Published online May 14, 2012. doi: 10.3748/wjg.v18.i18.2262
Revised: March 23, 2012
Accepted: March 29, 2012
Published online: May 14, 2012
AIM: To establish a rat ethanol gastritis model, we evaluated the effects of ethanol on gastric mucosa and studied the preventive effects of geranylgeranylacetone on ethanol-induced chronic gastritis.
METHODS: One hundred male Sprague-Dawley rats were randomly divided into 4 equal groups: normal control group, undergoing gastric perfusion of normal saline (NS) by gastrogavage; model control group and 2 model therapy groups that underwent gastric perfusion with ethanol (distillate spirits with 56% ethanol content) by gastrogavage for 4 wk. Low or high doses of geranylgeranylacetone were added 1 h before ethanol perfusion in the 2 model therapy groups, while the same amount of NS, instead of geranylgeranylacetone was used in that model control group. The rats were then sacrificed and stomachs were removed. The injury level of the gastric mucosa was observed by light and electron microscopy, and the levels of prostaglandin 2 (PGE2), endothelin-1 (ET-1) and nitric oxide (NO) were measured by radioimmunoassay and the Griess method.
RESULTS: The gastric mucosal epidermal damage score (EDS; 4.5) and ulcer index (UI; 12.0) of the model control group were significantly higher than that of the normal control group (0 and 0 respectively, all P = 0.000). The gastric mucosal EDS and UI of the 2 model therapy groups (EDS: 2.5 and 2.0; UI: 3.5 and 3.0) were significantly lower than that of the model control group (all P < 0.01). There was no statistically significant difference between the low-dose and high-dose model therapy groups. The expression value of plasma ET-1 of the model control group was higher than that of the normal control group (P < 0.01) and the 2 model therapy groups (all P < 0.01). The expression values of gastric mucosal PGE2 and serum NO of the model control group were lower than those of the normal control group (all P < 0.05) and the 2 model therapy groups (all P < 0.05). The thickness of the gastric mucous layerand the hexosamine content in the model control group were significantly lower than that in the normal control group (all P < 0.01) and the 2 model therapy groups (all P < 0.05). Scanning and transmission electron microscopy observation showed that in the model control group, the epithelial junctions were vague, the intercellular joints disappeared and damage of the intracellular organelles were significantly worse than those in the normal control group. However, in the 2 model therapy groups, damage to the intercellular joints and organelles was ameliorate relative to the model control group.
CONCLUSION: Administration of geranylgeranylacetone was correlated with a more favorable pattern of gastric mucosa damage after ethanol perfusion. The mechanism could be related to regulation of ET-1, NO and PGE2.
- Citation: Ning JW, Lin GB, Ji F, Xu J, Sharify N. Preventive effects of geranylgeranylacetone on rat ethanol-induced gastritis. World J Gastroenterol 2012; 18(18): 2262-2269
- URL: https://www.wjgnet.com/1007-9327/full/v18/i18/2262.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i18.2262
The liver is a main organ for ethanol metabolism. The gastrointestinal tract is also involved in the ethanol metabolic process. Research[1] has reported that binge drinking or long-term drinking can cause acute or chronic gastric mucosal injury. Ethanol can be converted into acetaldehyde in first-pass metabolism in the gastrointestinal tract, which may have a carcinogenic affect on the gastrointestinal tract through local toxic effects. Geranylgeranylacetone is a derivative of terpenes. Studies have shown that geranylgeranylacetone has a therapeutic effect on chronic gastritis, digestive ulcers and portal hypertensive gastropathy[2-5]. There have been few studies on the repair mechanisms of gastric mucosal damage caused by ethanol. By using a rat ethanol gastritis model, we aimed to study the effect and mechanisms of geranylgeranylacetone on repairing gastric mucosa by observing the histological and ultrastructure changes and detecting the expression levels of plasma endothelin-1 (ET-1), serum nitric oxide (NO) and gastric mucosal prostaglandin 2 (PGE2) in this research.
Adult male Sprague-Dawley (SD) rats, 8 wk old, weighing 200 ± 50 g, were purchased from the Animal Center of Zhejiang University of Traditional Chinese Medicine. They were fed in a specific pathogen free environment with 12 h of light a day with unlimited drinking water. Geranylgeranylacetone was manufactured by Japan Eisai Co., Ltd., and packed by the Suzhou Eisai Pharmaceutical Co. A kind of liquor (Trade name: Red Star Erguotou, with 56% ethanol, manufactured by a general Beijing brewing factory) was used for gastrogavage to establish an ethanol-induced gastritis model in rats. The ET-1 radioimmunoassay determination kit was purchased from Beijing North Institute of Biotechnology Technology. The PGE2 radioimmunoassay kit was purchased from the Beijing Huaying Biotechnology Research Institute.
Groups: One hundred adult male SD rats were randomly divided into four groups, including the normal control group, the model control group, the low-dose model therapy group (50 mg/kg) and the high-dose model therapy group (200 mg/kg). The ethanol gastritis model was built by following an established procedure[6]. Red Star Erguotou liquor with 56% ethanol content was used for feeding the laboratory rats by gastrogavage. On every Tuesday and Friday, the rats were fed with ethanol after fasting for 12 h (food was removed on every Monday and Thursday at 9 pm, and gastrogavage was performed on the next day at 9 am). The dose of ethanol was 8 g/kg body weight. The conversion formula was: weight of ethanol (A) = liquor volume (mL) × ethanol content (vol/vol) × ethanol density (8 g/kg). The normal control group received the same amount of normal saline instead of ethanol. All laboratory rats were administered treatment for 4 wk. In the model therapy group, geranylgeranylacetone was dissolved in pure water and was administered by gastrogavage 1 h before ethanol feeding each time. In the normal control and model control groups, only normal saline, instead of geranylgeranylacetone was administered.
Specimen collection: Observations were made of the reactivity, activity and death of rats in each group during the experiment. The animals were killed by cervical dislocation after administration of an overdose of sodium pentobarbital on the 4th weekend of the experiment. The abdomen was opeded immediately. The whole stomach was cut and removed 1.5 cm away from the cardia and the pylorus. Dissection was done along the greater curvature for general specimen observation. The obviously damaged gastric mucosa specimen was rinsed in cold saline solution. Then, the specimen was placed in formaldehyde and glutaraldehyde, and stored in liquid nitrogen solution for later observation. All operations of all specimens were assigned to the same experienced professional laboratory personnel. All animal studies were approved by the Animal Care and Use Committee of Zhejiang University in accordance with the Chinese guidelines for the care and use of laboratory animals.
Determination of gastric mucosal injury index: The length and the width of the injured gastric mucosa region were measured with a vernier caliper. The gastric mucosa ulcer index (UI) was determined according to the Guth standard[7]: spot erosion was recorded as 1 point, erosion length < 1 mm was recorded as 2 points, 1-2 mm was recorded as 3 points, 2-3 mm was recorded as 4 points, and > 3 mm was recorded as 5 points, the score doubled if the erosion width was > 1 mm.
Determination of the thickness of the gastric mucous layer and the mucus glycoprotein content: The thickness of the gastric mucous layer in each group was measured by converted fluorescence microscopy with a thick smear method (using an ink staining method to enhance the contrast). The thickness of the gastric mucous gel layer was detected by measuring the thickness of the centric bright area with a micrometer eyepiece. Detection of the levels of hexosamine (the main component of mucus glycoprotein) was performed by colorimetric assay using a spectrophotometer.
Histopathology: Four percent formalin-fixed gastric mucosa tissues were embedded by paraffin after gradient dehydration, 4 μm serial sections were obtained and HE staining was performed. We used the epithelial damage score (EDS) to rate morphological changes in the gastric mucosa under light microscopy: normal gastric mucosa was is recorded as 1 point, mucosal epithelial cell damage was recorded as 2 points, damage involving the glandular cells was recorded as 3 points, and mucosal erosion, bleeding or ulceration was recorded as 4 points. We observed a 1 cm length in each slice under light microscopy, and then calculated the cumulative score for each slice. Light microscopy was used to evaluate the degree of gastric damage, which was performed by two pathologists who were unaware of the treatment.
Observation of the ultrastructure of the gastric mucosa: We took 5 mm × 5 mm specimens close to the gastric antrum. According to the electron microscopy procedure, specimens were double fixed by 2.5% glutaraldehyde and 2% osmium tetroxide and subjected to conventional ethanol dehydration, and iso-amyl acetate transition. Critical point drying was carried out on a HCP-2 type critical point drying apparatus. The sample was stuck and a gilded target alloy was placed on the specimens using an IB-5 ion sputter coater. Specimens were observed on the sample stage of the electron microscope. We observed the cell morphology of the gastric mucosa, the junctions of the gastric mucosa epithelial cells, the shape of the gastric pit, and the ultrastructural changes of the intracellular mitochondria as well as the Golgi apparatus and other organelles in all of the laboratory rats by scanning electron microscopy and transmission electron microscopy.
Detection of the levels of serum nitric oxide, plasma endothelin-1, and gastric mucosal prostaglandin 2: We drawed 2 mL of blood from the abdominal aortic vein and injected it into the anticoagulant tube containing 30 μL 10% ethylenediaminetetraacetic acid disodium and 800 U aprotinin and mixed it well. We carried out centrifugal separation of the plasma at 4 °C at 3000 r/min for 30 min. Detection of plasma ET-1 levels and gastric mucosal PGE2 content was carried out by a radioimmunoassay method with strict attention to the instructions. Detection of serum NO levels was carried out by a chemiluminescence method (according to the Griess method).
All values are expressed as mean ± SE. The Tukey test or the Student’s t-test for unpaired results was used to evaluate differences between more than three groups or between two groups, respectively. Differences were considered to be significant for values of P < 0.05.
In the model control group, 3 of 25 rats died and most of the others were listless, had a bad appetite, and were unresponsive to external stimuli. In the normal control group and the model therapy groups, most of the rats had alert consciousness, normal appetites, agile responses to outside stimuli, and no deaths. The differences between the model control group and the other groups were significant.
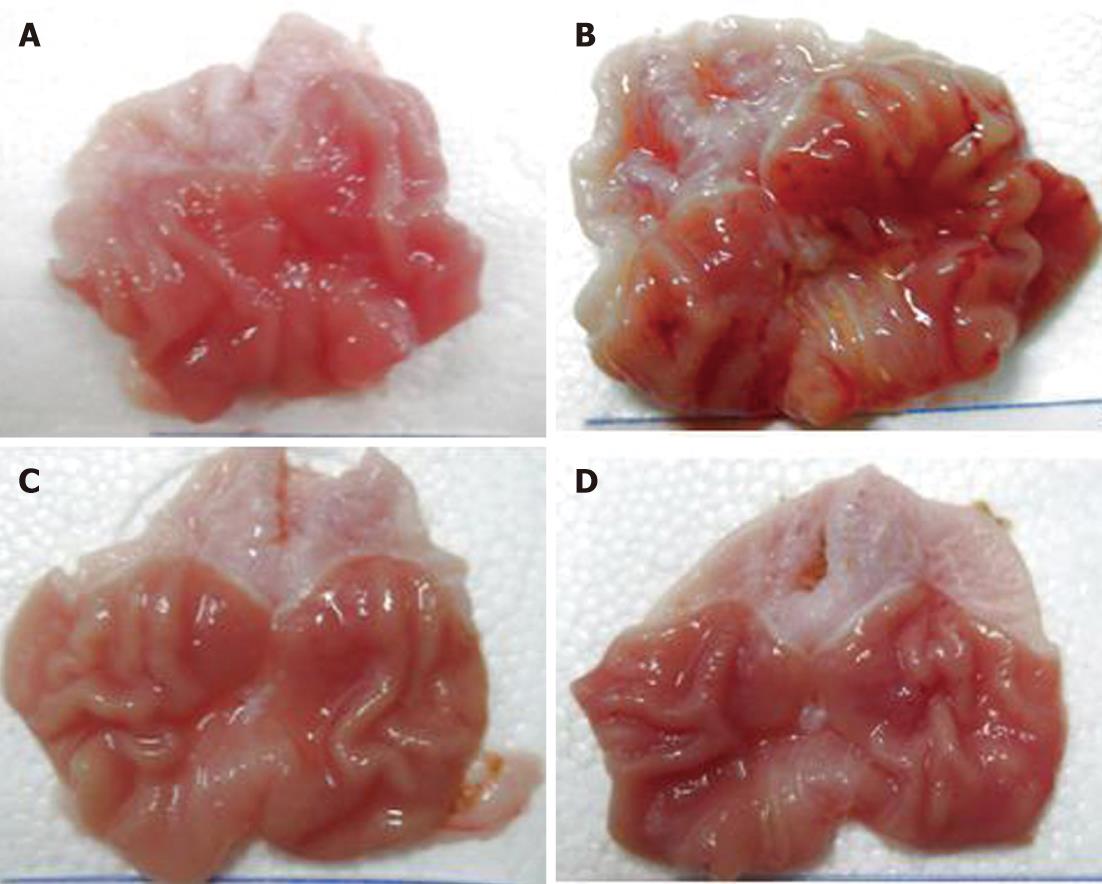
In the normal control group, there were only 2 rats (2/25, 8%) that had punctate erosion on the gastric body mucosa. The mucosa of the others was not damaged and the UI was 0. In the model control group, the erosion and ulcers on the gastric mucosa were obvious and the median ulcer index was 12.0, which was significantly different (P < 0.01) from the normal control group. The gastric mucosal damage of the low-dose and high-dose model therapy groups was significantly reduced and the UIs were decreased (3.5 and 3.0, respectively). The results were statistically significant difference (P < 0.01) compared with the model control group. But there was no statistically significant difference between the low-dose and high-dose model therapy groups(P> 0.05). In the model control group, the EDS values (median 4.5) were higher than that in the normal control group (median 0, P < 0.01). However, in the low-dose and high-dose therapy groups, the EDS values (median 2.5 and 2.0 respectively) were lower than the model control group (median 4.5, P < 0.05). The EDS values of the low-dose and the high-dose model therapy groups were not statistically significantly different (P > 0.05) (Figure 1 and Table 1).
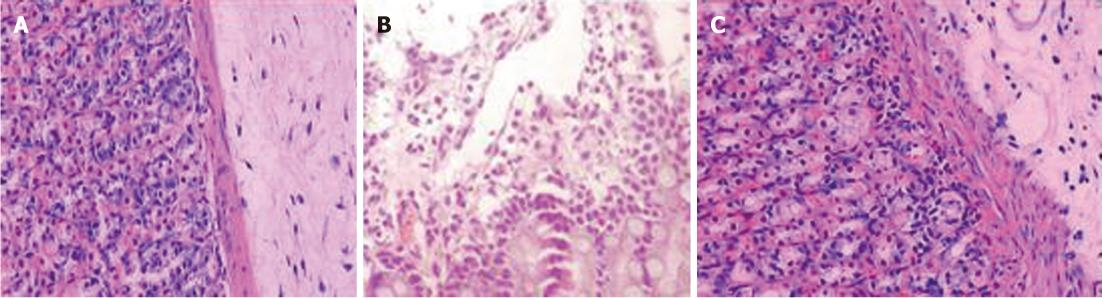
In the control group, the gastric mucosa was smooth, the layers of the gastric mucosa had clear boundaries under high-power microscopy, and there was no significant inflammatory cell infiltration and edema. In the model control groups, the gastric mucosal surface was uneven with erosion, ulcers and bleeding, and under high-power microscopy the gastric mucosa were congested and had edema. With telangiectasia, the surface mucus layers were damaged and the submucosal gastric glands were incomplete. In the model therapy groups, the gastric mucosal injuries were obviously slighter than the model control group. In the model control group, there was only scattered mucosal damage and local congestion. Under high-power microscopy, the gastric surface mucus layer in the model therapy groups was basically intact and the submucosal layers were slight congested and had less inflammatory cell infiltration, especially in the high-dose model therapy groups (Figure 2).
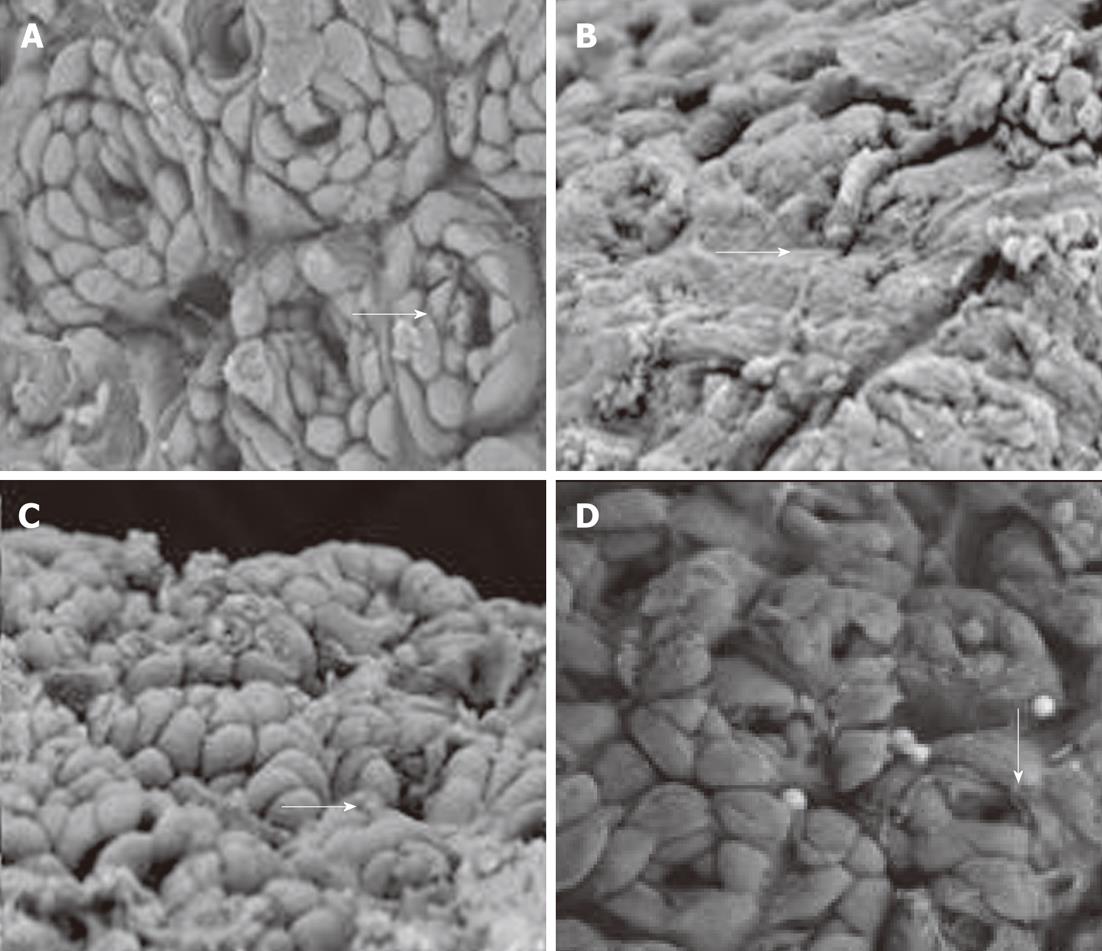
Ultrastructural changes under scanning electron microscopy: In the normal control group, the gastric mucosa epithelial cells were closely joined and were ring-wise arranged around the gastric gland openings. The gastric pits were clear with ordered cells (according to the arrow). The model control group showed extensive gastric epithelial cell loss, disappearance of gastric pits, and revealed the glandular epithelium (according to the arrow). In the low-dose and high-dose model therapy groups, the gastric epithelial cells showed a basically complete structure and a small amount of ruptured epithelial cells (according to the arrow) (Figure 3).
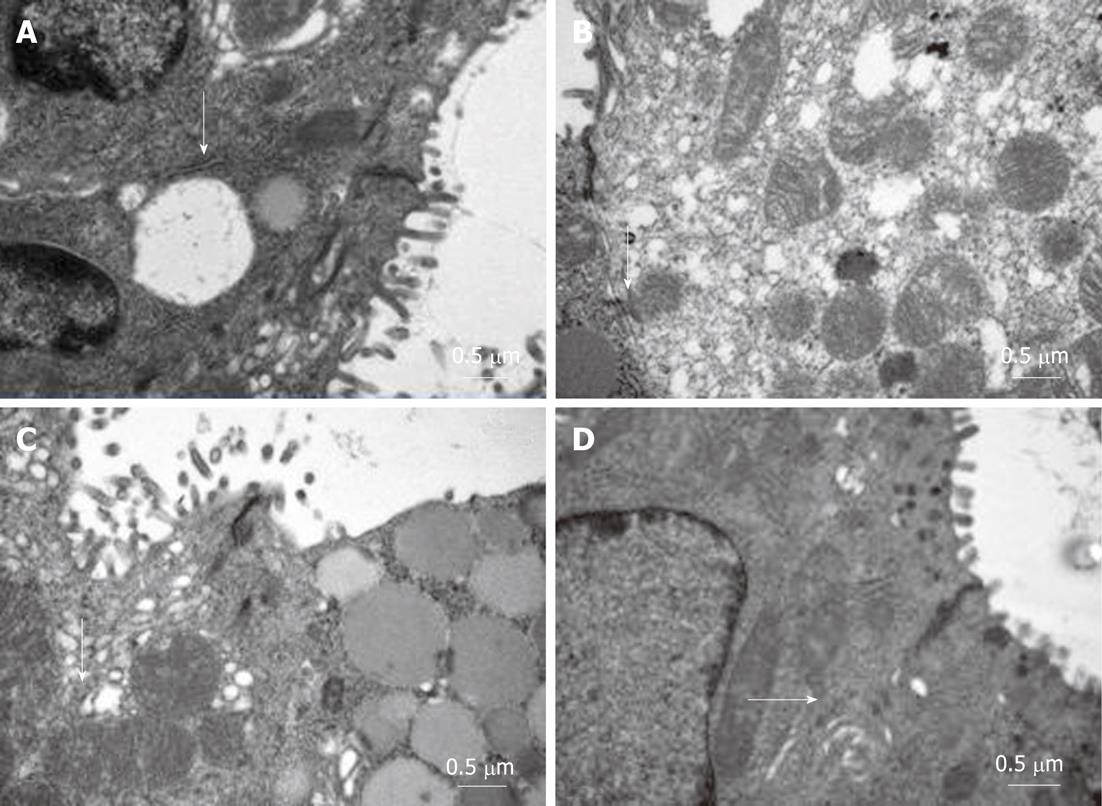
Ultrastructural changes under transmission electron microscopy: In the normal control group, the gastric mucosal organelles had integrated structures with no degeneration, and the microvillis were arranged in neat rows with no loss. The model control group showed widened cell gaps, vague intercellular junctions, sparse and deciduous microvillis, and swollen mitochondria and endoplasmic reticulum. In the model therapy groups, the cells were arranged in neat rows and the intercellular junctions were clear. The structures of the mitochondria and endoplasmic reticulum were clear with mild swelling (Figure 4).
The thickness of the gastric mucous layer and the contents of the hexosamine in the model control group were significantly lower than the normal control group (P < 0.01). While the thickness of the gastric mucous layer and the contents of the hexosamine in the model therapy groups were lower than the normal control group, they obviously were higher than the model control group, and the differences was statistically significant (P < 0.05) (Table 2).
| Group | The thickness of gastric mucous layer (μm) | The content of hexosamine (mg/g protein) | Plasma endothelin-1 (pg/mL) | Serum NO (μmol/L) | Gastric mucosal PGE2 (pg/mg) |
| Normal control group | 86.25 ± 3.21 | 65.57 ± 3.85 | 52.19 ± 2.82 | 30.20 ± 2.39 | 298.7 ± 9.28 |
| Model control group | 66.18 ± 5.11b | 21.51 ± 4.54b | 74.65 ± 8.84b | 17.6 ± 3.37a | 163.2 ± 8.84a |
| Low-dose geranylgeranylacetone | 79.43 ± 6.67ac | 31.78 ± 5.78ac | 35.98 ± 4.78ad | 50.60 ± 10.68c | 205.7 ± 10.39c |
| High-dose geranylgeranylacetone | 81.34 ± 5.98ac | 37.78 ± 4.98ac | 26.87 ± 4.87ad | 69.10 ± 9.56c | 265.5 ± 13.39c |
Plasma endothelin-1 levels: Compared with the normal control group, the levels of plasma ET-1 were significantly higher in the model control group (P < 0.01) and were significantly lower in the high-dose model therapy group (P < 0.05). While the levels of plasma ET-1 in the model therapy groups (including both the high-dose and low-dose groups) were significantly decreased compared to the model control group (P < 0.01) (Table 2).
Serum nitric oxide levels and gastric mucosal prostaglandin 2 levels: Compared with the normal control group, the levels of serum NO and gastric mucosal PGE2 were significantly decreased in the model control group (P < 0.05). In both model therapy groups, the content was significantly higher than in the model control group (P < 0.05), and this was especially the case in the high-dose model therapy group (Table 2).
The integrity of the gastric mucosa depends on the protection of the gastric mucosal barrier (including, for example, the mucus-bicarbonate barrier and mucosal microcirculation) and can be damaged by a variety of factors (internal or external) with the production of a number of inflammatory mediators and cytokines, resulting in secondary mucosal damage[8-10]. Of the damaging factors, ethanol is an important external factor. With both hydrophobic alkyl and hydrophilic hydroxyl in its molecular stucture, ethanol can damage the gastric mucosal barrier defense system, diminish the capacity of the gastric mucosa to defend the invasion of gastric acid, bile and many digestive enzymes, causing mucosal edema, erosion, hemorrhage and necrosis. In this study, excessive ethanol intake can cause laboratory rats to become apathetic, lose appetites, have slow responses, and have increased mortality. In our study, damage of the gastric mucosa in the model control group, including the general view, microscopic structures and ultrastructure changes, was significantly serious compared to the normal control group.
The gastric mucous layer is the first defensive line of the gastric mucosa to against external stimuli. Studies[11-13] have shown that changes in thickness and content; i.e., mucous glycoprotein (hexosamine) can reflect the anti-invasive ability of the gastric mucous. The results of this study showed that excessive ethanol intake can significantly reduce the thickness of the gastric mucous layer, reduce the content of hexosamine in the mucus gel layer, and finally result in the decline of the anti-invasive ability of the gastric mucosa to ethanol and other external attacks.
Studies[14,15] in recent years have shown that the impaired microcirculation is one of the pathological reasons for gastric mucosal barrier damage, which is accompanied by elevated levels of ET-1, and declining levels NO and PGE2 in blood and the gastric mucosa. NO and PGE2 are recognized vasodilator factors in vivo, which can inhibit platelet aggregation and thrombosis, accelerate the flow of the gastric mucosal microcirculation, promote the secretion of bicarbonate, mediate the adaptive immune protective function, increase protein synthesis and cell renewal, and finally enhance the repair ability of the damaged gastric mucosa. ET-1 is the strongest vasoconstrictor in vivo. Lazaratos et al[16] reported that after injection of exogenous ET-1 in the rat gastric artery, the gastric mucosa was obviously damaged, while injection of an endothelin receptor antagonist in advance can significantly reduce the gastric mucosal damage. Under physiological conditions, these factors work together to regulate the gastric mucosal microcirculation and maintain homeostasis. However, imbalanced regulation caused by various factors will disorder the gastric mucosal microcirculation, affect the integrity of the gastric mucosa, and thus lead to a variety of gastrointestinal diseases. This study[16] showed that the content of gastric mucosal PGE2 and serum NO were decreased in the model control group compared with the normal control group, and were negatively correlated with the gastric mucosal UI and EDS. The study also found that the levels of plasma ET-1 in excessive ethanol intake rats were higher than that in the normal control group, and were positively correlated with the UI and EDS. All these results suggested that the role of ethanol in damage to the gastric mucosa and weakening of its ability to repair may be caused by it stimulating the secretion of ET-1, and inhibiting the synthesis and secretion of endogenous NO and PGE2.
Geranylgeranylacetone (a derivatives of terpenes with a molecular formula of C23H38O) has been a widely used gastrointestinal mucosa protective agent in recent years. It can stimulate the synthesis and secretion of macromolecule glycoprotein and phospholipids in the gastric mucus layer and maintain the normal structure and function of the gastric mucus layer. It therefore has a strong role in renovation of various experimental and clinical gastric mucosal lesions. Studies[17,18] have shown that geranylgeranylacetone can stimulate the regenerated gastric mucosal cells to secrete hexosamine and carry out the biosynthesis of gastric mucosal PGE2. The mechanism may be related to the fact that geranylgeranylacetone changes the fluidity of membrane phospholipids, and increases the production of phospholipase A2, which is an important intermediate product for the PGE2 and hexosamine. Hexosamine is an essential component of polymer glycoprotein in the gastric mucosa gel layer, and PGE2 is a local hormone in the gastric mucosa. Studies have reported that PGE2 is involved in the improvement of gastric mucosal microcirculation, and the continuous secretion of PGE2 under the external stimulation helps to renovate gastric mucosa lesions[19-21]. Studies[22-24] have also shown that NO takes part in the process of geranylgeranylacetone inducing gastric mucus synthesis, in which NO synthase plays an important role, Meanwhile the synthesis of ET-1 is inhibited. Geranylgeranylacetone was used to pre-treat ethanol gastritis in the rat model in this study. We also showed that geranylgeranylacetone can elevate the serum NO and gastric mucosal PGE2 content and decrease the plasma ET-1 content to varying degrees with different dosages. Meanwhile, the gastric anti-invasion ability of ethanol showed a corresponding increase. In conclusion, Administration of GGA was correlated with a more favourable pattern of gastric mucosa damage after alcohol perfusion. The mechanism could be related to regulation of ET-1, NO and PGE2. The molecular pathways and mechanisms, however, need to be studied further.
Binge drinking or long-term drinking can cause acute or chronic gastric mucosal injury. Ethanol can be converted into acetaldehyde in the first-pass metabolism in the gastrointestinal tract, which may have a carcinogenic affect on the gastrointestinal tract through local toxic effects. Studies have shown that geranylgeranylacetone has a therapeutic effect on chronic gastritis, digestive ulcers and portal hypertensive gastropathy.
By establishing rat ethanol gastritis model, the authors evaluated the effects of ethanol on the gastric mucosa and studied the preventive effects of geranylgeranylacetone on ethanol-induced chronic gastritis.
There have been few studies on repair mechanisms for gastric mucosal damage caused by ethanol. Moreover, the effect and mechanisms of geranylgeranylacetone on repairing ethanol-induced gastritis have seldom been evaluated. This study demonstrated the administration of geranylgeranylacetone was correlated with a more favorable pattern of gastric mucosa damage after ethanol perfusion. The mechanism could be related to regulation of prostaglandin 2 (PGE2), endothelin-1 (ET-1) and nitric oxide (NO).
The study results suggest that geranylgeranylacetone can protect the rat gastric mucosa from ethanol-induced injury by changing the mobility of the cell membrane phospholipid bilayer, which further promotes the synthesis of endogenous PGE2 and NO, and inhibits the secretion of ET-1.
Geranylgeranylacetone is a derivative of terpenes, which has a therapeutic effect on chronic gastritis, digestive ulcers and portal hypertensive gastropathy. NO and PGE2 are recognized vasodilator factors in vivo, which can inhibit platelet aggregation and thrombosis, accelerate the flow of the gastric mucosal microcirculation, promote the secretion of bicarbonate, mediate the adaptive immune protective function and increase protein synthesis and cell renewal. ET-1 is the strongest vasoconstrictor in vivo.
It is an interesting study that confirms alcohol can damage the gastric mucosa. The study demonstrated the protective effect of geranylgeranylacetone on alcohol damage to the gastric mucosa and it elucidated the underlying mechanisms of this protective action.
| 1. | Salih BA, Abasiyanik MF, Bayyurt N, Sander E. H pylori infection and other risk factors associated with peptic ulcers in Turkish patients: a retrospective study. World J Gastroenterol. 2007;13:3245-3248. [PubMed] |
| 2. | Sakamoto C, Ogoshi K, Saigenji K, Narisawa R, Nagura H, Mine T, Tada M, Umegaki E, Maekawa T, Maekawa R. Comparison of the effectiveness of geranylgeranylacetone with cimetidine in gastritis patients with dyspeptic symptoms and gastric lesions: a randomized, double-blind trial in Japan. Digestion. 2007;75:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Liu X, Jia B, Lin S. [Teprenone in the treatment of chronic superficial gastritis, a multicentre study]. Zhonghua Neike Zazhi. 1996;35:12-14. [PubMed] |
| 4. | Niwa Y, Nakamura M, Miyahara R, Ohmiya N, Watanabe O, Ando T, Kawashima H, Itoh A, Hirooka Y, Goto H. Geranylgeranylacetone protects against diclofenac-induced gastric and small intestinal mucosal injuries in healthy subjects: a prospective randomized placebo-controlled double-blind cross-over study. Digestion. 2009;80:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Kai S, Ohta M, Tominaga M, Matsumoto T, Bandoh T, Kitano S. Reduction of ethanol-induced injury in portal hypertensive gastric mucosa of rats by induction of heat shock protein 72 by geranylgeranylacetone. Wound Repair Regen. 2007;15:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Vázquez-Ramírez R, Olguín-Martínez M, Kubli-Garfias C, Hernández-Muñoz R. Reversing gastric mucosal alterations during ethanol-induced chronic gastritis in rats by oral administration of Opuntia ficus-indica mucilage. World J Gastroenterol. 2006;12:4318-4324. [PubMed] |
| 7. | Guth PH, Aures D, Paulsen G. Topical aspirin plus HCl gastric lesions in the rat. Cytoprotective effect of prostaglandin, cimetidine, and probanthine. Gastroenterology. 1979;76:88-93. [PubMed] |
| 8. | Ma SY, Xiong LS, Dong YG, Yang XY, Gao XR, He JG, Liang LQ, Cui Y, Chen MH. [Side effects of non-steroidal anti-inflammatory drugs on gastric mucosa and preventive effects of teprenone]. Zhonghua Yixue Zazhi. 2009;89:1122-1125. [PubMed] |
| 9. | Choi SR, Lee SA, Kim YJ, Ok CY, Lee HJ, Hahm KB. Role of heat shock proteins in gastric inflammation and ulcer healing. J Physiol Pharmacol. 2009;60 Suppl 7:5-17. [PubMed] |
| 10. | Orlando RC. The integrity of the esophageal mucosa. Balance between offensive and defensive mechanisms. Best Pract Res Clin Gastroenterol. 2010;24:873-882. [PubMed] |
| 11. | Lü B, Zhang L, Fan YH, Meng LN, Zhang S. [Protection of gastric mucosa against steroids-induced damage by teprenone]. Zhonghua Yixue Zazhi. 2005;85:2749-2753. [PubMed] |
| 12. | Chaturvedi A, Kumar MM, Bhawani G, Chaturvedi H, Kumar M, Goel RK. Effect of ethanolic extract of Eugenia jambolana seeds on gastric ulceration and secretion in rats. Indian J Physiol Pharmacol. 2007;51:131-140. [PubMed] |
| 13. | Sharaev PN, Afanas'ev SS, Shkliaeva EV, Gileva OG. [Age-related changes in the exchange of hexosamine-containing biopolymers in rats under immobilization stress]. Patol Fiziol Eksp Ter. 2007;11-12. [PubMed] |
| 14. | Wallace JL, Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 284] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Morais TC, Pinto NB, Carvalho KM, Rios JB, Ricardo NM, Trevisan MT, Rao VS, Santos FA. Protective effect of anacardic acids from cashew (Anacardium occidentale) on ethanol-induced gastric damage in mice. Chem Biol Interact. 2010;183:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Lazaratos S, Irukayama-Tomobe Y, Miyauchi T, Goto K, Nakahara A. Oxygen radicals mediate the final exacerbation of endothelin-1-induced gastric ulcer in rat. Eur J Pharmacol. 2001;413:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Shiotani A, Haruma K, Nishi R, Fujita M, Kamada T, Honda K, Kusunoki H, Hata J, Graham DY. Randomized, double-blind, pilot study of geranylgeranylacetone versus placebo in patients taking low-dose enteric-coated aspirin. Low-dose aspirin-induced small bowel damage. Scand J Gastroenterol. 2010;45:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Suemasu S, Tanaka K, Namba T, Ishihara T, Katsu T, Fujimoto M, Adachi H, Sobue G, Takeuchi K, Nakai A. A role for HSP70 in protecting against indomethacin-induced gastric lesions. J Biol Chem. 2009;284:19705-19715. [PubMed] |
| 19. | Hattori Y, Ohno T, Ae T, Saeki T, Arai K, Mizuguchi S, Saigenji K, Majima M. Gastric mucosal protection against ethanol by EP2 and EP4 signaling through the inhibition of leukotriene C4 production. Am J Physiol Gastrointest Liver Physiol. 2008;294:G80-G87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Nishida T, Yabe Y, Fu HY, Hayashi Y, Asahi K, Eguchi H, Tsuji S, Tsujii M, Hayashi N, Kawano S. Geranylgeranylacetone induces cyclooxygenase-2 expression in cultured rat gastric epithelial cells through NF-kappaB. Dig Dis Sci. 2007;52:1890-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Funatsu T, Chono K, Hirata T, Keto Y, Kimoto A, Sasamata M. Mucosal acid causes gastric mucosal microcirculatory disturbance in nonsteroidal anti-inflammatory drug-treated rats. Eur J Pharmacol. 2007;554:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 22. | Fujimura N, Jitsuiki D, Maruhashi T, Mikami S, Iwamoto Y, Kajikawa M, Chayama K, Kihara Y, Noma K, Goto C. Geranylgeranylacetone, heat shock protein 90/AMP-activated protein kinase/endothelial nitric oxide synthase/nitric oxide pathway, and endothelial function in humans. Arterioscler Thromb Vasc Biol. 2012;32:153-160. [PubMed] |
| 23. | Nam SY, Kim N, Lee CS, Choi KD, Lee HS, Jung HC, Song IS. Gastric mucosal protection via enhancement of MUC5AC and MUC6 by geranylgeranylacetone. Dig Dis Sci. 2005;50:2110-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Yamamoto K, Sarukawa M, Ito T, Aoki H, Ichida M, Shimada K. An anti-ulcer drug, geranylgeranylacetone, suppresses inducible nitric oxide synthase in cultured vascular smooth muscle cells. J Hypertens. 2005;23:1847-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Peer reviewer: Dr. Pantelis Antonodimitrakis, Division of Endocrine Oncology, University Hospital of Uppsala, 76031 Uppsala, Sweden
S- Editor Gou SX L- Editor A E- Editor Xiong L