Published online Jan 14, 2012. doi: 10.3748/wjg.v18.i2.136
Revised: June 21, 2011
Accepted: June 28, 2011
Published online: January 14, 2012
AIM: To develop orthotopic gastric cancer mouse models from different cell lines and characterize the tumor features to assist further in preclinical trials and clinical treatment strategies.
METHODS: Human gastric cancer SGC-7901 and BGC-823 cell suspensions were injected subcutaneously into nude mice to develop solid tumors, and tumor tissue pieces were then implanted under the serous coat of the stomach. An autopsy was performed on all animals of the SGC-7901 and BGC-823 models to observe the primary tumor growth and metastases using pathological and immunohistochemical methods.
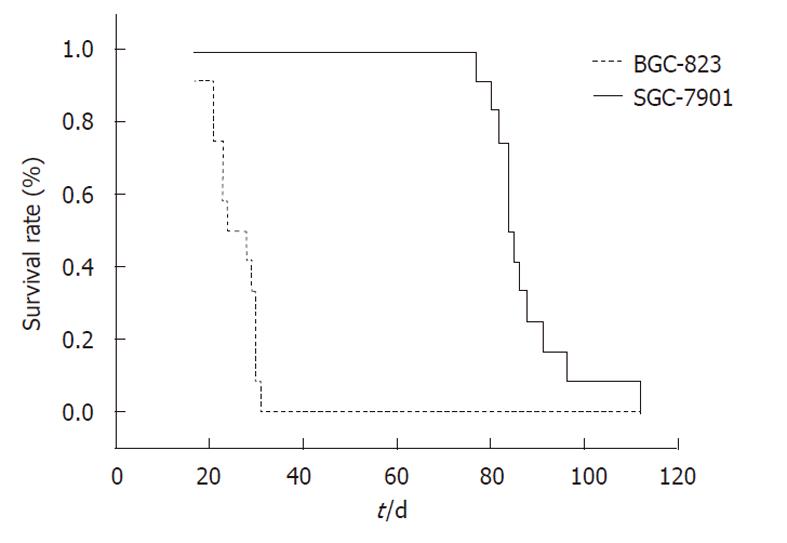
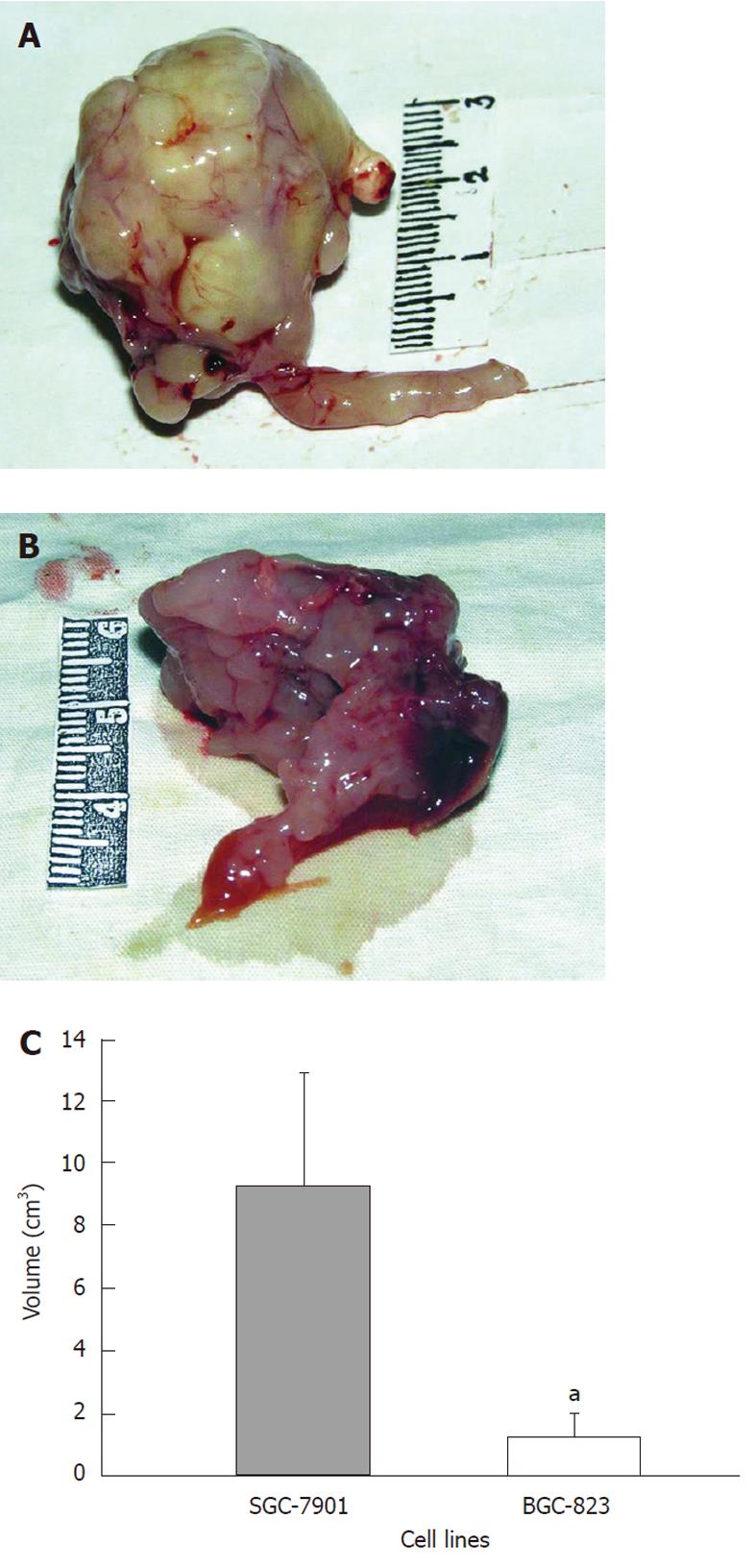
RESULTS: Both models showed large tumors in situ resulting in pressure and infiltration of the adjacent organs. The gastric cavity became smaller, along with stenosis of the cardia or pylorus. There were biological and statistical differences between the two models. The metastasis rate in involved organs (lymph nodes, kidney, spleen, testis) was significantly higher in the BGC-823 model compared to the SGC-7901 model (P < 0.05 or P < 0.01). The median survival of the BGC-823 model was shorter than that of SGC-7901 (23 d vs 84 d, P < 0.05). Histopathologically, the primary tumor and metastatic lesions of the two models showed obvious atypia and mucus in the cytoplasm. Compared with the SGC-7901 model, BGC-823 appeared more poorly differentiated (absence of adenoid structure), had a smaller volume, and richer capillary structure. Immunohistochemical staining revealed cytokeratin 20 and epithelial membrane antigen expression was positive in the SGC-7901 tumors, while negative in BGC-823 ones.
CONCLUSION: Models using the SGC-7901 and BGC-823 cell lines were established which could function in gastric cancer research on carcinogenesis mechanism and drug discovery. The two models showed different tumor behavior and the latter was more malignant than the former.
- Citation: Li Y, Li B, Xiang CP, Zhang Y, Li YY, Wu XL. Characterization of gastric cancer models from different cell lines orthotopically constructed using improved implantation techniques. World J Gastroenterol 2012; 18(2): 136-143
- URL: https://www.wjgnet.com/1007-9327/full/v18/i2/136.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i2.136
Gastric cancer is still the second most common malignant tumor in the world, and patients with gastric cancer generally have metastasis when clinically examined. There is a low five-year survival rate and poor quality of life even after tumor resection. We need to develop animal models of gastric cancer for exploring the mechanisms of carcinogenesis and clinical treatment strategy. Since the 1990s, orthotopic implantation techniques have gained popularity in establishing animal models of various tumors[1-4]. Many researchers have constructed orthotopic models of gastric cancer with different methods[5-11]. Of these methods, orthotopic transplantation using tumor pieces is useful because it not only simulates clinical cancer behavior but also promotes metastasis[12]. In the past, researchers mainly employed the “sewing” method to develop stomach cancer models[6,13]. However, there are disadvantages; such as a difficult operation, a time-consuming process, and low survival rates, and the sewing technique is not commonly used. In recent years, the procedure has been improved using tissue adhesive adhering to tumor pieces, which greatly facilitates surgical performance and decreases the mortality rate resulting from surgical operation[5,14]. Based on previous research, we intended to develop gastric cancer models from the SGC-7901 and BGC-823 cell lines and observe their biological characteristics to aid preclinical trials and therapy strategies. The human gastric cell line SGC-7901 was first established from the metastatic lymph node of a 56-year-old female patient suffering gastric adenocarcinoma. The BGC-823 cell line was derived from a specimen from a male patient who was 62 years of age, which was conserved in the Tumor Institute of Tianjin in China. The two gastric cell lines are poorly differentiated. This is the first time that a gastric cancer model of BGC-823 cell line was orthotopically constructed with histological tumor tissue via the “adhering” method.
Human gastric cancer cell lines SGC-7901 and BGC-823 were used for this study. The cells were purchased from the Centre of Cell Cultures of Chinese Academy of Medical Sciences, Shanghai, China, and cultured at 37 °C in a humidified atmosphere with 5% CO2, in RPMI-1640 medium (Gibco, Grand Island, NY) supplemented with 10% fetal calf serum (FCS; Gibco, Grand Island, NY), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L glutamine and 1 mmol/L sodium pyruvate. Cells were maintained by serial passaging after trypsinization with 0.1% trypsin.
Five- to six-week-old male Balb/c nu-nu mice (weight 18-20 g) were obtained from the Shanghai Experimental Animal Center of the Chinese Academy of Medical Sciences, China. They were kept in cages in a pathogen-free environment (temperature 25 °C-27 °C, humidity 45%-50%) and supplied with food and water ad libitum. All animal experiments were approved by the ethical committee of the Chongqing Medical University, and conformed to National and International Policies on Human Care and Use of Laboratory Animals.
Subcutaneous tumors were grown and then tumor tissue pieces used in surgical orthotopic implantation (SOI). Two cell lines were collected in the log phase and injected subcutaneously at 107/0.2 mL into the bilateral croup of Balb/c nu-nu mice. After 2 wk, the resulting tumors from the croup were harvested under strict aseptic conditions following removal of necrotic tissue from the central tumor areas, and cut into small pieces of approximately 1 mm3.
Nude mice were divided into 2 groups of 12 animals each, and were explanted with tumor fragments[5] from the SCG-7901 and BGC-823 cell lines, respectively. All procedures were performed under anesthesia with Sumianxin II (0.02 mL per animal; China Academy of Military Medical Science) at the benchtop. After a left-side upper abdominal incision was made, the stomach of the nude mouse was gently exteriorized. One small tissue pocket was prepared in the middle wall of the greater curvature using an ophthalmic scissor, and then one tumor piece was placed into the pocket following fixation with a drop of medical tissue adhesive (gifts from Shunkang Corporation of Biological Adhesive, Beijing, China). To avoid adhesion to adjacent normal tissue, the quantity of the tissue adhesive was strictly measured. The stomach was then returned to the peritoneal cavity, and the abdominal wall was closed with 4-0 absorbable sutures. The mice were given special care and fed in cages as usual after surgery.
All mice of the two groups were closely observed. At time of death, an autopsy was carried out to examine the tumor growth. The volume of primary tumor was calculated by the following formula: V = 0.4 × ab2 (a: maximum diameter; b: minimum diameter)[15]. Primary tumor, lymph nodes (gastroepiploic plexus, hilus pulmonis and mesenterium), and other organs (liver, lung, kidney, testis, spleen, etc.) involving infiltration or metastasis were sampled. The sampled tissues were fixed in 10% formalin, embedded in paraffin, sectioned to 3 μm thick, and stained with hematoxylin and eosin for microscopic examination. The ascitic fluid was centrifuged (1000 r, 5 min), and a cytologic smear was prepared for microscopic examination of malignant tumor cells.
The expression of cytokeratin (CK; High MW), cytokeratin 20 (CK-20) and epithelial membrane antigen (EMA) was studied by using mAbs 34βE12, KS20.8 and GP1.4 (MAB-0052, 0057, 0061; Maixin Inc., Fuzhou, China). An ultrasensitive SP kit (KIT-9710; Maixin Inc., Fuzhou, China) and a DAB kit (DAB-0031; Maixin Inc., Fuzhou, China) were employed according to the manufacturer’s instructions. The expression of CK, CK-20 and EMA proteins was defined as positive if the stained area of tumor cells was predominant in their cytoplasm.
Data are presented as mean ± SD. The median survival was analyzed using the Wilcoxon Rank-Sum test. The volume of primary tumor was analyzed by the student t test. The incidence of metastasis in both groups was analyzed using Fisher exact test. Differences were judged statistically significant at a P value < 0.05.
The tumor uptake rate after orthotopic implantation in both of the two groups was 100% (12/12). The median survival for the SGC-7901 and BGC-823 groups were 84 d and 23 d, respectively (P < 0.05, Figure 1). Mice of the SGC-7901 group had obvious cachexia (emaciation, retardation, ascites formation, etc.), whereas none of the BGC-823 group showed a decline in their general health.
The average tumor volume of the BGC-823 group was 1.24 ± 0.73 cm3, while it was 9.30 ± 3.62 cm3 in the SGC-7901 group (Figure 2A and B). There was a statistical significance in the difference (P < 0.05, Figure 2C). In addition, observations of the primary tumor and related characteristics in the two groups are presented in Table 1 in detail.
| Items | SGC-7901 group | BGC-823 group | |
| Primary tumor | Shape | Irregularly lobular | Irregularly lobular |
| Color | Grayish yellow | Gray-white | |
| Texture | Stiffer | Softer | |
| Vascularity | Rich | Richer | |
| Section of stomach tumor | Gastric cavity | Narrow or vanished | Narrow or vanished |
| Greater and lesser curvature | Undistinguishable | Undistinguishable | |
| Pylorus | Partly or totally obstructed | Totally obstructed | |
| Effects on adjacent organs | Abdominal cavity | Occupying whole abdomen | Occupying upper abdomen |
| Adhering extent | Adhering to liver lobes | Adhering to many organs | |
| Compressed status | Severely compressed | Partly compressed | |
| Ascites | Liquid quantity | Little | Much |
| Property | Clear and yellowish | Bloody fluid | |
Metastasis, which was always located in lymph nodes, liver, kidney, lung, peritoneum or diaphragm, occurred in both groups of gastric cancer models. Additionally, other organs (spleen and testicle) were also found to have metastatic lesions in the BGC-823 group. The incidence of metastasis from various organs in the BGC-823 group was higher than that in the SGC-7901 group, and these differences (except for liver, lung, peritoneum or diaphragm) were considered statistically significant (P < 0.05 or P < 0.01, Table 2).
The two groups differed in histological appearance of subcutaneous tumor samples, though the cell lines forming tumors were both poorly differentiated adenocarcinomas. In the SGC-7901 group, tumor cells spread with nest-like structures, characterized by nuclear polymorphism, nuclear hyperchromatism, many red nucleoli, and rich pathological karyokinesis. Mucus was observed in the cytoplasm of part of the tumor cells which resulted in mucus lakes, and adenoid structures and rich blood vessels formed in tumor areas. In the BGC-823 group, histopathologic examination confirmed a different phenotype of the BGC-823 tumor compared to the SGC-7901 tumor. The tumor cells mostly showed medullary growth with characteristics of ample cells, fewer fibroblasts, and rich vascularity. The neoplasm revealed the signs of undifferentiated structure, small size, nuclear hyperchromatism, and rich pathological karyokinesis. Mucus was found in the cytoplasm of part of the neoplastic cells, but there was an absence of glandular differentiation.
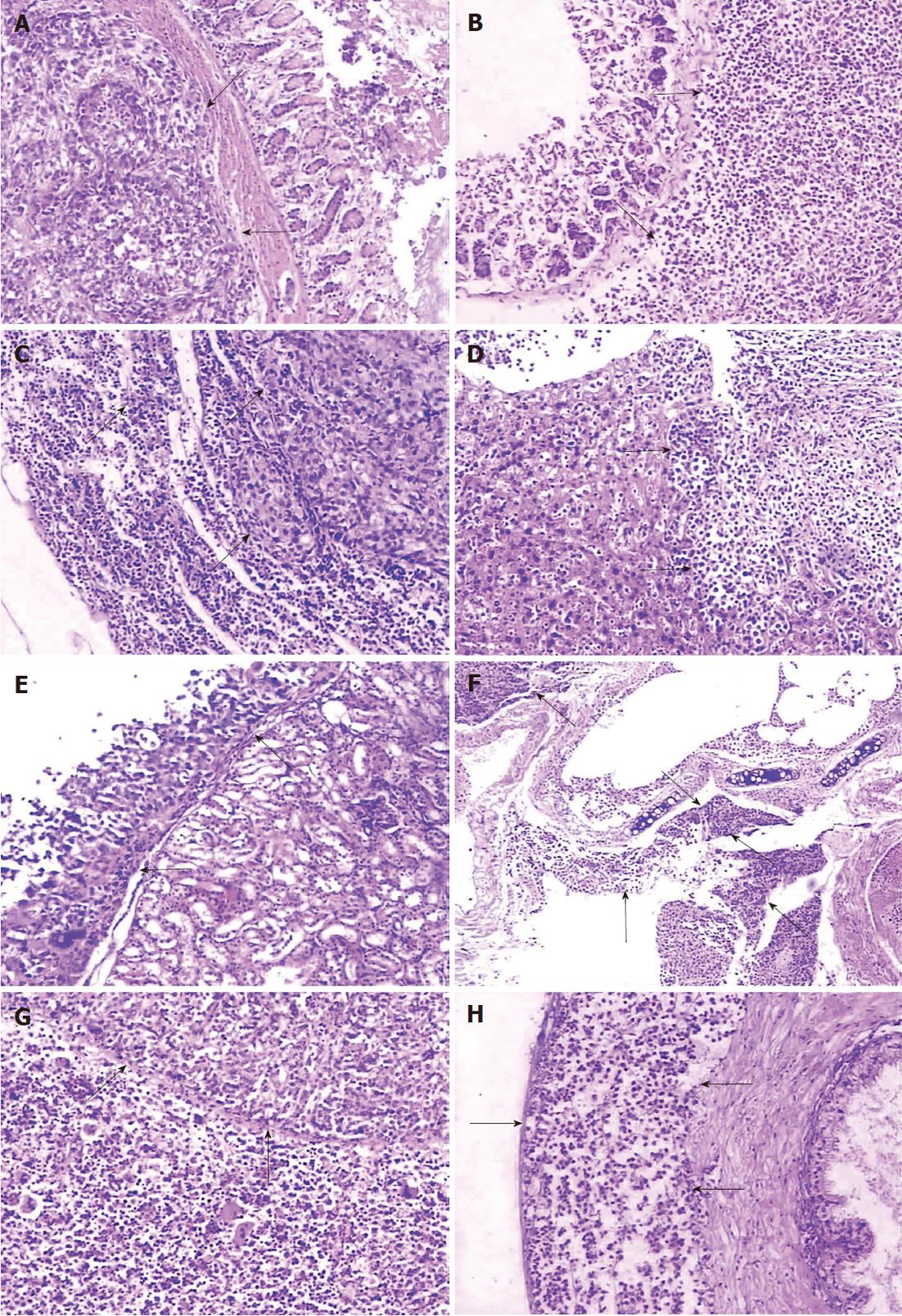
Both primary tumors and metastases, whose tumor cells were microscopically identical to the subcutaneous tumor, of the two group models showed similarity in histopathologic characteristics. The stomach cancer of the two models infiltrated the various layers of gastric wall with disruption of the integrity of the mucous layer or muscularis mucosae (Figure 3A and B). Widespread infiltration of tumor cells was found in the subcapsular, the cortical and medullary areas of lymph nodes, while extension into the medullary parts the lymph sinus was diminished (Figure 3C). In some of the lymph nodes, the nodal parenchyma was even totally replaced by neoplastic tumor. Metastases were detected in the liver (Figure 3D) and the kidney (Figure 3E) with nest-like structures or a glandular appearance, occasionally in the adrenal gland or pancreas (not shown). Metastatic lesions were always separated from adjacent normal tissue by fibrous capsules, and lymphoid infiltration in peripheral tumor areas was also seen. Tumors metastasized to the lung and destroyed bronchi or bronchioles (Figure 3F). In addition, local invasion was observed within the abdominal muscle or diaphragmatic muscle. Organs such as the spleen and the testicle (Figure 3G and H) were not spared in the BGC-823 models. Histological examination revealed that the spleen parenchyma was infiltrated and the seminiferous tubules of the testis or ductus epididymidis, and the prostate, were invaded by the metastatic tumor.
Smears of cast-off cells from ascites in both groups confirmed that the malignant cells originated from the primary adenocarcinoma (not shown).
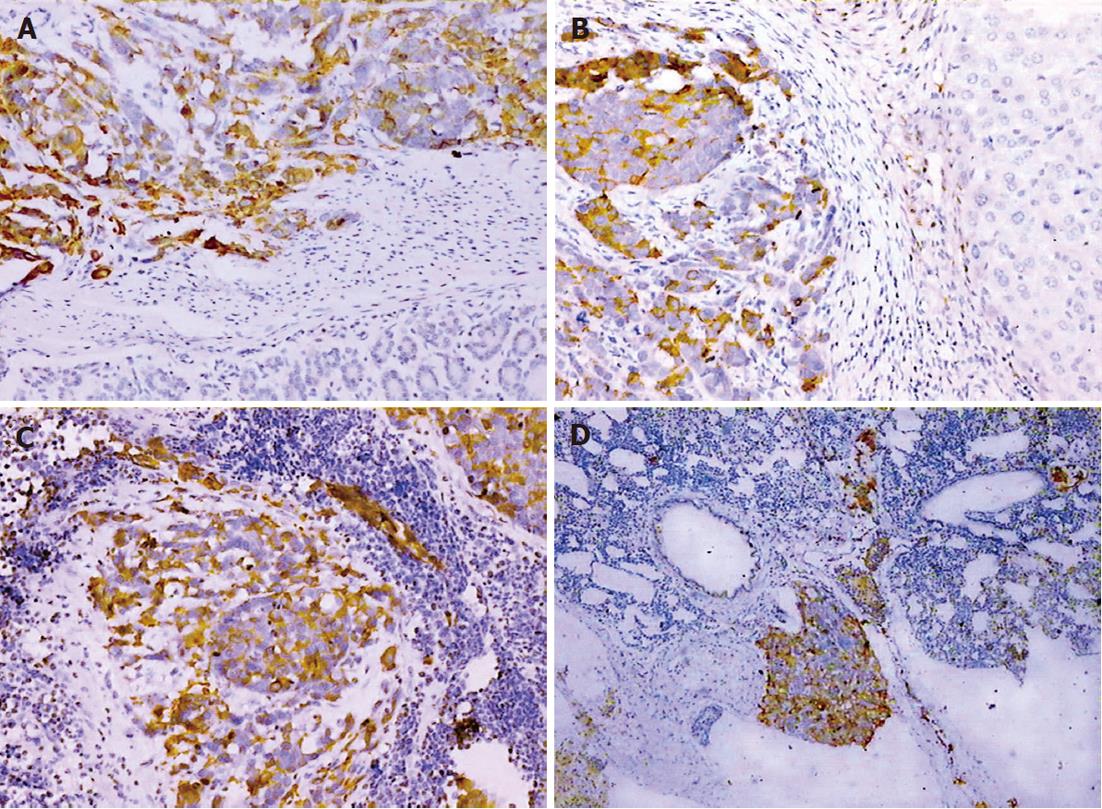
The expression of CK-20 and EMA protein was positive within the primary tumor and metastases in the SGC-7901 group, whereas CK expression was negative in the same tissues (Figure 4). In the BGC-823 group, CK, CK-20 and EMA immunostaining were negative.
The purpose of our study was to develop gastric cancer models from different cell lines with intact tumor pieces orthotopically implanted into the stomach wall by tissue glue adhesion. In the present study, we could replicate similar results of the SCG-7901 orthotopic models which have been established by other researchers in China. This is the first time that an animal model has been created from the BGC-823 gastric cancer cell line with SOI of tumor tissue.
Interestingly, we find the two models reveal significant differences in biological behavior. It has been reported that heterogeneity resulting from differences of histopathological type, grade, and individual factors reside in different tumor cell lines[5,16]. Thus, animal models derived from different tumor cell lines present different characteristics. Bhargava et al[5] orthotopically established gastric cancer models in two sites of the stomach using tumor fragments from three cell lines of differently differentiated extent. The findings showed that the tumor uptake rate and metastases were all different among the three cell lines, between two sites, and at various time points. Fujihara et al[13] developed a gastric cancer cell line subtype named OCUM-2M LN, characterized by high incidence of lymph node metastasis, which was obtained from the OCUM-2M cell line by serial passage selection in animals. The ability to metastasize to lymph nodes in subtype OCUM-2M LN was significantly higher than that in parental cell line OCUM-2M. In keeping with these authors above, we also found that heterogeneity among different cell lines contributed to differences of tumor behavior in both gastric cancer models.
Histopathological characteristics of malignant neoplasms were reflected in both cancer models, characterized by neoplastic atypia of varying degrees, and rich blood vessels promoting tumor growth and metastasis. However, the two types of tumor showed differences in pathologic phenotype. The SGC-7901 tumors showed nest-like structures and glandular differentiation, while the BGC-823 tumors presented medullary structures and no adenoid differentiation, which might be explained by the fact that the BGC-823 cell line develops variation and partly changed neoplastic properties during passage[9,13]. Microscopically, mucous secretion in both types of tumor cells, especially in the SGC-7901 tumor, was consistent with the characteristics of mucous adenocarcinoma. Meanwhile, the negative result of CK expression in the two models also excluded the differentiation of squamous carcinoma. The expression of CK-20 and EMA by immunohistochemical staining was positive in the SGC-7901 tumors, but negative in BGC-823. Histopathology and immunohistochemistry analysis all confirmed that the BGC-823 carcinoma had poorer differentiation than SGC-7901. In addition, all epithelial markers in BGC-823 tumors were negative which might suggest an epithelial-to-mesenchymal transition phase. We shall perform immunohistochemical staining with a mesenchymal marker, such as vimentin, to verify this presumption in our next experiments. It is reported that survival prognosis has close correlation with histopathologic grading of tumor[17-19]. The results in our study demonstrated that median survival course in the BGC-823 model was shorter than that in SGC-7901 (P < 0.05), which is compatible with histological examination.
Retrospective studies have suggested that there is cor-relation between tumor volume size and lymph node metastasis or five-year survival[19-22]. In our experimental results, the original tumor in both models presented a large-volume lesion invading adjacent organs and destroying the stomach cavity. There is no theoretical significance regarding difference of the tumor volume in the SGC-7901 and BGC-823 groups, though the former is obviously larger than the latter (P < 0.05) mainly resulting from the different course of primary tumor growth. However, the SGC-7901 tumor texture was harder than that of BGC-823, which was possibly linked with rich stromal component in the former but ample tumor cells and rich blood vessels in the latter. Angiogenesis is a prerequisite for metastatic spread[15,23], which also explains why vascular metastasis is a frequent occurrence in the BGC-823 model.
Metastasis is not only linked with the prognosis of patients but also the dilemma of cancer therapy. In the field of experimental research, metastatic events are assumed to cover the factors of the anatomic site of implantation[12,13,24,25] or status (suspension or fragment) of tumor sample used in implantation[10,23]. Orthotopic transplantation in animal models of various cancers has played a key role in mimicking the clinical tumor behavior[5,23,24]. There has been variable success at achieving good tumor uptake rate and metastatic incidence with implantation procedures using suspension injections and tumor pieces[10,23]. Many studies indicate that the properties on the tumor cell surfaces are preserved by the implantation of tumor pieces, but destroyed after the cell suspensions have been treated by trypsinization resulting in changes of malignant character and further contributing to decline of tumor growth and metastatic rate[13,24]. In the present study, the models of orthotopic implantation into the gastric wall with intact tumor fragments showed not only high uptake rate but also metastasis through various pathways, which was consistent with the previous research. It is well known that the general metastasis in terminal patients with gastric cancer mainly involves direct infiltration, lymphatic metastasis, vascular spread, and implantation dissemination. The aggressive behavior in the two model groups resembles clinical patients suffering from gastric cancer. In the SGC-7901 group, direct infiltration and lymphatic metastasis were frequently observed. However, multiple steps were assumed to be involved in the metastases of the BGC-823 group. In BGC-823 models, we could not identify the accurate pathway of metastasis through local invasion in the liver, kidney, spleen and testicle. The total involvement of multiple steps facilitating metastases requires further studies.
Compared with the SGC-7901 group, metastasis occurrence is earlier (P < 0.05), metastasis incidence in different organs higher (P < 0.05), and the number of involved organs greater in the BGC-823 group. The deaths in the SGC-7901 group were considered as multi-organ failure caused by cachexia, while the BGC-823 mice mostly died of early metastases and high metastatic rates without visible cachexia. Taken together, the results in our study demonstrate that the BGC-823 gastric cancer model is more aggressive than the SGC-7901 one with the support of the comprehensive factors discussed above.
We have successfully developed two types of gastric cancer model with different tumor behavior by orthotopic implantation, which are characterized by advantages such as low cost and resemblance to clinical gastric cancer. The two models are both suitable for preclinical research on gastric cancer, and their different characteristics may aid with different needs of experimental research. Considering comprehensive factors, surgical orthotopic implantation is still a desirable technique compared with other methods of constructing gastric cancer models[26-29].
Gastric cancer is still the most common malignant tumor in the world, and detection is difficult before metastasis occurrence, so people need to develop animal models of gastric cancer for exploring the mechanisms of carcinogenesis and clinical treatment strategy. Gastric cancer models of orthotopic implantation with intact tumor tissue have been well established. Moreover, orthotopic implantation performance has been improved from the “sewing” method to the “adhering” one. SCG-7901 orthotopic models have been established by other researchers, whereas this is the first time that an animal model was created from the BGC-823 gastric cancer cell line with surgical orthotopic implantation of tumor tissue.
Orthotopic implantation technique plays an important role in establishing an animal model of various tumors, including gastric cancer, which can not only simulate clinical cancer development but also facilitate metastasis occurrence. Moreover, the procedure of orthotopic implantation has been improved from the “sewing” method to the “adhering” one. The latter’s performance has greatly simplified the surgical operation course. In addition, the BGC-823 gastric cancer model has not been constructed by others, and shows different tumor characteristics compares with the SCG-7901 model.
Orthotopic implantation with “glue paste technique” is a new, popular and convenient method used for the establishment of orthotopic animal models in recent years. The results regarding the BGC-823 gastric cancer model in this study are the first to be reported, and make it possible that researchers can learn the different biological characteristics of gastric cancer models from different cell lines.
Establishment of the BGC-823 gastric cancer model provides evidence for researchers to learn the biological differences between animal models from different gastric cancer cell lines, which can help them to choose an appropriate model to tailor their research.
Surgical orthotopic implantation is defined as tumor cells in the form of suspension or fragments which are orthotopically implanted into the related organs.
The study is aimed to develop gastric cancer models from different cell lines and analyze the difference of biological behavior in many aspects, in order to provide evidence for researchers to choose an appropriate experimental carrier and therapy target for clinical trials. The present research is valuable for clinical doctors and researchers.
| 1. | Rashidi B, Gamagami R, Sasson A, Sun FX, Geller J, Moossa AR, Hoffman RM. An orthotopic mouse model of remetastasis of human colon cancer liver metastasis. Clin Cancer Res. 2000;6:2556-2561. [PubMed] |
| 2. | Yang M, Hasegawa S, Jiang P, Wang X, Tan Y, Chishima T, Shimada H, Moossa AR, Hoffman RM. Widespread skeletal metastatic potential of human lung cancer revealed by green fluorescent protein expression. Cancer Res. 1998;58:4217-4221. [PubMed] |
| 3. | Kurebayashi J, Nukatsuka M, Fujioka A, Saito H, Takeda S, Unemi N, Fukumori H, Kurosumi M, Sonoo H, Dickson RB. Postsurgical oral administration of uracil and tegafur inhibits progression of micrometastasis of human breast cancer cells in nude mice. Clin Cancer Res. 1997;3:653-659. [PubMed] |
| 4. | Pettaway CA, Pathak S, Greene G, Ramirez E, Wilson MR, Killion JJ, Fidler IJ. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res. 1996;2:1627-1636. [PubMed] |
| 5. | Bhargava S, Hotz B, Buhr HJ, Hotz HG. An orthotopic nude mouse model for preclinical research of gastric cardia cancer. Int J Colorectal Dis. 2009;24:31-39. [PubMed] |
| 6. | Illert B, Otto C, Braendlein S, Thiede A, Timmermann W. Optimization of a metastasizing human gastric cancer model in nude mice. Microsurgery. 2003;23:508-512. [PubMed] |
| 7. | Yang B, Tuo S, Tuo CW, Zhang N, Liu QZ. A liver-metastatic model of human primary gastric lymphoma in nude mice orthotopically constructed by using histologically intact patient specimens. Chin J Cancer. 2010;29:579-584. [PubMed] |
| 8. | Illert B, Otto C, Thiede A, Timmermann W. Detection of disseminated tumor cells in nude mice with human gastric cancer. Clin Exp Metastasis. 2003;20:549-554. [PubMed] |
| 9. | Takemura S, Yashiro M, Sunami T, Tendo M, Hirakawa K. Novel models for human scirrhous gastric carcinoma in vivo. Cancer Sci. 2004;95:893-900. [PubMed] |
| 10. | Furukawa T, Fu X, Kubota T, Watanabe M, Kitajima M, Hoffman . Nude mouse metastatic models of human stomach cancer constructed using orthotopic implantation of histologically intact tissue. Cancer Res. 1993;53:1204-1208. |
| 11. | Koyama T, Tsubota A, Nariai K, Yoshikawa T, Mitsunaga M, Sumi M, Nimura H, Yanaga K, Yumoto Y, Mabashi Y. Detection of sentinel nodes by a novel red-fluorescent dye, ATX-S10Na (II), in an orthotopic xenograft rat model of human gastric carcinoma. Lasers Surg Med. 2007;39:76-82. [PubMed] |
| 12. | Kozlowski JM, Fidler IJ, Campbell D, Xu ZL, Kaighn ME, Hart IR. Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res. 1984;44:3522-3529. [PubMed] |
| 13. | Fujihara T, Sawada T, Hirakawa K, Chung YS, Yashiro M, Inoue T, Sowa M. Establishment of lymph node metastatic model for human gastric cancer in nude mice and analysis of factors associated with metastasis. Clin Exp Metastasis. 1998;16:389-398. [PubMed] |
| 14. | Shi J, Wei PK, Zhang S, Qin ZF, Li J, Sun DZ, Xiao Y, Yu ZH, Lin HM, Zheng GJ. OB glue paste technique for establishing nude mouse human gastric cancer orthotopic transplantation models. World J Gastroenterol. 2008;14:4800-4804. [PubMed] |
| 15. | Kyriazis AP, Kyriazis AA, McCombs WB, Kereiakes JA. Biological behavior of human malignant tumors grown in the nude mouse. Cancer Res. 1981;41:3995-4000. [PubMed] |
| 16. | Hanna N, Fidler IJ. Expression of metastatic potential of allogeneic and xenogeneic neoplasms in young nude mice. Cancer Res. 1981;41:438-444. |
| 17. | Archie V, Kauh J, Jones DV, Cruz V, Karpeh MS, Thomas CR. Gastric cancer: standards for the 21st century. Crit Rev Oncol Hematol. 2006;57:123-131. [PubMed] |
| 18. | Shiraishi N, Sato K, Yasuda K, Inomata M, Kitano S. Multivariate prognostic study on large gastric cancer. J Surg Oncol. 2007;96:14-18. [PubMed] |
| 19. | de Liano AD, Yarnoz C, Aguilar R, Atieda C, Ortiz H. Surgical treatment of recurrent gastric cancer. Gastric Cancer. 2008;11:10-14. |
| 20. | Msika S, Benhamiche AM, Jouve JL, Rat P, Faivre J. Prognostic factors after curative resection for gastric cancer. A population-based study. Eur J Cancer. 2000;36:390-396. [PubMed] |
| 21. | Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449-461. [PubMed] |
| 22. | Yokota T, Kunii Y, Teshima S, Yamada Y, Saito T, Takahashi M, Kikuchi S, Yamauchi H. Significant prognostic factors in patients with early gastric cancer. Int Surg. 2000;85:286-290. [PubMed] |
| 23. | An Z, Jiang P, Wang X, Moossa AR, Hoffman RM. Development of a high metastatic orthotopic model of human renal cell carcinoma in nude mice: benefits of fragment implantation compared to cell-suspension injection. Clin Exp Metastasis. 1999;17:265-270. [PubMed] |
| 24. | Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci USA. 1992;89:5645-5649. |
| 25. | Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs. 1999;17:343-359. [PubMed] |
| 26. | Kossoy G, Ben-Hur H, Elhayany A, Schneider DF, Zusman I. The morphological pathway for mouse forestomach cancer. Oncol Rep. 2006;15:479-483. |
| 27. | Pritchard DM, Przemeck SM. Review article: How useful are the rodent animal models of gastric adenocarcinoma? Aliment Pharmacol Ther. 2004;19:841-859. [PubMed] |
| 28. | Tsuzuki T, Egashira A, Igarashi H, Iwakuma T, Nakatsuru Y, Tominaga Y, Kawate H, Nakao K, Nakamura K, Ide F. Spontaneous tumorigenesis in mice defective in the MTH1 gene encoding 8-oxo-dGTPase. Proc Natl Acad Sci USA. 2001;98:11456-11461. [PubMed] |
| 29. | Koike K, Hinrichs SH, Isselbacher KJ, Jay G. Transgenic mouse model for human gastric carcinoma. Proc Natl Acad Sci USA. 1989;86:5615-5619. [PubMed] |
Peer reviewer: Dr. Courtney Houchen, MD, The University of Oklahoma Health Sciences Center, 975 NE 10th Street, Oklahoma City, OK 73104, United States
S- Editor Sun H L- Editor Logan S E- Editor Li JY