Published online Apr 28, 2012. doi: 10.3748/wjg.v18.i16.1940
Revised: December 20, 2011
Accepted: March 10, 2012
Published online: April 28, 2012
AIM: To evaluate safety and feasibility of microcoil embolization of the common hepatic artery under proper or distal balloon inflation in preoperative preparation for en bloc celiac axis resection for pancreatic body cancer.
METHODS: Fifteen patients (11 males, 4 females; median age, 67 years) with pancreatic body cancer involving the nerve plexus surrounding the celiac artery underwent microcoil embolization. To alter the total hepatic blood flow from superior mesenteric artery (SMA), microcoil embolization of the common hepatic artery (CHA) was conducted in 2 cases under balloon inflation at the proximal end of the CHA and in 13 cases under distal microballoon inflation at the distal end of the CHA.
RESULTS: Of the first two cases of microcoil embolization with proximal balloon inflation, the first was successful, but there was microcoil migration to the proper hepatic artery in the second. The migrated microcoil was withdrawn to the CHA by an inflated microballoon catheter. Microcoil embolization was successful in the other 13 cases with distal microballoon inflation, with no microcoil migration. Compact microcoil embolization under distal microballoon inflation created sufficient resistance against the vascular wall to prevent migration. Distal balloon inflation achieved the requisite 1 cm patency at the CHA end for vascular clamping. All patients underwent en bloc celiac axis resection without arterial reconstruction or liver ischemia.
CONCLUSION: To impede microcoil migration to the proper hepatic artery during CHA microcoil embolization, distal microballoon inflation is preferable to proximal balloon inflation.
- Citation: Takasaka I, Kawai N, Sato M, Tanihata H, Sonomura T, Minamiguchi H, Nakai M, Ikoma A, Nakata K, Sanda H. Preoperative microcoil embolization of the common hepatic artery for pancreatic body cancer. World J Gastroenterol 2012; 18(16): 1940-1945
- URL: https://www.wjgnet.com/1007-9327/full/v18/i16/1940.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i16.1940
The overall 5-year survival rate after surgical resection for pancreatic cancer is extremely poor (< 10%)[1-3]. There is an ongoing effort toward treating this difficult disease and improving survival. Surgery plays a main role for a complete cure of pancreatic body cancer. En bloc celiac axis resection (modified Appleby operation) has been introduced to expand the surgical treatment for pancreatic body cancer with celiac axis involvement[4,5], and Hirano et al[6] report a promising estimated 5-year survival rate of 42% for locally advanced pancreatic body cancer. This surgical procedure has the aim of en bloc lymphadenectomy together with resection of the spleen, pancreatic body, and tail by ligation of the celiac trunk artery and common hepatic artery (CHA). The safety of this operation is based on the rationale that hepatic arterial blood is supplied from the superior mesenteric artery via the pancreatico-duodenal arcades following ligation of the CHA. However, weak pulsation of the proper hepatic artery was observed in some patients during surgery, immediately after surgical ligation of the CHA[7-9]. When poor pulsation of the proper hepatic artery is observed after clamping of the CHA, arterial reconstruction is necessary because liver necrosis is fatal once it occurs[10-13]. To avoid this complicated procedure, Kondo et al[14] reported the preparatory technique of enlarging the collateral pathways from the SMA before surgery, by preoperative embolization of the CHA using interlocking detachable coils. Following this report, surgeons first asked interventional radiologists to embolize the CHA in preoperative management to enlarge the collateral pathways from the superior mesenteric artery (SMA). However, exact microcoil embolization of the short segment of the CHA is not easy, even for experienced interventional radiologists, because of its rapid arterial flow and, of particular concern, the possibility of coil migration to the proper hepatic artery. In our case, the surgeon also asked that we retain vascular lumen patency 1 cm from the distal end of the common hepatic artery and the proximal celiac artery trunk, to enable clamping of these vessels. In response to these requirements, we conducted microcoil embolization of the CHA under either proximal or distal microballoon inflation. The purpose of this clinical study is to describe these techniques and to evaluate their safety and feasibility.
Approval of the Institutional Ethics Committee of our institution was obtained for this clinical trial prior to initiation of the study. All patients were fully informed of the extent of their diseases and of the risks and benefits associated with preoperative CHA embolization and en bloc resection.
In cases of right or common hepatic artery branching from the SMA, it was not necessary to conduct microcoil embolization of the CHA. Between May 2007 and January 2010, 15 patients with pancreatic body cancer involving the nerve plexus surrounding the celiac artery were scheduled for surgical radical pancreatectomy and underwent microcoil embolization preoperatively. Tumor stage was T4 in 14 patients and T3 in 1 patient according to the tumor, node and metastasis classification of the Union for International Cancer Control (UICC)[15]. Eleven patients were male and 4 were female; age ranged from 46 to 79 years (median, 67 years) (Table 1). All patients suffered from severe back pain and/or abdominal pain. Enhanced computed tomography using contrast medium revealed tumors sized 10-76 mm located in the body to the tail of the pancreas and involving the celiac, splenic, and/or common hepatic arteries, but with no evidence of liver metastases or invasion to the superior mesenteric artery. Microcoil embolization of the common hepatic artery was performed 7 d to 14 d before surgery. Two interventional radiologists, each with more than 7 years experience in transcatheter arterial embolization, conducted the following procedure.
| Tumor | Coil used | ||||||||
| N | Age (yr) | Sex | Major axis (mm) | Stage (UICC TNM ver.6) | Balloon catheter inflation proximal/distal | Coil migration | CHA diameter (mm) | Detachable coil diameter (mm)/length (cm) × N | Fiber coil proximal/distal diameters (mm) × N |
| 1 | 51 | F | 10 | T4 | +/- | - | 7.0 | 8/20 | |
| 2 | 70 | M | 35 | T4 | +/- | + | 6.7 | 10/10 | |
| 3 | 67 | M | 40 | T4 | +/+ | - | 6.1 | 8/20 | 4/8 x 3 |
| 4 | 56 | M | 46 | T4 | -/+ | - | 5.6 | 7/10 | 4/8 × 2 |
| 5 | 64 | M | 30 | T4 | -/+ | - | 7.4 | 10/20, 7/20, 6/10 | |
| 6 | 71 | M | 61 | T4 | -/+ | - | 6.4 | 9/20, 7/20, 6/10 | |
| 7 | 71 | M | 35 | T4 | -/+ | - | 7.7 | 10/10 | 7/3 × 2 |
| 8 | 54 | M | 31 | T4 | -/+ | - | 4.5 | 6/10 × 2, 5/10 | 6/2 × 2 |
| 9 | 69 | F | 76 | T3 | -/+ | - | 5.1 | 7/10 | 7/3 × 2, 8/4 × 3 |
| 10 | 46 | M | 35 | T4 | -/+ | - | 4.4 | 6/10 | 6/2 × 4 |
| 11 | 78 | F | 42 | T4 | -/+ | - | 6.4 | 8/10, 6/10 | 6/2 |
| 12 | 64 | M | 45 | T4 | -/+ | - | 2.1 | 3/10 | 3/2 |
| 13 | 74 | F | 37 | T4 | -/+ | - | 3.6 | 5/10 | 6/2, 5/2 |
| 14 | 62 | M | 34 | T4 | -/+ | - | 4.4 | 6/10 | 7/3 × 3 |
| 15 | 79 | M | 45 | T4 | -/+ | - | 5.5 | 7/10 | 8/4 × 3 |
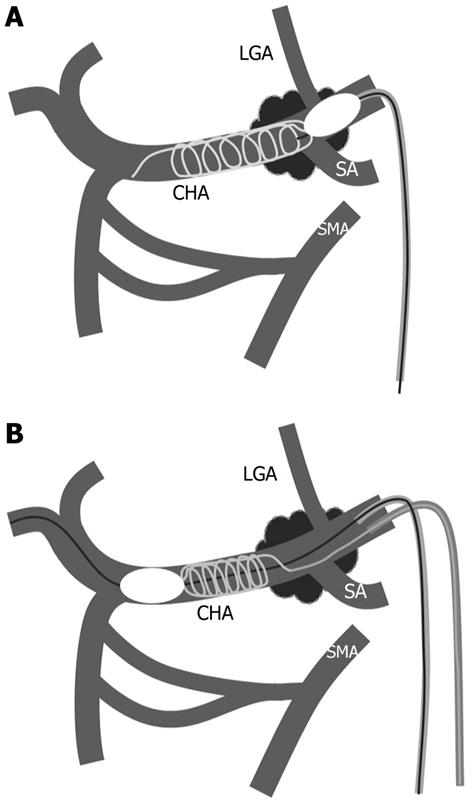
Microcoil embolization under proximal balloon inflation: A 5F balloon catheter (balloon diameter 10 mm; Selecon MP, Catheter Rosch II; Terumo, Tokyo, Japan) was inserted into the celiac artery through a 6F long sheath (Terumo) via the right femoral artery. After celiac arteriography, a 5F balloon catheter was advanced to the CHA using a guide wire (0.035, angle type; Radiofocus, Terumo). Under balloon inflation at the proximal end of the CHA, a microcatheter with two markers for detachable coil embolization (Rapidtransit, Johnson and Johnson, New Brunswick, NJ) was inserted coaxially and advanced to the distal CHA, 1 cm before the branching of the gastroduodenal artery. Detachable microcoils (interlocking detachable coil, Boston Scientific, Boston, MA) of diameter at least 1 mm greater than that of the CHA were deployed by making a lengthwise and/or sidewise frame (Figure 1A).
Microcoil embolization under distal balloon inflation: 6F and 4F long sheaths (Terumo) were inserted via the right and left femoral arteries, respectively. A 6F guiding catheter (Elway, Terumo Clinical Supply, Tochigi, Japan) was advanced through a 6F sheath to the celiac artery. After celiac arteriography, a 3.3F microballoon catheter (8 mm in maximum inflated diameter, 1 cm in length; Iiguman, Fuji System, Tokyo, Japan) was inserted into the common hepatic artery through a 6F guiding catheter and placed at the distal end of the CHA (Figure 1B). The microballoon was inflated, taking care not to interrupt blood flow in the gastroduodenal artery (Figure 2A). A 4F catheter (Rosch celiac type, Medikit, Tokyo, Japan) was advanced through a 4F sheath to catheterize the celiac artery, and a microcatheter with two markers was then advanced to the proximal end of the balloon inflation (Figure 2A). Detachable coils of diameter greater than that of the CHA were used initially, and fiber coils (Tornado, Boston Scientific) were added to fill the space if necessary. Microcoils were placed in the CHA from the proximal end of the microballoon inflation to the inlet of the left gastric artery (LGA) branching from the celiac trunk artery (Figure 2B). In fluoroscopic guidance, the tube angle that enabled the best visualization of the CHA or LGA was used. After microcoil embolization, the microballoon catheter was deflated and withdrawn.
Microcoil embolization under distal balloon inflation and proximal balloon inflation at the time of withdrawal: After microcoil embolization of the CHA under distal balloon inflation, as described above, a 5F balloon catheter (Selecon MP, Catheter Rosch II, Terumo) was inserted via the femoral artery to the celiac artery through a 6F long sheath. This 5F balloon catheter was used to prevent microcoil migration from the CHA to the celiac trunk artery. Specifically, when the distal balloon catheter was deflated and withdrawn, the 5F balloon catheter was inflated at the CHA proximal end or celiac trunk artery. After confirming no proximal coil migration, the proximal balloon catheter was deflated, and both balloon catheters were removed.
After coil embolization in each procedure, superior mesenteric arteriography was conducted to confirm the alteration of blood flow from the SMA to the hepatic artery (Figure 2C). We aimed for total hepatic arterial blood flow to be supplied from the superior mesenteric artery. When the left hepatic artery branched from the LGA, microcoil embolization of left gastric artery was also performed.
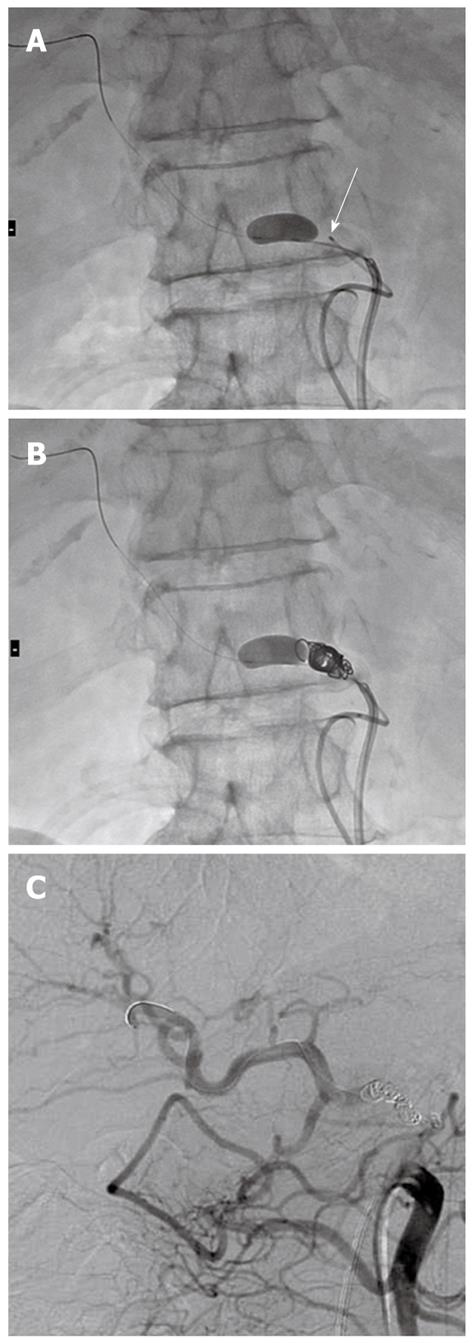
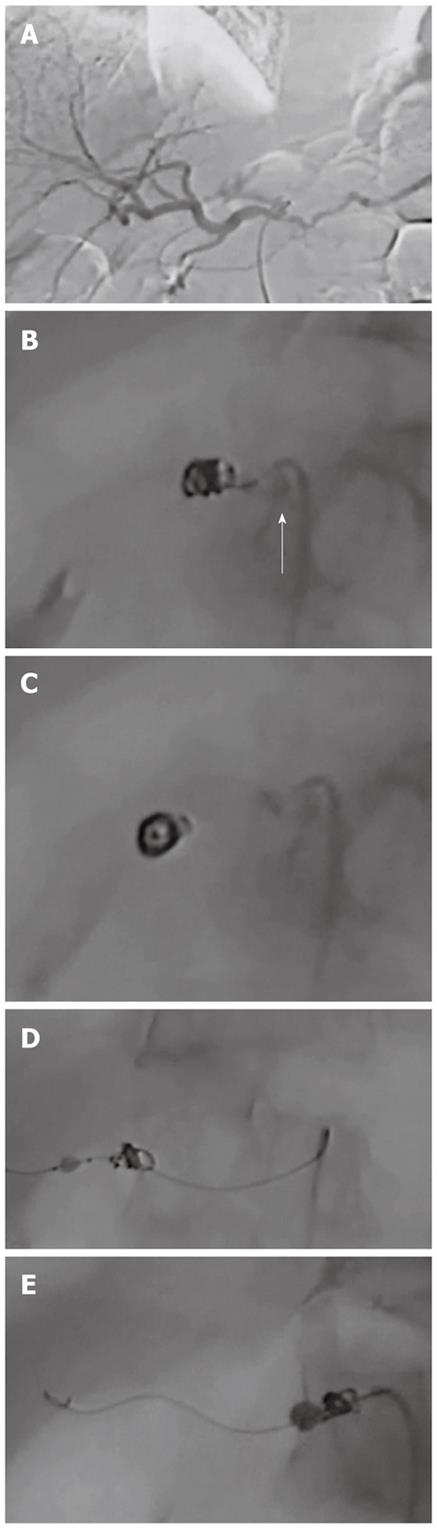
In the present series, the first two patients underwent microcoil embolization of the CHA under proximal balloon inflation. Successful microcoil embolization of the CHA was completed in the first case. However, in the second case, distal migration of the microcoils occurred from the CHA to the proper hepatic artery after deflation of the proximal balloon catheter following microcoil placement. When this occurred, we immediately inserted the deflated microballoon catheter into the proper hepatic artery, advanced the catheter beyond the migrated microcoil, inflated the balloon catheter, and successfully withdrew the migrated coil to the common hepatic artery (Figure 3).
The third case underwent microcoil embolization under distal microballoon inflation. At the time of withdrawing the distal deflated balloon catheter located at the distal end of the CHA, we inflated the proximal balloon at the proximal end of the CHA to prevent microcoil migration from the CHA to the celiac trunk artery. However, we realized that the second (proximal) balloon catheter was not necessary because of the rigid fixation of the microcoils against the vascular wall. Thereafter, the distal deflated microballoon catheter was withdrawn without the assistance of proximal balloon inflation. There was no microcoil migration in the following 12 cases under distal microballoon inflation (Table 1). Accordingly, distal balloon inflation achieved the 1 cm patency at the end of the CHA required for vascular clamping.
In three cases having the variation of left hepatic artery branching from the LGA, we performed additional embolization of the LGA trunk using microcoils. In two of these three cases, total hepatic arterial blood flow was confirmed in superior mesenteric arteriography to come from the SMA via intrahepatic communication between the right and left hepatic arteries. In the remaining case, there was communication between the LGA and the short gastric artery and left gastroepiploic artery, which branched from the splenic artery. In this case, LGA and the splenic artery were embolized, aiming to achieve total hepatic blood flow from the SMA. However, following these embolizations, hepato-petal blood flow did not come from the SMA but from the inferior phrenic artery. No evidence of liver ischemia was observed during surgery, enabling radical pancreatectomy to be performed.
Superior mesenteric arteriography after embolization showed good hepato-petal blood flow from the SMA to the proper hepatic artery in all cases except that described above. All patients successfully underwent radical pancreatectomy without liver or gastric ischemia, and experienced no problems or complications related to the microcoil embolization.
In the present series, microcoil embolization under distal balloon inflation was superior to that under proximal balloon inflation in terms of impeding distal embolization. White[16] described two techniques of coil placement in the pulmonary artery: an anchor technique in which the microcoil tip was hooked into the small branch, and a scaffold technique in which a long frame was created lengthwise to increase friction against the vessel wall and fill the feeding artery. There are no small branch arteries from the CHA; therefore, using the scaffold technique we made a lengthwise and sidewise frame, by pushing and pulling the coils. If the microcoils continued to push out under proximal balloon inflation, then they tended to move to a more peripheral site than intended; in this case, the microcoils needed to be pulled back. In the second case of the present series, under proximal balloon inflation the microcoils migrated to the proper hepatic artery despite the microcoils having a diameter 3 mm greater than that of the CHA; this probably occurred because the loose frame of placed microcoils did not create sufficient friction against the vascular wall. The migrated microcoils were pulled back by the distal inflated balloon catheter to the original CHA site. We found that microcoils placed under distal balloon inflation became more compact than those under proximal balloon inflation, creating enough friction against the vascular wall to prevent migration.
We anticipated that the microcoils could migrate from the CHA to the celiac trunk artery or abdominal aorta after retrieval of the deflated distal microballoon catheter following microcoil embolization. For this reason, in the third case of this series, an additional balloon catheter was inserted and inflated in the celiac trunk artery, with the aim of blocking proximal coil migration from the CHA to the celiac trunk. However, this precaution proved unnecessary. The rigid friction of the microcoils against the vessel wall resulted in no proximal migration when the distal deflated microballoon catheter was retrieved. The use of detachable coils of diameter 1-2 mm greater than that of the CHA was sufficient to create increased friction in the subsequent 12 cases under distal microballoon inflation.
As an additional positive outcome of the described technique, the long dimension of the inflated microballoon (10 mm) enables patency to be maintained at the distal CHA end. Accordingly, the distal microballoon inflation method fulfilled the surgeon’s requirement to retain 1 cm patency at the distal CHA end for vascular clamping.
It is a weakness of the distal microballoon catheter method that performing the procedure is somewhat complicated. However, the dual femoral artery approach is minimally invasive, and maneuvering the microballoon catheter does not have a steep learning curve.
In conclusion, the distal microballoon inflation method in CHA microcoil embolization was preferable to the proximal balloon inflation method, in terms of creating a compact microcoil frame that caused no coil migration to the proper hepatic artery, and of supplying a sufficient length of CHA patency to enable vascular clamping.
In preoperative management to avoid liver ischemia during surgery for pancreatic body cancer, the surgeons requested the interventional radiologists to embolize the common hepatic artery, to enlarge the collateral pathways from the superior mesenteric artery.
This surgical procedure has the aim of en bloc lymphadenectomy together with resection of the spleen, pancreatic body, and tail by ligation of the celiac trunk artery and common hepatic artery (CHA). The safety of this operation is based on the rationale that hepatic arterial blood is supplied from the superior mesenteric artery via the pancreatico-duodenal arcades following ligation of the CHA.
The previous method using microcoils was conducted to occlude CHA without a balloon catheter. The present study occluded the CHA using microcoil embolization with the assistance of a microballoon catheter.
As the actual application for CHA occlusion, the distal microballoon inflation method in CHA microcoil embolization was preferable to the proximal balloon inflation method, in terms of creating a compact microcoil frame that caused no coil migration to the proper hepatic artery, and of supplying a sufficient length of CHA patency to enable vascular clamping.
For surgical treatment for pancreas body cancer, en bloc lymphadenectomy is performed together with resection of the spleen, pancreatic body, and tail by ligation of the celiac trunk artery and CHA. Because of the risk of liver ischemia following ligation of the CHA during surgery, preoperative occlusion of the CHA using microcoil embolization is necessary.
This study describes an interesting variant of interventional radiology that allows to minimize ischemic hepatic damage in Appleby operation. All technical steps are well and clearly described. Results are encouraging and convincing. Figure are clear and pertaining. The study is important for surgeons and interventional radiologists, even if only 15 patients have been enrolled.
| 1. | Ohigashi H, Ishikawa O, Eguchi H, Takahashi H, Gotoh K, Yamada T, Yano M, Nakaizumi A, Uehara H, Tomita Y. Feasibility and efficacy of combination therapy with preoperative full-dose gemcitabine, concurrent three-dimensional conformal radiation, surgery, and postoperative liver perfusion chemotherapy for T3-pancreatic cancer. Ann Surg. 2009;250:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 2. | Imamura M, Doi R, Imaizumi T, Funakoshi A, Wakasugi H, Sunamura M, Ogata Y, Hishinuma S, Asano T, Aikou T. A randomized multicenter trial comparing resection and radiochemotherapy for resectable locally invasive pancreatic cancer. Surgery. 2004;136:1003-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Takao S, Shinchi H, Aikou T. Is Pylorus-preserving Pancreatico-duodenectomy an Adequate Operation for Stage IVa Cancer of the Head of the Pancreas? J Jpn Surg. 1999;32:2437-2442. |
| 4. | Takenaka H, Iwase K, Ohshima S, Hiranaka T. A new technique for the resection of gastric cancer: modified Appleby procedure with reconstruction of hepatic artery. World J Surg. 1992;16:947-951. [PubMed] |
| 5. | Appleby LH. The coeliac axis in the expansion of the operation for gastric carcinoma. Cancer. 1953;6:704-707. [PubMed] |
| 6. | Hirano S, Kondo S, Hara T, Ambo Y, Tanaka E, Shichinohe T, Suzuki O, Hazama K. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer: long-term results. Ann Surg. 2007;246:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 245] [Article Influence: 12.9] [Reference Citation Analysis (2)] |
| 7. | Furukawa H, Hiratsuka M, Iwanaga T. A rational technique for surgical operation on Borrmann type 4 gastric carcinoma: left upper abdominal evisceration plus Appleby's method. Br J Surg. 1988;75:116-119. [PubMed] |
| 8. | Gagandeep S, Artinyan A, Jabbour N, Mateo R, Matsuoka L, Sher L, Genyk Y, Selby R. Extended pancreatectomy with resection of the celiac axis: the modified Appleby operation. Am J Surg. 2006;192:330-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 9. | Sperti C, Berselli M, Pedrazzoli S. Distal pancreatectomy for body-tail pancreatic cancer: is there a role for celiac axis resection? Pancreatology. 2010;10:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 10. | Wu X, Tao R, Lei R, Han B, Cheng D, Shen B, Peng C. Distal pancreatectomy combined with celiac axis resection in treatment of carcinoma of the body/tail of the pancreas: a single-center experience. Ann Surg Oncol. 2010;17:1359-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Stitzenberg KB, Watson JC, Roberts A, Kagan SA, Cohen SJ, Konski AA, Hoffman JP. Survival after pancreatectomy with major arterial resection and reconstruction. Ann Surg Oncol. 2008;15:1399-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (2)] |
| 12. | Amano H, Miura F, Toyota N, Wada K, Katoh K, Hayano K, Kadowaki S, Shibuya M, Maeno S, Eguchi T. Is pancreatectomy with arterial reconstruction a safe and useful procedure for locally advanced pancreatic cancer? J Hepatobiliary Pancreat Surg. 2009;16:850-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Iizuka I, Katyama K, Tanaka Y, Konishi T, Idezuki Y, Maruyama Y, Wada T. Postoperative Complications of Appleby’s Operation: Complications due to Visceral Ischemia and their Prevention. Jpn J Gastroenterol Surg. 1987;20:40-48. |
| 14. | Kondo S, Katoh H, Shimizu T, Omi M, Hirano S, Ambo Y, Okushiba S, Morikawa T. Preoperative embolization of the common hepatic artery in preparation for radical pancreatectomy for pancreas body cancer. Hepatogastroenterology. 2000;47:1447-1449. [PubMed] |
| 15. | UICC TNM Classification of Malignant Tumors. 6th Ed. Sobin L, Wittekind C, editors. New York: Wiley-Liss 2002; 93-96. |
| 16. | White RI. Pulmonary arteriovenous malformations: how do I embolize? Tech Vasc Interv Radiol. 2007;10:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
Peer reviewer: Paolo Aurello, MD, PhD, Department of Surgery, University of Rome “La Sapienza”, Faculty of Medicine 2,
S- Editor Shi ZF L- Editor A E- Editor Zhang DN