. The experimental protocol was approved by the Ethical Committee of Medicine Faculty, Chulalongkorn University, Thailand. The animals were housed in Macrolon cages (5 animals per cage), given food and tap water ad libitum at room temperature (18 °C-22 °C), humidity 55%, and a 12/12 h-light/dark cycle.
Experimental protocol: The rats were randomly divided into four experimental groups (eight rats each group) as follows. Group 1: Rats were fed phosphate buffered saline (PBS) 1 mL/rat by gavage twice a day at an interval of four hours for three consecutive days. Then, they were housed with free access to water and standard food for 1 wk. After that, the animals were treated with PBS 1 mL/rat by gavage once daily for 1 wk. Group 2: Rats were inoculated with H. pylori using the method of Thong-Ngam et al[13]. Briefly, the rats were pre-treated with streptomycin suspended in tap water (5 mg/mL) for three days before H. pylori inoculation. The H. pylori suspension (5 × 1010 CFUs/mL) in PBS was administered (1 mL/rat) by gavage twice daily at an interval of four hours for three consecutive days. One week after the inoculation, the animals were treated with PBS (1 mL/rat) by gavage once daily for one week. Group 3: One week after H. pylori inoculation, the rats were treated by gavage with L. plantarum B7 106 CFUs/mL suspended in PBS once daily for 1 wk. Group 4: One week after H. pylori inoculation, the rats were treated by gavage with L. plantarum B7 1010 CFUs/mL suspended in PBS once daily for 1 wk.
At the end of the experiment, animals were sacrificedby an overdose of intraperitoneal thiopental sodium. Blood samples were then collected for TNF-α determination using enzyme-linked immunosorbent assay (ELISA). The stomach was removed. One-half of the stomach was frozen in liquid nitrogen, and stored at -80 °C for MDA analysis. The remainder of the stomach was fixed in 4% paraformaldehyde in phosphate buffer solution to determine histopathology and epithelial cell apoptosis.
Determination of serum cytokine levels: Blood samples were taken by cardiac puncture, allowed to clot for two hours at room temperature before centrifuging for 20 min at approximately 1000 ×g. Then, the serum was removed and stored at -80 °C for determination of TNF-α level using an ELISA kit (R and D Systems, United States).
Assessment of H. pylori infection and examination of histopathology: The presence of H. pylori infection in the rats was determined by the urease test and histopathological examination by a blinded pathologist. After completing the experiment, the rats were sacrificed. The stomach was removed and 2 mm2 of gastric mucosa from the antrum was immediately dissected and placed in the urease tube to examine urease activity.
The remaining tissue from the gastric antrum biopsy was fixed in 4% paraformaldehyde in phosphate buffer solution at pH 7.4 and room temperature. The tissue was processed and stained with hematoxylin-eosin. The slides were observed by light microscopy and the presence of H. pylori was detected by Warthin-Starry staining in unclear cases. The level of bacterial colonization was evaluated using a grading system as follows. Score 0: No bacteria detected; Score 1: Mild colonization in some gastric crypts; Score 2: Mild colonization in most gastric crypts; Score 3: Moderate colonization in all gastric crypts. The results are presented as the bacterial colonization scores for each group. In addition to H. pylori colonization, the gastric inflammation level was estimated and scored following the updated Sydney System[14]. The infiltration of polymorphonuclear leukocytes in the gastric mucosa, defining the inflammatory scores, was recorded. Scores from 0 to 3 represented normal, mild, moderate and marked histopathological changes, respectively.
Determination of gastric malondialdehyde: Gastric MDA level was measured using the thiobarbituric acid (TBA) reactive substances assay kit (Cayman, United States). The principle is that the reaction of one molecule of MDA and two molecules of TBA form a red MDA-TBA complex under high temperature (90 °C-100 °C) and acidic conditions, which can be quantitated using a spectrophotometer at 532 nm. The assay procedures were performed as described. The content of MDA was expressed in terms of nmol/mg protein.
Determination of gastric epithelial cell apoptosis: Apoptosis was measured by the identification of apoptotic nuclei in sections of stomachusing fragment end labeling of DNA (Apoptosis detectionkit, Chemicon, United States). In brief, the DNA fragments were allowed to bind an antidigoxigenin antibody that was conjugated to a peroxidase. Diaminobenzidine was applied to develop a dark brown color and the slides were counterstained with hematoxylin. The positive stained cells showed dark brown nuclei under light microscopy. To verify the incidence of apoptosis, the dark brown-stained cells were counted. One thousand gastric epithelial cells were counted for each rat. The data were shown as a percentage (%) of apoptotic cells calculated as: the percentage of apoptotic cells (%) = (numbers of positive stained cells × 100)/1000.
All data are presented as mean ± SD. The means were compared by one-way analysis of variance followed by least significant different post hoc test. All statistical tests were performed using SPSS for Windows version 13.0 (SPSS Inc, Chicago, IL, United States). Differences were considered statistically significant at P < 0.05.
Disk diffusion method: At intact pH 4, mean inhibitory zone diameters of 8.5 ± 0.7 mm and 13 ± 0 mm were noted at the concentrations of 5× and 10× of L. plantarum B7 supernatant disks, respectively. At adjusted pH 7, mean inhibitory zone diameters of 6.5 ± 0.7 mm and 11 ± 1.4 mm were noted at the concentrations of 5× and 10× of L. plantarum B7 supernatant disks, respectively (Table 1). Both intact pH 4 and adjusted pH 7 of L. plantarum B7 supernatants showed dose-dependent anti-H. pylori activity. The supernatant of pH 4 L. plantarum B7 at the concentration of 10× showed the clearest inhibition (Figure 1).
| Concentration of L. plantarum | Diameters of inhibition zone (mm) | |
| B7 supernatant | Intact pH 4 | Adjusted pH 7 |
| MRS (negative control) | 0 | 0 |
| 1× | 0 | 0 |
| 5× | 8.5 ± 0.7 | 6.5 ± 0.7 |
| 10× | 13 ± 0 | 11 ± 1.4 |
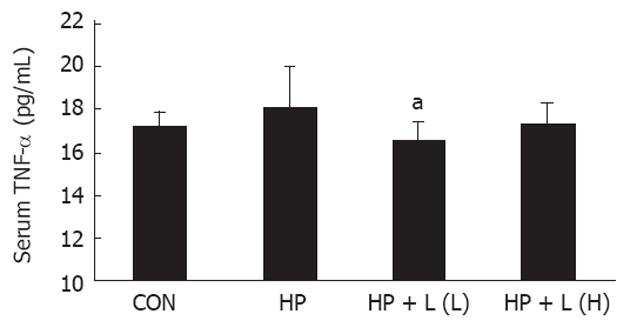
Changes in TNF-αlevel: The serum TNF-α level was not significantly different between the control group and H. pylori infected group. However, in the L. plantarum B7 106 CFUs/mL treated group, a significant decrease in serum TNF-α level was noted compared with the H. pylori infected group (P = 0.019). The average concentrations of serum TNF-α were 17.22 ± 0.63 pg/mL, 18.05 ± 1.94 pg/mL, and 16.52 ± 0.84 pg/mL in the control, H. pylori infected, and in the L. plantarum B7 106 CFUs/mL treated group, respectively. The average serum TNF-α levels in all groups are shown in Figure 2.
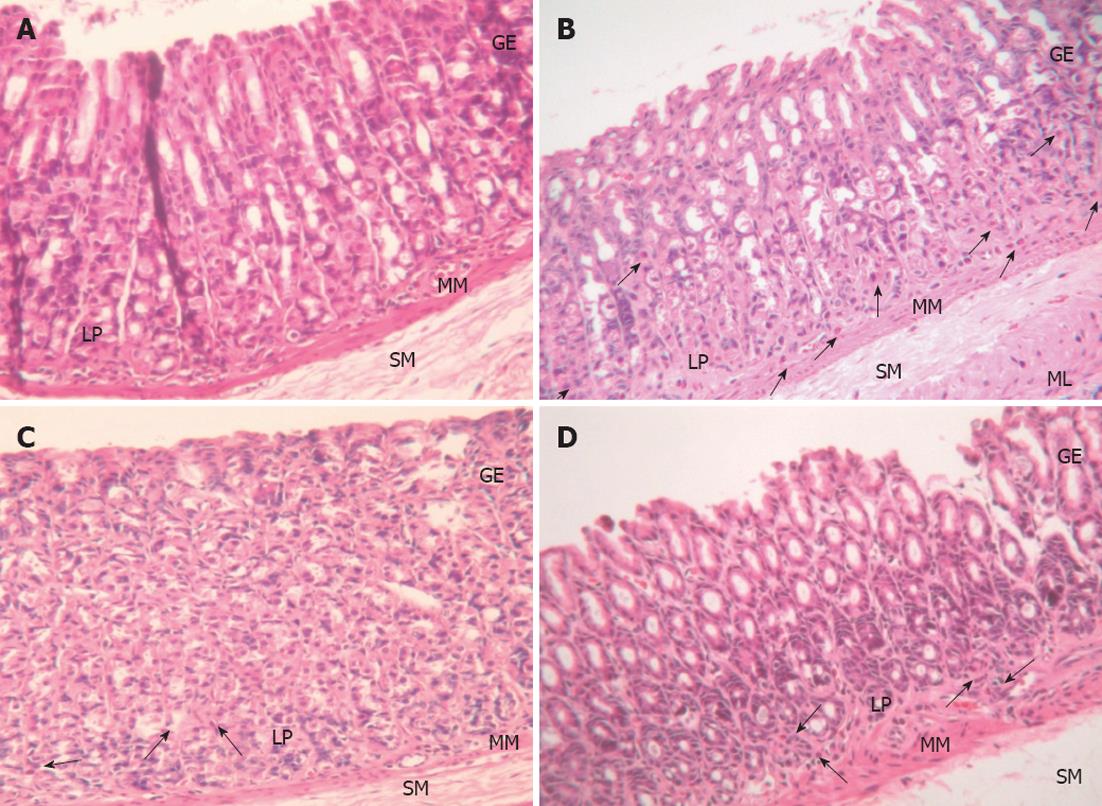
Histopathological examination:H. pylori infection in rats was determined by the urease test and histopathology. Histopathology in the control group was normal, while in the H. pylori infected group there was moderate H. pylori colonization and inflammation. The L. plantarum B7 106 CFUs/mL treated and L. plantarum B7 1010 CFUs/mL treated groups showed reduced H. pylori colonization and improved stomach inflammation (Figures 3 and 4). The histology scores for H. pylori colonization and gastric inflammation are summarized in Table 2.
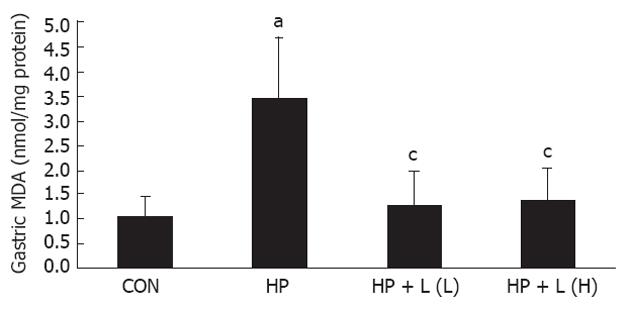
Determination of gastric malondialdehyde: The level of gastric MDA increased significantly in the H. pylori infected compared with the control group (3.46 ± 1.25 nmol/mg vs 1.05 ± 0.41 nmol/mg protein, P = 0.000, respectively). After one week of 106 CFUs/mL or 1010 CFUs/mL of L. plantarum B7 suspension, there was a significant decrease in elevated gastric MDA level in both L. plantarum B7 treated groups compared with the H. pylori infected group (1.28 ± 0.69, 1.37 ± 0.66 nmol/mg vs 3.46 ± 1.25 nmol/mg protein, P = 0.000, respectively) (Figure 5).
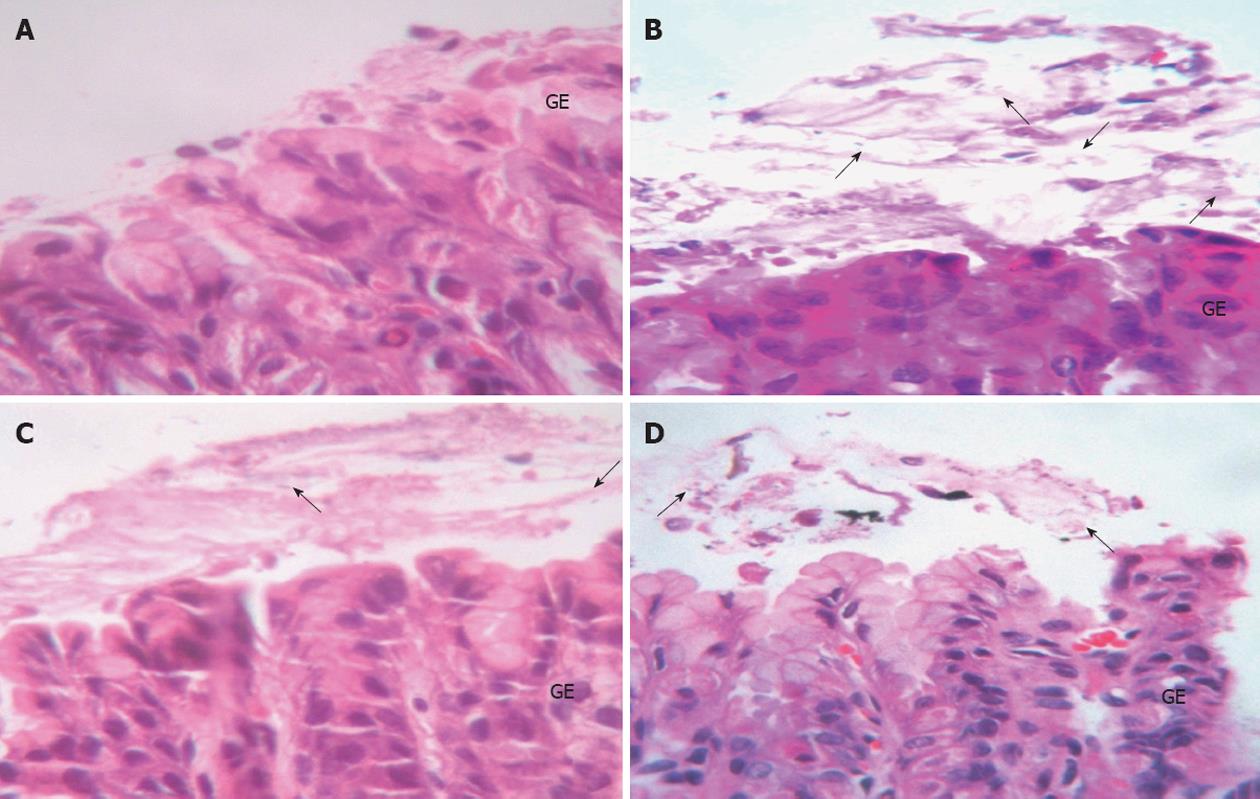
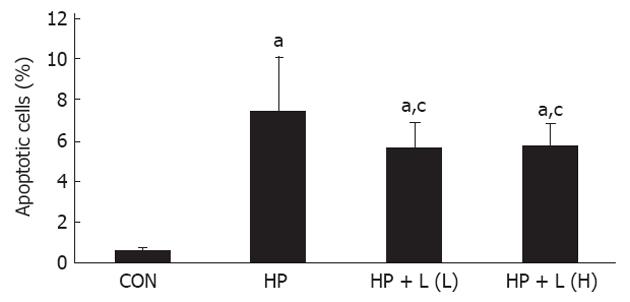
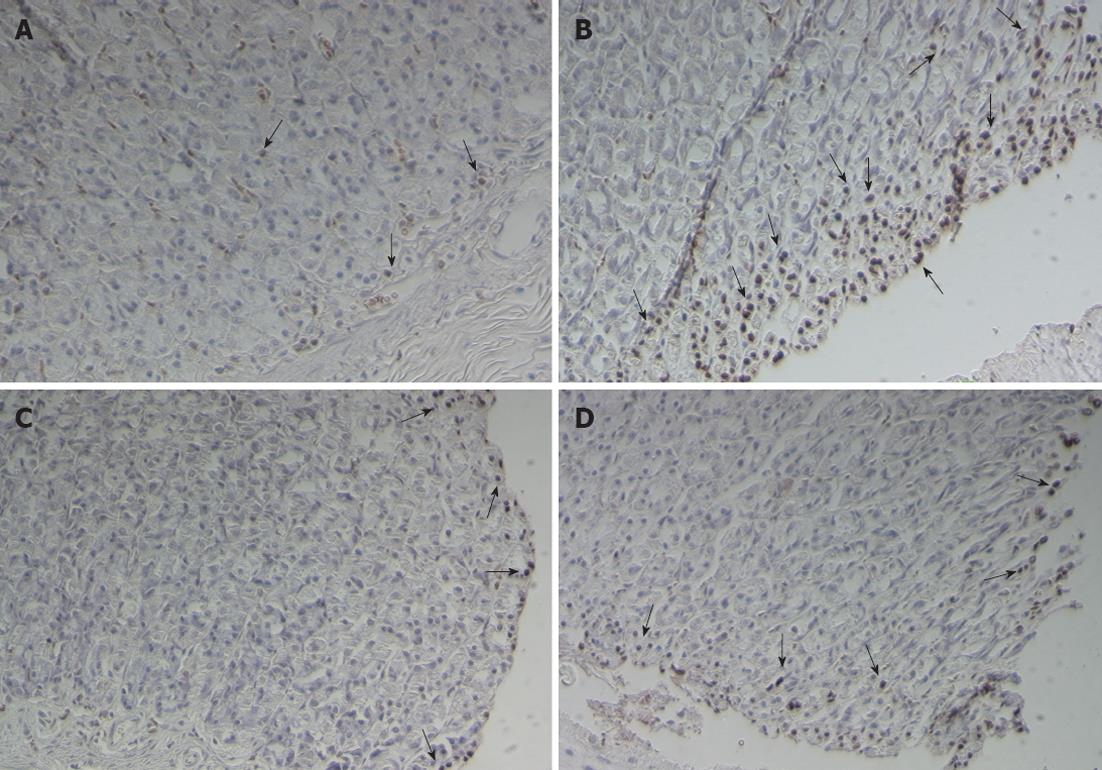
Determination of gastric epithelial cell apoptosis: The percentage of apoptotic cells was significantly increased in the H. pylori infected group when compared with the control group (7.44 ± 2.65 vs 0.58 ± 0.13, P = 0.0001, respectively). After treatment with 106 CFUs/mL or 1010 CFUs/mL of L. plantarum B7 suspension, the percentage of apoptotic cells was significantly decreased at 106 CFUs/mL (P = 0.027) and 1010 CFUs/mL (P = 0.038) compared with the H. pylori infected group. The average percentages of apoptotic cells in all the groups are shown in Figure 6. Figure 7 shows gastric sections processed for apoptosis by the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) reaction.
The in vitro study with intact pH 4 and adjusted pH 7 of L. plantarum B7 supernatants showed concentration-dependent anti-H. pylori activity, however, the culture supernatants of intact pH 4 L. plantarum B7 supernatant showed higher inhibition. This implied that low pH values are important for anti-H. pylori activity. In a study by Boyanova et al[15], the anti-Helicobacter activity of L. delbrueckii subsp. bulgaricus cultures was strain-dependent and better at their native pH.
It is known that Lactobacillus secretes metabolic products such as lactic acid which exerts activity against H. pylori[16]. Lactic acid inhibits the urease activity and viability of H. pylori. Several studies have reported that bacteriocin, peroxide, proteinase, exopolysaccharide and cell wall components, called Lactobacillus-inhibitory factors, have antibacterial effects[17,18]. In addition, Coconnier et al[19] showed that a heat-stable antimicrobial substance secreted by L. acidophilus LB was active against H. pylori infection.
In summary, our in vitro study found that L. plantarum B7 supernatant inhibited H. pylori growth in a dose-dependent manner and was better at intact pH 4 indicating that the amount of antimicrobial substance released by L. plantarum B7 correlated with the intensity of the inhibitory effect against H. pylori. Furthermore, the anti-H. pylori activity of this substance was supported by low pH values.
The present in vivo study showed that the gastric histopathology in the H. pylori infected group revealed mild to moderate H. pylori colonization and inflammation as well as increased gastric MDA and gastric epithelial cell apoptosis.
H. pylori induces a host inflammatory response including production of cytokines, resulting in mucosal damage. The produced cytokines lead to infiltration of inflammatory cells, namely polymorphonuclear neutrophils (PMNs), lymphocytes and macrophages, at the site of infection. These inflammatory cells then release large amounts of ROS, causing tissue injury. Wilson et al[20] showed that the gastric mucosal levels of proinflammatory cytokines, such as TNF-α, IL-1β, IL-6 and IL-8, were significantly higher in H. pylori positive patients than in H. pylori negative patients. Crabtree et al[21] showed that increased gastric mucosal production of TNF-α and IL-6 was associated with H. pylori gastritis. Moreover, they implied that inflammatory cytokines generated locally within the gastric mucosa can be relevant to the gastric physiology of H. pylori infection.
As mentioned above, infection with H. pylori in the gastric mucosa is known to activate the production of many proinflammatory cytokines including TNF-α, IL-1β, IL-6 and IL-8. The production of these proinflammatory cytokines is not limited to the local site of infection, as these cytokines are produced in numbers and contribute to the systemic circulation. In 2006, Prabjone et al[22] investigated the effects of chronic H. pylori infection on serum TNF-α level in rats. They found a significant increase in serum TNF-α in the H. pylori infected groups compared with the control groups. In the present study, no significant increase in serum TNF-α level was observed in the H. pylori infected group.
Several studies have shown that H. pylori strains with the cagA+/vacAs1 genotype are more virulent than strains with other genotypes[23]. Similarly, Azuma et al[24] reported that H. pylori cagA+ strains were involved in more intense tissue responses than cagA- strains. Moreover, epidemiological studies have shown that colonization with cagA+ H. pylori is associated with an increased risk for the development of both peptic ulcer disease and gastric cancer. In an in vitro study, Zhang et al[25] demonstrated that H. pylori cagA+ strains induced an increased oxidative burst in PMNs with higher ROS production. Recently, studies have shown that ROS production in gastric mucosa is enhanced by infection with cagA+ H. pylori species with an extensive accumulation of neutrophils in both patients with chronic gastritis and gastric ulcer[4,5]. In this study, rats infected with H. pylori cagA+, vacA+ strains were found to have significantly increased gastric MDA levels, suggesting that oxidative stress may be associated with the cagA+ status of H. pylori.
Furthermore, we demonstrated that H. pylori cagA+, vacA+ strains can induce epithelial cell apoptosis in rats. The cagA gene or expression of VacA might be involved in gastroduodenal diseases by affecting apoptosis. The cagA gene is a marker of the presence of the pathogenicity island that encodes disease-associated virulence factors and is associated with the expression of VacA[26]. In 2006, Cabral et al[27] showed that the expression of pro-apoptotic proteins such as Bax and Bak was higher than anti-apoptotic proteins including Bcl-2 and Bcl-XL in most gastric biopsies from patients with H. pylori gastritis and was significantly higher in patients infected by cagA+ strains than in those infected by cagA-. Moreover, they found that Bak expression was higher at the lesser curvature (antrum and incisura) than in the other regions and was correlated with atrophy. These results suggest that in addition to cagA, vacA plays a crucial role in the induction of apoptosis. In the present study, our data also showed that infection with H. pylori cagA+, vacA+ strains leads to elevated gastric MDA levels, as previously mentioned. MDA, a major product of lipid peroxidation, can react with DNA to form MDA-DNA adducts, resulting in DNA damage.
Several previous investigations have shown the anti-inflammatory properties of Lactobacillus. A study by Johnson-Henry et al[28] found that the probiotic combination containing L. rhamnosus R0011 and L. acidophilus R0052 decreased the effects of H. pylori infection in a C57BL/6 mouse model of infection by reducing H. pylori colonization and alleviating H. pylori-induced gastric mucosa inflammation. In 2003, Peña et al[29] showed that L. rhamnosus GG was able to antagonize H. pylori LPS-induced TNF-α production in murine macrophages in vitro by a contact-independent mechanism. Ko et al[12] reported that L. plantarum was capable of inhibiting epithelial barrier dysfunction and reducing IL-8 secretion induced by TNF-α. In addition to anti-inflammatory activity, several studies have shown that Lactobacillus also has effective antioxidative and anti-apoptotic properties. Truusalu et al[30] found that L. fermentum ME-3 suppressed excessive oxidative stress-associated inflammation induced by S. typhimurium infection in a mouse model. Using the same experimental typhoid fever model, they also showed that treatment with L. fermentum ME-3 alone or in combination with an antimicrobial quinolone (ofloxacin) leads to a significant decrease in lipid peroxidation and the glutathione redox ratio (GSSG/GSH). In 2010, Zhang et al[31] reported that oral L. plantarum treatment in rats with obstructive jaundice increased GSH levels in the liver and stimulated GSH biosynthesis, resulting in attenuated oxidative damage. Using the TUNEL assay, they also showed that treatment with L. plantarum significantly decreased hepatic apoptosis. In addition, Lam et al[32] showed that pre-treatment of rats with L. rhamnosus GG markedly reduced ethanol-induced mucosal lesion area and gastric cell apoptosis.
Interestingly, all of these studies were concordant with our results. In the current study, we found that L. plantarum B7 treatment resulted in improved stomach pathology, and decreased serum TNF-α level, gastric MDA level, and apoptotic epithelial cells. However, the mechanisms of action are unclear and require further investigation.
In conclusion, the present study showed that H. pylori infection induced gastric injury by increasing levels of H. pylori colonization and inflammation, gastric MDA and epithelial cell apoptosis. L. plantarum B7 may have anti-H. pylori activity in vitro and anti-inflammatory effects on H. pylori infection by improving stomach histopathology, and reducing serum TNF-α levels, gastric MDA and epithelial cell apoptosis.
Helicobacter pylori (H. pylori) infection induces the production of proinflammatory mediators such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-2, IL-6 and IL-8, and infiltration of the lamina propria with inflammatory cells as well as the generation of reactive oxygen species (ROS). However, these H. pylori-induced inflammatory responses do not appear to confer protective immunity, and may lead to the excess production of ROS, oxidative bursts caused by phagocytic cells, and gastric tissue damage. Lactobacillus plantarum (L. plantarum) B7 has anti-H. pylori activity in vitro and anti-inflammatory properties resulting in the alleviation of gastric injury in H. pylori-induced gastritis in rats.
L. plantarum is a non-pathogenic gram-positive bacterium that exerts anti-H. pylori activity and immunomodulatory effects. H. pylori infection can cause gastric mucosal damage by increasing H. pylori colonization and inflammation levels, gastric malondialdehyde (MDA) and epithelial cell apoptosis. The hallmark of this study was the interesting results which showed an inhibitory effect of L. plantarum B7 supernatant on H. pylori growth in vitro, and an improvement in stomach pathology, reduction in serum TNF-α level, gastric MDA and epithelial cell apoptosis following treatment with L. plantarum B7.
A previous study showed that L. plantarum B7 has anti-inflammatory properties in vitro. However, it is not clear whether L. plantarum B7 has in vivo effects on H. pylori-induced gastric inflammation. Therefore, in this study, the authors examined the anti-inflammatory effect of L. plantarum B7 in rats and found that L. plantarum B7 ameliorated H. pylori-induced gastritis by improving stomach pathology, and decreasing TNF-α production, gastric MDA level and epithelial cell apoptosis. Moreover, supernatants of L. plantarum B7 showed anti-H. pylori activity in vitro.
L. plantarum B7 may be beneficial in clinical application, and can be used as an adjunct to antibiotics to decrease H. pylori-induced gastric inflammation and reduce side effects of triple therapy.
This is an experimental study on the effect of H. pylori infection in gastric inflammation. This study shows the efficacy of L. plantarum B7 in treatment of H. pylori-induced gastritis reflects in attenuated levels of H. pylori colonization, gastric inflammation, cytokine production, gastric MDA, and apoptotic cells. Also, the results from in vitro study demonstrates the inhibitory effect of L. plantarum B7 supernatants on H. pylori growth.
| 2. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [PubMed] |
| 3. | McGee DJ, Mobley HL. Mechanisms of Helicobacter pylori infection: bacterial factors. Curr Top Microbiol Immunol. 1999;241:155-180. [PubMed] |
| 4. | Naito Y, Yoshikawa T. Molecular and cellular mechanisms involved in Helicobacter pylori-induced inflammation and oxidative stress. Free Radic Biol Med. 2002;33:323-336. [PubMed] |
| 5. | Augusto AC, Miguel F, Mendonça S, Pedrazzoli J, Gurgueira SA. Oxidative stress expression status associated to Helicobacter pylori virulence in gastric diseases. Clin Biochem. 2007;40:615-622. [PubMed] |
| 6. | Wagner S, Beil W, Westermann J, Logan RP, Bock CT, Trautwein C, Bleck JS, Manns MP. Regulation of gastric epithelial cell growth by Helicobacter pylori: offdence for a major role of apoptosis. Gastroenterology. 1997;113:1836-1847. [PubMed] |
| 7. | Moss SF, Calam J, Agarwal B, Wang S, Holt PR. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498-501. [PubMed] |
| 8. | Meyer JM, Silliman NP, Wang W, Siepman NY, Sugg JE, Morris D, Zhang J, Bhattacharyya H, King EC, Hopkins RJ. Risk factors for Helicobacter pylori resistance in the United States: the surveillance of H. pylori antimicrobial resistance partnership (SHARP) study, 1993-1999. Ann Intern Med. 2002;136:13-24. [PubMed] |
| 9. | Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365-378. [PubMed] |
| 10. | Saxelin M, Tynkkynen S, Mattila-Sandholm T, de Vos WM. Probiotic and other functional microbes: from markets to mechanisms. Curr Opin Biotechnol. 2005;16:204-211. [PubMed] |
| 11. | Rokka S, Pihlanto A, Korhonen H, Joutsjoki V. In vitro growth inhibition of Helicobacter pylori by lactobacilli belonging to the Lactobacillus plantarum group. Lett Appl Microbiol. 2006;43:508-513. [PubMed] |
| 12. | Ko JS, Yang HR, Chang JY, Seo JK. Lactobacillus plantarum inhibits epithelial barrier dysfunction and interleukin-8 secretion induced by tumor necrosis factor-alpha. World J Gastroenterol. 2007;13:1962-1965. [PubMed] |
| 13. | Thong-Ngam D, Prabjone R, Visedopas N, Chatsuwan T. A simple rat model of chronic Helicobacter pylori infection for research study. Thai J Gastroenterol. 2005;6:3-7. |
| 14. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] |
| 15. | Boyanova L, Stephanova-Kondratenko M, Mitov I. Anti-Helicobacter pylori activity of Lactobacillus delbrueckii subsp. bulgaricus strains: preliminary report. Lett Appl Microbiol. 2009;48:579-584. [PubMed] |
| 16. | Midolo PD, Lambert JR, Hull R, Luo F, Grayson ML. In vitro inhibition of Helicobacter pylori NCTC 11637 by organic acids and lactic acid bacteria. J Appl Bacteriol. 1995;79:475-479. [PubMed] |
| 17. | Silva M, Jacobus NV, Deneke C, Gorbach SL. Antimicrobial substance from a human Lactobacillus strain. Antimicrob Agents Chemother. 1987;31:1231-1233. [PubMed] |
| 18. | Pritchard GG, Coolbear T. The physiology and biochemistry of the proteolytic system in lactic acid bacteria. FEMS Microbiol Rev. 1993;12:179-206. [PubMed] |
| 19. | Coconnier MH, Lievin V, Hemery E, Servin AL. Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl Environ Microbiol. 1998;64:4573-4580. [PubMed] |
| 20. | Wilson M, Seymour R, Henderson B. Bacterial perturbation of cytokine networks. Infect Immun. 1998;66:2401-2409. [PubMed] |
| 21. | Crabtree JE, Lindley IJ. Mucosal interleukin-8 and Helicobacter pylori-associated gastroduodenal disease. Eur J Gastroenterol Hepatol. 1994;6 Suppl 1:S33-S38. [PubMed] |
| 22. | Prabjone R, Thong-Ngam D, Wisedopas N, Chatsuwan T, Patumraj S. Anti-inflammatory effects of Aloe vera on leukocyte-endothelium interaction in the gastric microcirculation of Helicobacter pylori-infected rats. Clin Hemorheol Microcirc. 2006;35:359-366. [PubMed] |
| 23. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [PubMed] |
| 24. | Azuma T, Ohtani M, Yamazaki Y, Higashi H, Hatakeyama M. Meta-analysis of the relationship between CagA seropositivity and gastric cancer. Gastroenterology. 2004;126:1926-1927; author reply 1926-1927. [PubMed] |
| 25. | Zhang Q, Dawodu JB, Etolhi G, Husain A, Gemmell CG, Russell RI. Relationship between the mucosal production of reactive oxygen radicals and density of Helicobacter pylori in patients with duodenal ulcer. Eur J Gastroenterol Hepatol. 1997;9:261-265. [PubMed] |
| 26. | Cover TL, Krishna US, Israel DA, Peek RM. Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 2003;63:951-957. [PubMed] |
| 27. | Cabral MM, Mendes CM, Castro LP, Cartelle CT, Guerra J, Queiroz DM, Nogueira AM. Apoptosis in Helicobacter pylori gastritis is related to cagA status. Helicobacter. 2006;11:469-476. [PubMed] |
| 28. | Johnson-Henry KC, Mitchell DJ, Avitzur Y, Galindo-Mata E, Jones NL, Sherman PM. Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-infected mice. Dig Dis Sci. 2004;49:1095-1102. [PubMed] |
| 29. | Peña JA, Versalovic J. Lactobacillus rhamnosus GG decreases TNF-alpha production in lipopolysaccharide-activated murine macrophages by a contact-independent mechanism. Cell Microbiol. 2003;5:277-285. [PubMed] |
| 30. | Truusalu K, Mikelsaar RH, Naaber P, Karki T, Kullisaar T, Zilmer M, Mikelsaar M. Eradication of Salmonella Typhimurium infection in a murine model of typhoid fever with the combination of probiotic Lactobacillus fermentum ME-3 and ofloxacin. BMC Microbiol. 2008;8:132. [PubMed] |
| 31. | Zhang L, Li N, Caicedo R, Neu J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-alpha-induced interleukin-8 production in Caco-2 cells. J Nutr. 2005;135:1752-1756. [PubMed] |
| 32. | Lam EK, Yu L, Wong HP, Wu WK, Shin VY, Tai EK, So WH, Woo PC, Cho CH. Probiotic Lactobacillus rhamnosus GG enhances gastric ulcer healing in rats. Eur J Pharmacol. 2007;565:171-179. [PubMed] |
Peer reviewer: Francesco Luzza, Professor, Dipartimento di Medicina Sperimentale e Clini, Università di Catanzaro, Via Tommaso Campanella 115, 88100 Catanzaro, Italy
S- Editor Gou SX L- Editor Webster JR E- Editor Li JY