Published online May 14, 2012. doi: 10.3748/wjg.v18.i18.2225
Revised: December 7, 2011
Accepted: March 10, 2012
Published online: May 14, 2012
AIM: To evaluate the effects of fucoidan, a complex sulfated polysaccharide extract from marine seaweed, on hepatitis C virus (HCV) RNA load both in vitro and in vivo.
METHODS: HCV-1b replicon-expressing cells were cultured in the presence of fucoidan obtained from Cladosiphon okamuranus Tokida cultivated in Okinawa, Japan, and quantified the level of HCV replication. In an open-label uncontrolled study, 15 patients with chronic hepatitis C, and HCV-related cirrhosis and hepatocellular carcinoma were treated with fucoidan (0.83 g/d) for 12 mo. The clinical symptoms, biochemical tests, and HCV RNA levels were assessed before, during, and after treatment.
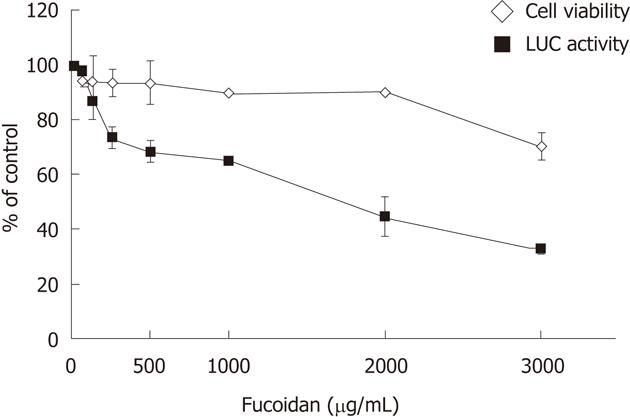
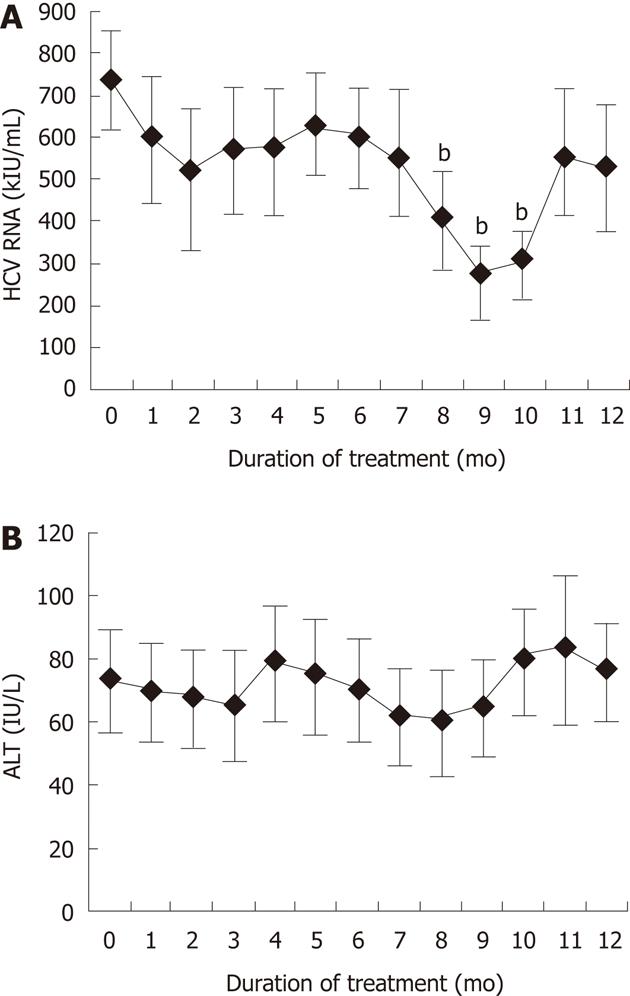
RESULTS: Fucoidan dose-dependently inhibited the expression of HCV replicon. At 8-10 mo of treatment with fucoidan, HCV RNA levels were significantly lower relative to the baseline. The same treatment also tended to lower serum alanine aminotransferase levels, and the latter correlated with HCV RNA levels. However, the improved laboratory tests did not translate into significant clinical improvement. Fucoidan had no serious adverse effects.
CONCLUSION: Our findings suggest that fucoidan is safe and useful in the treatment of patients with HCV-related chronic liver diseases. Further controlled clinical trials are needed to confirm the present findings.
- Citation: Mori N, Nakasone K, Tomimori K, Ishikawa C. Beneficial effects of fucoidan in patients with chronic hepatitis C virus infection. World J Gastroenterol 2012; 18(18): 2225-2230
- URL: https://www.wjgnet.com/1007-9327/full/v18/i18/2225.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i18.2225
Hepatitis C virus (HCV) infection often advances to chronic hepatitis due to the low viral clearance rate, leading to liver cirrhosis (LC) and subsequent development of hepatocellular carcinoma (HCC)[1,2]. The estimated global number of people infected with HCV is 170 million and more than 3.5 million new sufferers are diagnosed annually[3]. Currently, there is no vaccine available for prevention of HCV infection due to the extreme sequence variability within the HCV genome. The first-line treatment for chronic hepatitis C (CHC) includes the combination of pegylated α-interferon (IFN) and ribavirin, a broad spectrum antiviral drug[4,5]. Although the reported HCV eradication rate by this combination therapy is 75%-90% for genotypes II and III and 45%-52% for genotypes I and IV[6], these rates are still far from ideal. Because of the high rate of nonresponders among those infected with genotype I, the predominant strain in Japan, and because antiviral treatment causes frequent, unpleasant and sometimes serious adverse effects[7], the establishment of a new treatment modality without serious adverse effects is desirable[8].
Considering the prolonged period (20-30 years) required for development of LC and HCC in individuals infected with HCV, progression of the disease might be influenced by nutritional status and diet. Although herbal supplements, including silymarin (an extract of milk thistle), are frequently used by patients with chronic liver diseases[9,10], the available scientific evidence for the beneficial effects of these supplements is limited[11]. However, administration of EH0202, a mixture of four herbal extracts, is reported to induce IFN activity and reduce HCV RNA levels in patients with high viral titers[12]. Furthermore, a recent study reported the hepatoprotective effect of birch bark extract in patients with CHC[13].
Fucoidan is a sulfated polysaccharide extracted from marine brown seaweeds that possess some biological activities including anti-inflammatory properties[14,15]. Sulfated polysaccharides, including fucoidan, are also reported to inhibit the replication of viruses such as herpes simplex virus, Sindbis virus, human immunodeficiency virus, parainfluenza virus type II, and dengue virus[16-18]. We have also reported recently that oral administration of fucoidan for 12 mo resulted in 42.4% decrease in the human T-cell leukemia virus type I proviral load in patients with human T-cell leukemia virus type I-associated neurological disease[19]. Since fucoidan shows no toxicity or irritation in humans, it may be useful also as an anti-HCV agent.
To our knowledge, there are no data on the anti-HCV effect of fucoidan. In the present study, we examined the anti-HCV activity of fucoidan extracted from the marine alga, Cladosiphon okamuranus Tokida (C. okamuranus Tokida) cultivated in Okinawa, Japan. Our pilot study is the first clinical trial that investigated the effect of fucoidan in patients with HCV-related chronic liver diseases.
The unsalted brown seaweed C. okamuranus Tokida cultivated in Okinawa, Japan, was suspended in water, 0.57% (w/v) citric acid was added to the solution, and then heated at 90 °C for 40 min. The suspension was neutralized with NaOH and cooled to 40 °C. It was centrifuged at 3500 g by decantation centrifugal separator. The supernatant was collected, filtered using Cohlo filter, and concentrated by ultrafiltration (molecular weight cutoff 6000). The extracts were dried by spraydrier. They were composed of carbohydrates (72%), uronic acids (24%), and sulfate (8%). Total carbohydrates were determined by the phenol-H2SO4 method using fucose as the standard. Uronic acids were determined by the carbazole-H2SO4 method using D-glucuronic acid as the standard. The sulfate contents were measured by ion chromatography. The main carbohydrates were fucose. Fucoidan content determined by high-performance liquid chromatography was 83% and the molecular weight was 21-kDa. Fucoidan was dissolved in phosphate-buffered saline at a concentration of 30 mg/mL.
Fucoidan was added to Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum of HCV subgenomic replicon cells FLR3-1 (genotype Ib, Con-1;)[20] at a final concentration of 62.5, 125, 250, 500, 1000, 2000, and 3000 μg/mL. FLR3-1 cells were established from human hepatoma HuH-7 cells[21] by stable transfection with subgenomic selectable RNA in which the encoding HCV structural proteins were replaced by the firefly luciferase gene, the internal ribosome entry site of the Encephalomyocarditis virus, and the neomycin phosphotransferase gene[22]. After 72 h-incubation, the cells were washed in phosphate buffered saline and lysed in reporter lysis buffer (Promega, Madison, WI). Lysates were assayed for luciferase activity with the luciferase assay system (Promega) using the instructions provided by the manufacturer. With this HCV subgenome, the efficiency of subgenomic HCV expression could be estimated by measuring luciferase activity in the replicon cells.
Cell viability was measured using the cell proliferation reagent, WST-8 (Wako Pure Chemicals, Osaka, Japan). This method relies on mitochondrial dehydrogenase cleavage of WST-8 to formazan dye to estimate the level of cell viability. Briefly, FLR3-1 cells were incubated in a 96-well microculture plate. After 24 h incubation, fucoidan was added to the cells at various concentrations. After 72 h culture, WST-8 (5 μL) was added for the last 4 h of incubation and absorbance at 450 nm was measured using an automated microplate reader. WST-8 solution was added to the media-only wells to correct for background.
Table 1 lists the characteristics of the patients. The subjects included in the study were 15 patients with chronic liver diseases (7 men and 8 women; age, 66.1 ± 11.1 years; mean ± SD, range, 42-86), who visited the Nakasonekazu Medical Clinic. This study was carried out as an open-label study. All patients were infected with HCV genotype Ib, with a serum viral load in excess of 105 copies/mL. Nine patients had been diagnosed with CHC, 4 with HCV-related LC, and 2 with HCV-related cirrhosis and HCC. Eight patients were not eligible for IFN treatment because of LC, complication (depression), or advanced age. Seven patients had received IFN therapy in the past. Six patients were non-responders to IFN and 1 discontinued therapy because of side effect (depression). All patients assessed the tolerability as excellent.
| Patient No. | Age (yr) | Gender | Diagnosis | Previous IFN therapy | Other medications |
| 1 | 73 | F | LC | Not eligible | Glycyrrhizin |
| 2 | 78 | F | LC | Not eligible | Glycyrrhizin |
| 3 | 49 | M | LC | Not eligible | Glycyrrhizin |
| 4 | 72 | M | LC + HCC | Not eligible | Glycyrrhizin |
| 5 | 66 | M | LC + HCC | Not eligible | Glycyrrhizin |
| 6 | 70 | M | LC | Not eligible | None |
| 7 | 70 | F | CHC | Not eligible | Glycyrrhizin |
| 8 | 86 | F | CHC | Not eligible | Glycyrrhizin |
| 9 | 55 | M | CHC | Intolerant | None |
| 10 | 69 | M | CHC | Non-responder | Glycyrrhizin |
| 11 | 71 | M | CHC | Non-responder | Glycyrrhizin |
| 12 | 68 | F | CHC | Non-responder | Glycyrrhizin |
| 13 | 61 | F | CHC | Non-responder | Glycyrrhizin |
| 14 | 62 | F | CHC | Non-responder | None |
| 15 | 42 | F | CHC | Non-responder | None |
During fucoidan treatment, 11 patients received a glycyrrhizin preparation. Fucoidan (provided by Kanehide Bio Co., Okinawa, Japan) was given orally as capsules containing 166 mg of dry extract from C. okamuranus Tokida per capsule in a dose of five capsules daily for 12 mo. Informed consent was obtained from all patients enrolled in the study, after a thorough explanation of the aims, risks, and benefits of this therapy.
The outcome parameters included the course of alanine aminotransferase (ALT), aspartate aminotransferase (AST), quantitative HCV RNA levels, subjective symptoms associated with CHC, LC, and HCC (such as fatigue, abdominal discomfort, depression, and dyspepsia), safety, and compliance. Data on all clinical parameters were documented at each visit. HCV RNA levels were determined using the AMPLICOR GT HCV Monitor test (Roche Diagnostics, Basel, Switzerland), which has a lower limit of quantitation of 0.5 kIU/mL at a linear range up to 850 kIU/mL.
Data are expressed as mean ± SD. The results of biochemical tests and HCV RNA levels were compared by the Student’s t test. A P < 0.05 was considered significant.
To assess the effects of fucoidan on intracellular replication of the HCV genome, HCV subgenomic replicon cells FLR3-1 were cultured in the presence of various concentrations of fucoidan in the medium. The luciferase activities of the FLR3-1 cells showed a concentration-dependent suppression of replication of HCV replicon by fucoidan. The WST-8 assay showed that fucoidan had negligible effect on cell viability (Figure 1). These results suggest that fucoidan inhibits HCV replication but does not have cytotoxic effects.
Changes in HCV RNA and serum ALT levels in patients treated with fucoidan are shown in Figure 2. The mean HCV RNA for the 15 patients was 736 ± 118 kIU/mL (range, 100-850 kIU/mL) before fucoidan therapy. As shown in Figure 2A, fucoidan tended to reduce the mean HCV RNA level with time relative to the baseline, with significant falls registered at 8-10 mo of treatment. However, HCV RNA increased after 11 and 12 mo. Biochemical tests showed that the mean serum ALT level, but not AST, correlated with the mean HCV RNA level, although the decrease in ALT level was not significant relative to the baseline (Figure 2B). Whereas the above changes were not associated with improvement in clinical symptoms in every patient, none of the patients showed progression of LC or adverse events.
It is estimated that 170 million people worldwide are infected with HCV[3], and some 2 million (1%) of these reside in Japan[23]. Of the HCC cases in Japan, around 80% are caused by HCV infection. The increase in the number of HCC patients contributes to the increase in total deaths in Japan from HCC. This trend is expected to continue until 2015[23]. The general strategies followed in the treatment of CHC include eradication of HCV and suppression of hepatitis.
Sulfated polysaccharides including fucoidan are reported to inhibit the growth of various enveloped viruses[16-18]. Fucoidan is thought to inhibit virus adsorption to the cell surface by binding to the cell surface, with subsequent prevention of cell infection[18]. In addition, fucoidan interacts directly with the envelope glycoprotein on dengue virus type II[17]. In the present study, we demonstrated a novel mechanism of action for fucoidan. Using the HCV replicon system, we demonstrated here that fucoidan inhibits intracellular replication of the HCV genome in vitro.
To our knowledge, this is the first clinical study to investigate the effects of fucoidan in patients with liver diseases. The rationale for this study was stimulated by experimental data showing the efficiency of fucoidan in cell cultures. Patients with chronic HCV infection, who were not eligible for, did not respond to, or were intolerant of IFN treatment, were treated for 12 mo with fucoidan at 830 mg/d to investigate the effect of this treatment on HCV RNA level. In Case 6 (baseline HCV RNA 380 kIU/mL), fucoidan treatment successfully eradicated HCV at 9 mo, although HCV RNA was 5 kIU/mL at 10 mo. Thus, fucoidan was effective in lowering HCV RNA level in this study, although its effect was temporary. There was a significant decrease in HCV RNA at month 8, 9 and 10 of fucoidan commencement (P < 0.01). However, the level increased later at month 12 to become equivalent to the baseline.
We also measured serum IFNα levels to determine the indirect effect of fucoidan on IFNs, especially whether it increases the antiviral activity of IFNs. However, IFNα could not be detected in the serum of patients treated with fucoidan. Furthermore, fucoidan did not enhance IFNs expression in FLR3-1 replicon cells (data not shown). It has been reported that the protective effect of fucoidan is based on direct inhibition of viral replication and stimulation of both innate and adaptive immune defense functions[24]. We are currently investigating the effect of fucoidan on the host immune system including natural killer cell cytotoxic activity.
Our study has certain limitations. First, the study comprised only a small number of patients, including 6 patients who were known non-responders to IFN therapy. Second, all patients harbored HCV virus genotype Ib and 6 had cirrhosis. Thus, at least some patients in this cohort could be classified as likely non-responders to IFN therapy[25,26]. Thus, the selection criteria employed in the present study may have favored a poor response to fucoidan.
The abnormally high levels of ALT tended to decrease temporarily during fucoidan treatment, suggesting a correlation between viral load and indices of hepatic dysfunction. Thus, fucoidan may be effective in the management of HCV-related chronic liver diseases, although long-term clinical improvement was not observed in the present study. Importantly, no adverse events were observed in all patients, similar to the results reported in a previously study on fucoidan[19], suggesting that daily oral administration of fucoidan for 12 mo is safe and tolerable.
There is no doubt that patients who fail to respond to conventional treatments often seek alternative therapies. In conclusion, our study demonstrated that fucoidan from C. okamuranus Tokida has HCV replication suppressive effects in a replicon cell system. Furthermore, our relatively small uncontrolled pilot study showed that fucoidan has temporary but beneficial effects on HCV RNA levels in HCV infected patients. The preliminary findings suggest that fucoidan may be a useful health-food additive with antiviral activity to be used in the treatment of chronic liver diseases. To suppress the viral titer as much and for as long as possible, we need to define the daily effective dosage. Further studies on the mechanism of fucoidan-induced HCV inhibition may provide alternative strategies for the design of novel anti-HCV drugs.
We thank Dr. Michinori Kohara for providing HCV replicon cells and technical assistance. We are grateful to Kanehide Bio Co., Ltd., for providing fucoidan.
Hepatitis C virus (HCV) is a major cause of chronic liver diseases including chronic hepatitis, cirrhosis, and hepatocellular carcinoma. The standard care for chronic hepatitis C involves the administration of pegylated α-interferon in combination with the nucleotide analog ribavirin. However, this regimen has limited success rate for genotype I and IV, and unfavorable side effects. Thus, it is important to discover more effective and safer agents to improve the clinical treatment on HCV carriers. Fucoidan, a sulfated polysaccharide, has significant biological activities, such as antiviral and anti-inflammatory effects. Nevertheless, there has been no investigation on the efficacy of fucoidan against HCV infection.
Natural products have been used for the treatment of various diseases as an alternative to conventional chemical agents. So far, several natural products have been screened for their antiviral effect against various viral infections. Screening of natural potent inhibitors for HCV has also become a research hotspot.
Previous studies have shown the efficacy of natural products against HCV replication in a cell-based HCV replicon system. However, there have been few clinical studies that evaluated the safety and efficacy of these natural products. In the present study, the authors investigated the anti-HCV activity of fucoidan obtained from the Cladosiphon okamuranus Tokida cultivated in Okinawa, Japan, both in vitro and in vivo. This pilot study is the first clinical trial that investigated the effect of fucoidan in patients with HCV-related chronic liver diseases.
Fucoidan inhibited HCV RNA replication in the HCV replicon assay system. The experimental data on fucoidan efficiency in cell culture stimulated the rationale for clinical study. Oral fucoidan administration resulted in temporary reduction of viral loads of genotype Ib in patients with chronic HCV infection, who were not eligible for, did not respond to, or were intolerant of interferon therapy. Fucoidan is well tolerated and no serious adverse events were observed in any of the patients. Fucoidan exhibited antiviral properties against HCV both in vitro and in vivo, and would be expected to become a new strategy for HCV infection. Further controlled clinical trials will be required to confirm the present findings.
Fucoidan is a complex sulfated polysaccharide found in the cell walls of several edible brown algae, including Fucus vesiculosus. The HCV replicon system replicates a modified HCV genome containing luciferase gene to high levels in human hepatoma cells. The efficacy of subgenomic HCV expression was estimated by measuring luciferase activity in the replicon cells. This system provides a powerful tool for studying virus replication and for screening anti-HCV drugs.
The paper studied the effects of fucoidan, a complex sulfated polysaccharide extracted from marine seaweed, on HCV RNA load in vitro and in vivo. The research is of significance because of the high rate of nonresponders in HCV genotype I, which is the predominant strain in Japan. Moreover, antiviral treatment causes frequent, unpleasant and sometimes serious adverse effects, Thus the search for a new treatment modality without serious adverse effects is desirable.
| 1. | Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436-2441. [PubMed] |
| 2. | Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107-115. [PubMed] |
| 3. | Gao X, Cui Q, Shi X, Su J, Peng Z, Chen X, Lei N, Ding K, Wang L, Yu R. Prevalence and trend of hepatitis C virus infection among blood donors in Chinese mainland: a systematic review and meta-analysis. BMC Infect Dis. 2011;11:88. [PubMed] |
| 4. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [PubMed] |
| 5. | Brok J, Gluud LL, Gluud C. Meta-analysis: ribavirin plus interferon vs. interferon monotherapy for chronic hepatitic C - an updated Cochrane review. Aliment Pharmacol Ther. 2010;32:840-850. [PubMed] |
| 6. | Deutsch M, Hadziyannis SJ. Old and emerging therapies in chronic hepatitis C: an update. J Viral Hepat. 2008;15:2-11. [PubMed] |
| 7. | Cornberg M, Wedemeyer H, Manns MP. Treatment of chronic hepatitis C with PEGylated interferon and ribavirin. Curr Gastroenterol Rep. 2002;4:23-30. [PubMed] |
| 8. | Vermehren J, Sarrazin C. New hepatitis C therapies in clinical development. Eur J Med Res. 2011;16:303-314. [PubMed] |
| 9. | Siddiqui U, Weinshel EH, Bini EJ. Prevalence and predictors of herbal medication use in veterans with chronic hepatitis C. J Clin Gastroenterol. 2004;38:605-610. [PubMed] |
| 10. | Polyak SJ, Morishima C, Lohmann V, Pal S, Lee DY, Liu Y, Graf TN, Oberlies NH. Identification of hepatoprotective flavonolignans from silymarin. Proc Natl Acad Sci USA. 2010;107:5995-5999. [PubMed] |
| 11. | Seeff LB, Curto TM, Szabo G, Everson GT, Bonkovsky HL, Dienstag JL, Shiffman ML, Lindsay KL, Lok AS, Di Bisceglie AM. Herbal product use by persons enrolled in the hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) Trial. Hepatology. 2008;47:605-612. [PubMed] |
| 12. | Kaji K, Yoshida S, Nagata N, Yamashita T, Mizukoshi E, Honda M, Kojima Y, Kaneko S. An open-label study of administration of EH0202, a health-food additive, to patients with chronic hepatitis C. J Gastroenterol. 2004;39:873-878. [PubMed] |
| 13. | Shikov AN, Djachuk GI, Sergeev DV, Pozharitskaya ON, Esaulenko EV, Kosman VM, Makarov VG. Birch bark extract as therapy for chronic hepatitis C--a pilot study. Phytomedicine. 2011;18:807-810. [PubMed] |
| 14. | Berteau O, Mulloy B. Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology. 2003;13:29R-40R. [PubMed] |
| 15. | Park HY, Han MH, Park C, Jin CY, Kim GY, Choi IW, Kim ND, Nam TJ, Kwon TK, Choi YH. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem Toxicol. 2011;49:1745-1752. [PubMed] |
| 16. | Baba M, Snoeck R, Pauwels R, de Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother. 1988;32:1742-1745. [PubMed] |
| 17. | Hidari KI, Takahashi N, Arihara M, Nagaoka M, Morita K, Suzuki T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem Biophys Res Commun. 2008;376:91-95. [PubMed] |
| 18. | Taoda N, Shinji E, Nishii K, Nishioka S, Yonezawa Y, Uematsu J, Hattori E, Yamamoto H, Kawano M, Tsurudome M. Fucoidan inhibits parainfluenza virus type 2 infection to LLCMK2 cells. Biomed Res. 2008;29:331-334. [PubMed] |
| 19. | Araya N, Takahashi K, Sato T, Nakamura T, Sawa C, Hasegawa D, Ando H, Aratani S, Yagishita N, Fujii R. Fucoidan therapy decreases the proviral load in patients with human T-lymphotropic virus type-1-associated neurological disease. Antivir Ther. 2011;16:89-98. [PubMed] |
| 20. | Sakamoto H, Okamoto K, Aoki M, Kato H, Katsume A, Ohta A, Tsukuda T, Shimma N, Aoki Y, Arisawa M. Host sphingolipid biosynthesis as a target for hepatitis C virus therapy. Nat Chem Biol. 2005;1:333-337. [PubMed] |
| 21. | Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858-3863. [PubMed] |
| 22. | Nakagawa S, Umehara T, Matsuda C, Kuge S, Sudoh M, Kohara M. Hsp90 inhibitors suppress HCV replication in replicon cells and humanized liver mice. Biochem Biophys Res Commun. 2007;353:882-888. [PubMed] |
| 23. | Yoshizawa H. Hepatocellular carcinoma associated with hepatitis C virus infection in Japan: projection to other countries in the foreseeable future. Oncology. 2002;62 Suppl 1:8-17. [PubMed] |
| 24. | Hayashi K, Nakano T, Hashimoto M, Kanekiyo K, Hayashi T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int Immunopharmacol. 2008;8:109-116. [PubMed] |
| 25. | Martinot-Peignoux M, Marcellin P, Pouteau M, Castelnau C, Boyer N, Poliquin M, Degott C, Descombes I, Le Breton V, Milotova V. Pretreatment serum hepatitis C virus RNA levels and hepatitis C virus genotype are the main and independent prognostic factors of sustained response to interferon alfa therapy in chronic hepatitis C. Hepatology. 1995;22:1050-1056. [PubMed] |
| 26. | Chevaliez S, Asselah T. Mechanisms of non-response to antiviral treatment in chronic hepatitis C. Clin Res Hepatol Gastroenterol. 2011;35 Suppl 1:S31-S41. [PubMed] |
Peer reviewers: Arturo Panduro, MD, PhD, Head of the Department of Molecular Biology in Medicine, Civil Hospital of Guadalajara Fray Antonio Alcalde/University of Guadalajara, Hospital No. 278 S.H., Guadalajara, Jalisco 44280, Mexico; Runu Chakravarty, PhD, ICMR Virus Unit Kolkata, GB4, ID and BG Hospital Campus, Kolkata 700010, India
S- Editor Shi ZF L- Editor A E- Editor Zheng XM