Published online May 14, 2012. doi: 10.3748/wjg.v18.i18.2197
Revised: January 16, 2012
Accepted: December 29, 2011
Published online: May 14, 2012
AIM: To investigate the hepatic protective effects of 5-methoxypsoralen (5-MOP) and to learn if 5-MOP causes hepatotoxicity at protective doses.
METHODS: C57BL/6J mice were administrated orally with 5-MOP at doses of 12.5, 25 and 50 mg/kg body weight respectively every morning for 4 d before given acetaminophen (APAP) subcutaneously at a dose of 500 mg/kg. The 5-MOP alone group was treated with 5-MOP orally at a dose of 50 mg/kg body weight for 4 d without APAP. Twenty-four hours after APAP administration, blood samples of mice were analyzed for serum enzyme alanine transaminase (ALT), aspartate transaminase (AST), lactate dehydrogenase (LDH) levels, and malondialdehyde (MDA), reduced glutathione (GSH) and oxidized glutathione (GSSG) of liver tissues were measured and histopathologic changes of the liver were observed.
RESULTS: Compared with the vehicle control group, the serum levels (IU/L) of ALT, AST and LDH were all increased significantly in APAP group (8355 ± 3940 vs 30 ± 21, P < 0.05; 6482 ± 4018 vs 146 ± 58, P < 0.05; 24627 ± 10975 vs 1504 ± 410, P < 0.05). Compared with APAP group, the serum ALT levels (IU/L) (1674 ± 1810 vs 8355 ± 3940, P < 0.05; 54 ± 39 vs 8355 ± 3940, P < 0.05; 19 ± 9 vs 8355 ± 3940, P < 0.05), AST levels (IU/L) (729 ± 685 vs 6482 ± 4108, P < 0.05; 187 ± 149 vs 6482 ± 4108, P < 0.05; 141 ± 12 vs 6482 ± 4108, P < 0.05) and LDH levels (IU/L) (7220 ± 6317 vs 24 627 ± 10 975, P < 0.05; 1618 ± 719 vs 24 627 ± 10 975, P < 0.05; 1394 ± 469 vs 24 627 ± 10 975, P < 0.05) were all decreased drastically in the three-dosage 5-MOP pretreatment groups. Pretreatment of 5-MOP could attenuate histopathologic changes induced by APAP, including hepatocellular necrosis and infiltration of inflammatory cells, and the effect was dose-dependent. MDA levels (nmol/mg) were decreased by 5-MOP in a dose-dependent manner (0.98 ± 0.45 vs 2.15 ± 1.07, P > 0.05; 0.59 ± 0.07 vs 2.15 ± 1.07, P < 0.05; 0.47 ± 0.06 vs 2.15 ± 1.07, P < 0.05). The pretreatment of 5-MOP could also increase the GSH/GSSG ratio (3.834 ± 0.340 vs 3.306 ± 0.282, P > 0.05; 5.330 ± 0.421 vs 3.306 ± 0.282, P < 0.05; 6.180 ± 0.212 vs 3.306 ± 0.282, P < 0.05). In the group treated with 5-MOP but without APAP, the serum enzyme levels, the liver histopathologic manifestation, and the values of MDA and GSH/GSSG ratio were all normal.
CONCLUSION: 5-MOP can effectively protect C57BL/6J mice from APAP-induced hepatotoxicity and possesses an antioxidative activity, and does not cause liver injury at the protective doses.
- Citation: Liu WX, Jia FL, He YY, Zhang BX. Protective effects of 5-methoxypsoralen against acetaminophen-induced hepatotoxicity in mice. World J Gastroenterol 2012; 18(18): 2197-2202
- URL: https://www.wjgnet.com/1007-9327/full/v18/i18/2197.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i18.2197
Acetaminophen (APAP), a widely used antipyretic and analgesic drug, could induce hepatotoxicity and even acute liver failure (ALF) when taken at overdose[1]. APAP overdose is a common cause of adult and children ALF in the United States and other countries[2-5]. APAP can be metabolized by cytochrome P450 enzymes (CYPs) to N-acetyl-P-benzoquinoneimine (NAPQI)[6]. At overdoses of APAP, a large number of NAPQIs is generated, which can deplete reduced glutathione (GSH) and then bind to mitochondrial proteins to cause mitochondrial dysfunction and oxidant stress[7,8], leading to hepatocellular damage and centrilobular hepatic necrosis. In this process, APAP can increase the level of malondialdehyde (MDA) both in the liver and plasma[9] and NAPQI is capable of lowering GSH/oxidized glutathione (GSSG) ratio by oxidizing the thiol group of GSH[10]. Oxidant stress plays a central role in the hepatic damage induced by APAP[11].
5-methoxypsoralen (5-MOP), a furocoumarin found in many medicinal plants, possesses slight antioxidative activity evidenced from researches in vitro[12,13]. 5-MOP has been used in combination with UV radiation in skin photochemotherapy for decades[14], and some studies also found that it has anticancer[15-19], antidepressant[20-24], anticonvulsion[25] and anti-inflammatory effects[26,27], but none of previous studies have shown that 5-MOP could prevent hepatotoxicity.
In addition, some patients suffered from toxic hepatitis induced by 5-MOP when it was used as photochemotherapeutic agent[28,29], and one animal experiment demonstrated that high doses of 5-MOP can induce hepatotoxicity in mice[30]. So it is essential to examine if 5-MOP can cause liver injury at therapeutic doses.
This study was designed to determine the protective effects of 5-MOP in APAP-induced hepatotoxicity using mouse hepatotoxic models, and to investigate if 5-MOP can cause hepatotoxicity in mice at effective doses.
5-MOP was purchased from Tokyo Chemical Industry (Tokyo, Japan), and APAP from Jiaozuo Xin’An Science and Technology Company (Henan, China). Tween 80, which was used to prepare 5-MOP suspension, was bought from Biodee Biotechnology Company (Beijing, China). APAP was dissolved in normal saline before use. GSH and N-ethylmathione gained from Lizhudongfeng Biotechnology Company (Shanghai, China), GSSG from Hongxing Biotechnology Company (Beijing, China), o- phthalaldehyde (OPT) from Jinlong Chemical Company (Beijing, China), and thiobarbituric acid (TBA) from Acros (United States).
Male C57BL/6J mice, 18-22 g in weight, were purchased from Peking University Laboratory Animal Department, Beijing, China. They were housed in a well-ventilated room and the room temperature was controlled at 21 °C-23 °C and humidity at 65%-70% with a 12 h light-12 h dark cycle. All the mice were fed adaptively for three d before experiment, and they had free access to water and were fed with forage supplied by Laboratory Animal Center of Military Medical Science Academy.
5-MOP was suspended in 1% Tween 80 at different concentrations of 1.25 mg/mL, 2.5 mg/mL, and 5 mg/mL, and all of these suspensions were administrated to mice at 10 mL/kg body weight; that is, mice were administrated with 5-MOP at doses of 12.5 mg/kg, 25 mg/kg and 50 mg/kg, respectively. A 5-d experiment was performed with 36 mice which were randomly divided into 6 groups by weight. Group 1 was the vehicle control group and group 2 was APAP alone group, both groups were orally treated with 1% Tween 80 (10 mL/kg body weight) every morning for 4 d. Groups 3, 4 and 5 were 5-MOP multiple-dose groups administered with oral 5-MOP at doses of 12.5, 25 and 50 mg/kg body weight respectively every morning for 4 d. Group 6 was 5-MOP alone group treated with oral 5-MOP at a dose of 50 mg/kg body weight also for 4 d. Thirty minutes after the administrations, all mice except those in the vehicle control group and 5-MOP alone group were subcutaneously administrated with APAP (500 mg/kg body weight). Twenty hours after APAP administration, blood samples were collected from orbital venous plexus of the mice. After the mice were sacrificed, their livers were dissected out immediately and washed with normal saline, dried on a filter paper and weighted. Then the livers were prepared immediately for further examinations.
The animal care and surgical procedures were performed in compliance with the Guidelines for Animal Care and Use of Peking University.
The blood samples were collected to determine serum enzyme [alanine transaminase (ALT), aspartate transaminase (AST), lactate dehydrogenase (LDH)] levels by HITACHI-7170A automatic analyzer. The liver tissues were homogenized with potassium chloride (KCl) solution (0.15 mol/L) on ice to yield a 5% (w/v) homogenates for MDA test. The hepatic MDA levels were determined as thiobarbituric acid reactive substances levels using a published colorimetric method[31].
The liver tissues were homogenized with phosphate buffered solution on ice to yield a 5% (w/v) homogenates for glutathione test. The GSH and GSSG levels in liver tissues were measured by the improved Hission method[32], a fluorometric method that uses OPT as a fluorescent reagent. Then GSH/GSSG ratio was calculated.
The left liver lobes were scissored out and fixed in 10% formalin solution for 48 h. The liver samples were then cut into thin transverse sections with the help of microtome and permanent slides were prepared with HE staining. Liver histopathologic changes were examined under an optical microscope with the original magnification × 200.
The experimental results were expressed as mean ± SE (standard error). Statistical comparison between groups was performed by one-way analysis of variance with SPSS 13.0 statistical software. A P value < 0.05 was indicated as a statistically significant difference.
The hepatocellular damage induced by a toxic dose (500 mg/kg) of APAP and the effects of pretreatment with 5-MOP were investigated by measuring the serum levels of ALT, AST and LDH. As shown in Table 1, APAP significantly increased the serum ALT, AST and LDH levels compared with the control group, and the multiple-dose 5-MOP pretreatment significantly prevented the increases of serum enzyme levels. The effect of 5-MOP was dose-dependent, and in the highest dose group, serum levels of ALT, AST and LDH were close to the normal levels as compared with the vehicle control group (P > 0.05).
| Treatments groups | ALT (IU/L) | AST (IU/L) | LDH (IU/L) | MDA (nmol/mg) | GSH/GSSH |
| Vehicle control | 30 ± 21 | 146 ± 58 | 1504 ± 410 | 0.18 ± 0.11 | 6.045 ± 0.629 |
| APAP alone at 500 mg/kg | 8355 ± 3940a | 6482 ± 4018a | 24 627 ± 10975a | 2.15 ± 1.07a | 3.306 ± 0.282a |
| 5-MOP at 12.5 mg/kg | 1674 ± 1810c | 729 ± 685c | 7220 ± 6317c | 0.98 ± 0.45 | 3.834 ± 0.340 |
| 5-MOP at 25 mg/kg | 54 ± 39c | 187 ± 149c | 1618 ± 719c | 0.59 ± 0.07c | 5.330 ± 0.421c |
| 5-MOP at 50 mg/kg | 19 ± 9c | 141 ± 12c | 1394 ± 469c | 0.47 ± 0.06c | 6.180 ± 0.212c |
| 5-MOP alone at 50 mg/kg | 37 ± 20 | 138 ± 22 | 1471 ± 191 | 0.15 ± 0.09 | 6.858 ± 0.678 |
The influences of 5-MOP alone on serum enzyme levels were also observed. There were no statistically significant differences in the serum levels of ALT, AST and LDH between 5-MOP alone group (50 mg/kg) and the vehicle control group (P > 0.05).
As seen in Table 1, compared with the vehicle control group, a toxic dose of APAP elevated liver MDA and lowered the hepatic GSH/GSSG ratio. With the escalating dose of 5-MOP (12.5, 25 and 50 mg/kg), the content of MDA decreased and ratio of GSH/GSSG increased.
In the 5-MOP alone group, the MDA level in liver was as low as that in the vehicle control group (0.15 ± 0.09 vs 0.18 ± 0.11, P > 0.05). In addition, the hepatic GSH/GSSG ratio in the 5-MOP alone group was not significantly changed as compared with that in the vehicle control group (6.858 ± 0.678 vs 6.045 ± 0.629, P > 0.05).
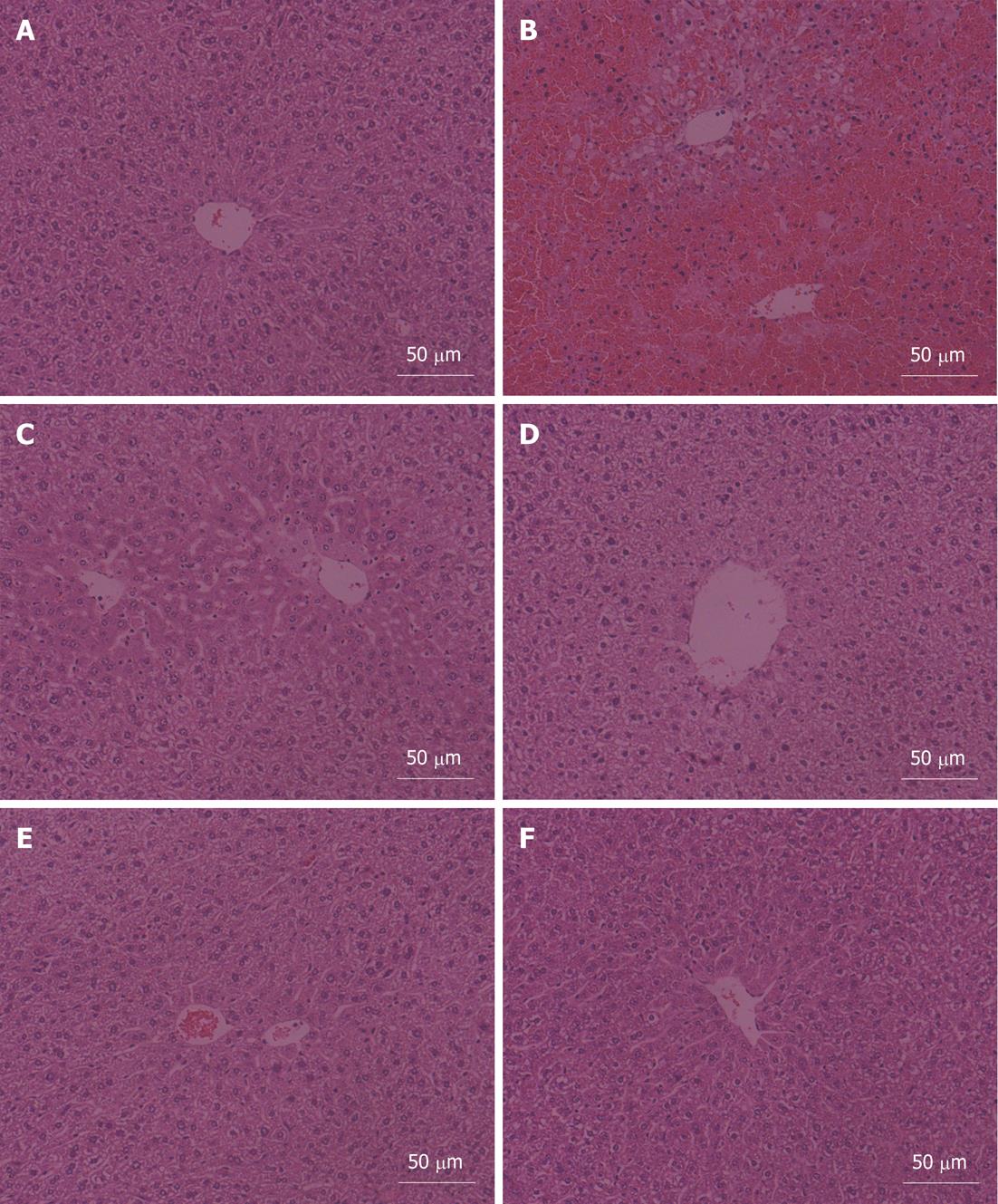
The liver histopathologic changes of mice in the six groups are shown in Figure 1. The liver sections displayed the representative hepatocellular morphological changes of each group.
In the vehicle control group, hepatocytes, presenting normal morphology, arranged around the central vein in a radical pattern, and liver lobule structures were clear and regular (Figure 1A). Normal liver lobule structures were damaged and collapsed in the APAP alone group. Large areas of hepatocellular necrosis and infiltration of inflammatory cells were also observed (Figure 1B). 5-MOP administration could alleviate the pathological injury induced by APAP in a dose-dependent manner. 5-MOP at a dose of 12.5 mg/kg could slightly relieve the pathological injury. In this group, no hepatocellular necrosis and infiltration of inflammatory cells were observed, but hepatocellular hydropic degeneration and sinusoidal dilation occurred (Figure 1C). There was no necrosis and hydropic degeneration of hepatocytes, no sinusoidal dilation and infiltration of inflammatory cells in the 25 mg/kg 5-MOP dose group. However, liver lobule structures were still not clear in this group (Figure 1D). 5-MOP at a dose of 50 mg/kg could significantly prevent APAP-induced hepatotoxicity with an almost normal lobular structure comparable to the vehicle control group (Figure 1E). There were no significant liver histopathologic changes in the 5-MOP alone group (Figure 1F).
The protective effect of 5-MOP against hepatocellular injury and oxidative stress, and the potential toxic effect of 5-MOP on the liver were investigated in this study. C57BL/6J mice were used because our previous research found that C57BL/6J mice were more susceptible to APAP[33]. The serum levels of ALT, AST and LDH are main indices of liver injury[34] and the levels of MDA, GSH and GSSG can be used as indices of oxidative stress[9]. We evaluated the hepatic protective effect of 5-MOP based on these indices. It is well known that any chemical can be toxic if its dose is high enough, so a 5-MOP alone group was designed to see if the highest therapeutic dose of 5-MOP could cause hepatotoxicity.
APAP used alone can significantly increase the serum levels of ALT, AST and LDH and cause pathological changes as compared with the vehicle control group. Oxidative stress also took place as shown by the increase of MDA level and decrease of GSH/GSSG ratio. The model of APAP-induced hepatotoxicity was successfully established in this experiment.
5-MOP can protect mice from APAP-induced acute liver injury based on the fact that it can decrease the serum ALT, AST and LDH levels in a dose-dependent manner and alleviate the liver histopathologic alterations. Moreover, 5-MOP decreased the MDA level and increased the GSH/GSSG ratio in a dose-related manner, which reflected that 5-MOP could significantly attenuate the oxidative stress induced by APAP and suggested that the hepatoprotective effect of 5-MOP may be associated with its antioxidative activity.
However, besides antioxidant activity, 5-MOP also possesses biological activities to inhibit the mouse and human CYPs both in vivo and in vitro[14]. And CYPs-catalyzed formation of NAPQI is the key mechanism in APAP-induced hepatotoxicity[35]. So we presume that inhibition of CYPs of 5-MOP may also account for the protective mechanism against APAP-induced hepatotoxicity, which should be further investigated.
In the 5-MOP alone group, the serum enzyme (ALT, AST and LDH) levels and histopathologic changes were as normal as in the vehicle control group, which indicated that 5-MOP could not cause liver injury at a dose of 50 mg/kg (the highest therapeutic dose used in this study). The MDA level and the GSH/GSSG ratio were not significantly changed as compared with the vehicle control group, which showed that 5-MOP did not influence the normal oxido-reduction levels.
In conclusion, 5-MOP could protect against APAP-induced hepatotoxicity in mice and had an antioxidative activity, and caused no hepatotoxicity at protective doses.
Overdose of acetaminophen (APAP) can induce hepatotoxicity and oxidative stress plays a central role in the hepatic damage. Though 5-methoxypsoralen (5-MOP) possesses antioxidative activity suggested by researches in vitro, none of previous studies has found that 5-MOP could prevent APAP-induced hepatotoxicity.
It is important to search for effective methods to protect human from APAP-induced hepatotoxicity. Despite the various applications of 5-MOP, no research has been conducted to determine if 5-MOP could prevent APAP-induced hepatotoxicity. In addition, although the antioxidative activity of 5-MOP has been evidenced from researches in vitro, this activity was not manifested in vivo. Besides, 5-MOP may also cause hepatotoxicity when taken at high doses.
This study manifested that 5-MOP could protect mice from APAP-induced hepatotoxicity in vivo, and this hepatoprotective effect was associated with its antioxidation activities. In addition, 5-MOP caused no hepatotoxicity at protective doses.
This study has suggested that 5-MOP can be used at appropriate doses as a drug against APAP-induced hepatotoxicity in human. However, before clinical use, more researches are needed to confirm the safety of APAP administration at protective doses.
The authors investigated the protective effects of 5-MOP against APAP-induced hepatotoxicity and whether 5-MOP could cause hepatotoxicity in mice. The results suggested that 5-MOP resisted APAP-induced hepatotoxicity, reduced APAP-induced oxidative stress, and did not cause liver injury at protective doses.
| 1. | Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1295] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 2. | Lee WM, Squires RH, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology. 2008;47:1401-1415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 515] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 3. | Murray KF, Hadzic N, Wirth S, Bassett M, Kelly D. Drug-related hepatotoxicity and acute liver failure. J Pediatr Gastroenterol Nutr. 2008;47:395-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Norris W, Paredes AH, Lewis JH. Drug-induced liver injury in 2007. Curr Opin Gastroenterol. 2008;24:287-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Craig DG, Ford AC, Hayes PC, Simpson KJ. Systematic review: prognostic tests of paracetamol-induced acute liver failure. Aliment Pharmacol Ther. 2010;31:1064-1076. [PubMed] |
| 6. | Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-Acetyl-P-benzoquinone imine; a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Nat Acad Sci USA. 1984;81:1327-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 707] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 7. | Ramachandran A, Lebofsky M, Weinman SA, Jaeschke H. The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2011;251:226-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Agarwal R, MacMillan-Crow LA, Rafferty TM, Saba H, Roberts DW, Fifer EK, James LP, Hinson JA. Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J Pharmacol Exp Ther. 2011;337:110-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Acharya M, Lau-Cam CA. Comparison of the protective actions of N-acetylcysteine, hypotaurine and taurine against acetaminophen-induced hepatotoxicity in the rat. J Biomed Sci. 2010;17 Suppl 1:S35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Bessems JG, Vermeulen NP. Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol. 2001;31:55-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 470] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 11. | Avila DS, Palma AS, Colle D, Scolari R, Manarin F, da Silveira AF, Nogueira CW, Rocha JB, Soares FA. Hepatoprotective activity of a vinylic telluride against acute exposure to acetaminophen. Eur J Pharmacol. 2011;661:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Ng TB, Liu F, Wang ZT. Antioxidative activity of natural products from plants. Life Sciences. 2000;66:709-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 341] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 13. | Yu J, Wang L, Walzem RL, Miller EG, Pike LM, Patil BS. Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J Agric Food Chem. 2005;53:2009-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 291] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 14. | Lee YM, Wu TH. Effects of 5-methoxypsoralen (5-MOP) on arylamine N-acetyltransferase activity in the stomach and colon of rats and human stomach and colon tumor cell lines. In Vivo. 2005;19:1061-1069. [PubMed] |
| 15. | Viola G, Vedaldi D, Dall'Acqua F, Fortunato E, Basso G, Bianchi N, Zuccato C, Borgatti M, Lampronti I, Gambari R. Induction of gamma-globin mRNA, erythroid differentiation and apoptosis in UVA-irradiated human erythroid cells in the presence of furocumarin derivatives. Biochem Pharmacol. 2008;75:810-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Guerrini A, Lampronti I, Bianchi N, Zuccato C, Breveglieri G, Salvatori F, Mancini I, Rossi D, Potenza R, Chiavilli F. Bergamot (Citrus bergamia Risso) fruit extracts as γ-globin gene expression inducers: phytochemical and functional perspectives. J Agric Food Chem. 2009;57:4103-4111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Salvador A, Dall'Acqua S, Sardo MS, Caffieri S, Vedaldi D, Dall'Acqua F, Borgatti M, Zuccato C, Bianchi N, Gambari R. Erythroid induction of chronic myelogenous leukemia K562 cells following treatment with a photoproduct derived from the UV-A irradiation of 5-methoxypsoralen. ChemMedChem. 2010;5:1506-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Panno ML, Giordano F, Mastroianni F, Palma MG, Bartella V, Carpino A, Aquila S, Andò S. Breast cancer cell survival signal is affected by bergapten combined with an ultraviolet irradiation. FEBS Lett. 2010;584:2321-2326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Lee YM, Wu TH, Chen SF, Chung JG. Effect of 5-methoxypsoralen (5-MOP) on cell apoptosis and cell cycle in human hepatocellular carcinoma cell line. Toxicol In vitro. 2003;17:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Souetre E, Salvati E, Belugou JL, de Galeani B, Krebs B, Ortonne JP, Darcourt G. 5-Methoxypsoralen increases the plasma melatonin levels in humans. J Invest Dermatol. 1987;89:152-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Souêtre E, Salvati E, Belugou JL, Robert P, Brunet G, Darcourt G. Antidepressant effect of 5-methoxypsoralen: a preliminary report. Psychopharmacology (Berl). 1988;95:430-431. [PubMed] |
| 22. | Souêtre E, Salvati E, Belugou JL, Krebs B, Darcourt G. 5-Methoxypsoralen increases evening sleepiness in humans: possible involvement of the melatonin secretion. Eur J Clin Pharmacol. 1989;36:91-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Souetre E, Salvati E, Belugou JL, Krebs B, Darcourt G. 5-Methoxypsoralen as a specific stimulating agent of melatonin secretion in humans. J Clin Endocrinol Metab. 1990;71:670-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Darcourt G, Feuillade P, Bistagnin Y, Robert P, Pringuey D, Touari M, Merdji Y, Bensmaïl B. Antidepressant effect of 5-methoxypsoralen: The melatonin synchronizer hypothesis. Eur Psychiatry. 1995;10:142-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Tosun F, Kızılay CA, Erol K, Kılıç FS, Kürkçüoğlu M, Başer KHC. Anticonvulsant activity of furanocoumarins and the essential oil obtained from the fruits of Heracleum crenatifolium. Food Chemistry. 2008;107:990-993. [RCA] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Nicolis E, Lampronti I, Dechecchi MC, Borgatti M, Tamanini A, Bezzerri V, Bianchi N, Mazzon M, Mancini I, Giri MG. Modulation of expression of IL-8 gene in bronchial epithelial cells by 5-methoxypsoralen. Int Immunopharmacol. 2009;9:1411-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Bose SK, Dewanjee S, Sahu R, Dey SP. Effect of bergapten from Heracleum nepalense root on production of proinflammatory cytokines. Nat Prod Res. 2011;25:1444-1449. [PubMed] |
| 28. | Berg M, Ros AM. Treatment of psoriasis with psoralens and ultraviolet A. A double-blind comparison of 8-methoxypsoralen and 5-methoxypsoralen. Photodermatol Photoimmunol Photomed. 1994;10:217-220. [PubMed] |
| 29. | Stephens RB, Cooper A. Hepatitis from 5-methoxypsoralen occurring in a patient with previous flucloxacillin hepatitis. Australas J Dermatol. 1999;40:217-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Diawara MM, Williams DE, Oganesian A, Spitsbergen J. Dietary psoralens induce hepatotoxicity in C57 mice. J Nat Toxins. 2000;9:179-195. [PubMed] |
| 31. | Pang ZJ, Zhou M, Chen A. Medical Methods of Free Radicals. Beijing: People’s Health Publishing House 2000; 61-64. |
| 32. | Shen HQ, Zhao LY, Qu QS, Jiang QG. Fluorescent method for determination of glutathione in tissue. Zhonghua Laodong Weisheng Zhiyebing Zazhi. 1988;6:103-108. |
| 33. | Zhang BX, Jia FL, Ruan M. Mechanism investigation of acetaminophen induced hepatotoxicity in mice. Weisheng Dulixue Zazhi. 2003;17:31-33. |
| 34. | Giboney PT. Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Physician. 2005;71:1105-1110. [PubMed] |
| 35. | Laine JE, Auriola S, Pasanen M, Juvonen RO. Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica. 2009;39:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
Peer reviewer: Dr. SV Rana, Professor, Department of Gastroenterology, Postgraduate Institute of Medical Education and Research, House No. 137, Sector 15-A, Chandigarh 160015, India
S- Editor Gou SX L- Editor Ma JY E- Editor Xiong L