Published online Apr 21, 2012. doi: 10.3748/wjg.v18.i15.1806
Revised: February 28, 2012
Accepted: March 20, 2012
Published online: April 21, 2012
AIM: To investigate the association of Rab27A and Rab27B expression with clinicopathological characteristics and prognosis of hepatocellular carcinoma (HCC).
METHODS: We used reverse transcription polymerase chain reaction (RT-PCR), real-time PCR, and Western blotting to detect Rab27A and Rab27B mRNA and protein expression in 5 human HCC lines and the immortalized hepatic HL-7702 cell line. We further examined 148 primary HCC samples matched with adjacent normal tissue and 80 non-HCC specimens by immunohistochemistry to evaluate the correlation of Rab27A and Rab27B expression with clinicopathological features and prognosis.
RESULTS: Our data showed that Rab27A and Rab27B were differentially expressed in cell lines and primary HCC tumors. Rab27A mRNA and protein were detected in 67% (4/6) of human cell lines and 80% (4/5) of HCC cell lines, while Rab27B was found in 50% (3/6) of human lines and 40% (2/5) of HCC lines. Rab27A expression was higher in primary HCC (46.2%, 66/143) than in matched adjacent tissue (24.3%, 33/136, P < 0.001), whereas immunopositivity for Rab27B was lower in primary HCC (57.4%, 81/141) than in matched adjacent tissue (87.5%, 119/136, P < 0.001). Analysis of clinicopathological characteristics of 148 HCC specimens revealed significant correlations between Rab27A and Rab27B expression and tumor tumor-node-metastasis (TNM) classification (P = 0.046 and P = 0.027, respectively), and between strong Rab27A expression and tumor differentiation grade (P = 0.008). Survival analyses revealed that patients with Rab27A+ or Rab27B+ tumors had significantly reduced overall survival compared with that of patients with Rab27A- or Rab27B- tumors (P = 0.015 and P = 0.005, respectively). Risk analyses revealed that Rab27B+ and TNM III-IV were independent poor prognosis factors associated with a 3.36- and 3.37- fold higher relative risk of death, respectively.
CONCLUSION: Rab27A and Rab27B expression were closely correlated with tumor progression and can be valuable prognostic indicators for HCC patients.
- Citation: Dong WW, Mou Q, Chen J, Cui JT, Li WM, Xiao WH. Differential expression of Rab27A/B correlates with clinical outcome in hepatocellular carcinoma. World J Gastroenterol 2012; 18(15): 1806-1813
- URL: https://www.wjgnet.com/1007-9327/full/v18/i15/1806.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i15.1806
Primary hepatocellular carcinoma (HCC) is one of the top 10 most frequent tumor types globally, carrying a high mortality rate and accounting for more than 1 million deaths annually[1]. The identification of novel biomarkers correlating with HCC progression is critical in order to optimize treatment strategies. A large body of evidence indicates that vesicle trafficking and exocytosis are important in tumorigenesis, with many reports implicating the Rab family of proteins[2-4]. Rabs are a ubiquitously expressed family of small (20-29 kDa) monomeric Ras-like GTPases[5] composed of more than 60 mammalian members, each thought to localize to a distinct subcellular organelle. Rabs function as molecular switches, oscillating between GTP- or GDP-bound conformations, which enables them to reversibly recruit GTP-dependent effectors and elicit their regulatory functions at multiple stages of vesicular transport[6]. Rab27A and Rab27B constitute the Rab27 subfamily and share 71% identity[7]. Rab27A is expressed in a wide variety of secretory cell types, including exocrine, endocrine, ovarian, and hematopoietic cells, most of which function specifically in regulated exocytic pathways[8,9]. Loss-of-function mutations in the human Rab27A gene result in Griscelli syndrome, a rare autosomal disorder characterized by a combination of partial cutaneous albinism and severe immunodeficiency[10,11]. Its clinical picture appears to be the manifestation of defects in 2 specialized lysosome-related organelles: the failure to distribute melanosomes in melanocytes and the inability to release the contents of lytic granules in cytotoxic T lymphocytes[12]. In contrast to Rab27A, Rab27B expression is much more restricted, and is mainly expressed in platelets, the stomach, large intestine, pancreas, pituitary, and bladder[13-16]. Unlike Rab27A, Rab27B has not been well characterized, and no human disease or animal strain with mutations in the Rab27B gene has been identified[8].
Several members of the Rab family have been well studied in cancer. Rab25, for example, has been shown to decrease apoptosis as well as increase the proliferation and aggressiveness of ovarian and breast cancer[17-19]. Increased expression of Rab25 has also been noted in prostate cancer[20], transitional cell carcinoma of the bladder[21], and in colon cancer cells[22]. Hou et al[23] showed that the Rab23 gene is over-expressed in gastric cancer, and has an important role in invasion. In addition, Rab11, Rab4, Rab14 and Rab35, among others, have been studied in various cancers[24]. However, research on Rab27 in cancer has been limited, and focused exclusively on breast cancer. These studies have shown that Rab27A, along with several other metastasis-associated genes, have vesicle trafficking roles, and were differentially expressed in murine xenograft models of breast cancer metastasis[25,26]. Wang et al[27] showed that Rab27A is associated with the invasive and metastatic potential of human breast cancer cells. Overexpression of Rab27A protein in breast cancer cells altered the cell cycle and increased the invasive and metastatic abilities both in vitro and in vivo. However, in another study, it was shown that Rab27B, not Rab27A, regulates invasive growth and metastasis in ER-positive breast cancer cell lines, with increased expression associated with poor prognosis in humans[28]. Despite the link between Rab27 and breast cancer, alterations in Rab27 expression have yet to be explored in other tumor types.
During a previous study of vesicle transport and metastasis in gastric cancer and colorectal cancer (unpublished data), we found a significant correlation between Rab27A and Rab27B expression and clinicopathological characteristics and prognosis. We then examined Rab27A and Rab27B expression in HCC to determine if a similar correlation was present. To accomplish this, we evaluated the expression of Rab27A and Rab27B mRNA and protein in 5 HCC cell lines and the human hepatic HL-7702 cell line. Additionally, we performed parallel immunohistochemical staining of Rab27A and Rab27B in 148 primary HCCs in order to analyze the association with clinicopathological characteristics and clarify the distinct roles of Rab27A and Rab27B in the progression of HCC.
One hundred forty-eight HCC specimens and 80 non-HCC specimens were collected from 182 men and 46 women (age, 29-72 years; mean ± SD, 51.6 ± 8.9 years) who were inpatients at the PLA General Hospital, Beijing, China, from 2005 to 2009. Survival data were available for 120 patients; 52 of 120 patients (43.3%) died of cancer metastasis or local recurrence after surgery. Patient data are shown in Table 1. All patients underwent surgery, and no patients had received chemotherapy or radiation therapy. Tissue microarray blocks containing formalin-fixed and paraffin-embedded human tissues were constructed in our laboratory as described previously[29]. Tumor stage was classified according to the American Joint Committee on Cancer tumor-node-metastasis (TNM) classification. The study was approved by the Research Ethics Boards of the hospitals, and informed consent was obtained from all patients.
| No. of cases | Positive | Negative | P value | |
| Rab27A clinicopathological features | ||||
| All patients | 148 | |||
| Gender | ||||
| Male | 108 (75.5) | 47 (43.5) | 61 (56.5) | |
| Female | 35 (24.5) | 19 (54.3) | 16 (45.7) | NS |
| Age at diagnosis | ||||
| < 60 | 116 (81.1) | 51 (44) | 65 (56) | |
| ≥ 60 | 27 (18.9) | 15 (56.6) | 12 (43.5) | NS |
| Carcinoma and adjacent tissue | ||||
| Carcinoma tissue | 143 (51) | 66 (46.2) | 77 (53.8) | |
| Adjacent tissue | 136 (49) | 33 (24.3) | 103 (75.7) | < 0.001 |
| Hepatic and cirrhosis tissue | ||||
| Normal hepatic tissue | 40 (44.4) | 10 (25) | 30 (75) | |
| Hepatitis and cirrhosis tissue | 50 (55.6) | 13 (26) | 37 (74) | NS |
| Degree of differentiation | ||||
| Well | 39 (27.3) | 2 (5.1) | 37 (94.9) | |
| Moderate | 91 (63.6) | 9 (9.9) | 82 (90.1) | |
| Poor | 13 (9.1) | 5 (38.5) | 8 (61.5) | 0.0081 |
| TNM classification | ||||
| Stage I/II | 106 (74.1) | 41 (38.7) | 65 (61.3) | |
| Stage III/IV | 37 (25.9) | 25 (67.7) | 12 (32.4) | 0.002 |
| HBV | ||||
| Negative | 26 (19.4) | 10 (38.5) | 16 (61.5) | |
| Positive | 108 (80.6) | 53 (49.1) | 55 (50.9) | NS |
| AFP | ||||
| Negative | 42 (32.5) | 15 (35.7) | 27 (64.3) | |
| Positive | 88 (67.7) | 43 (48.9) | 45 (51.1) | NS |
| Type of hepatoma | ||||
| Nodular | 84 (66.7) | 39 (46.4) | 45 (53.6) | |
| Massive | 33 (26.2) | 12 (36.4) | 21 (63.6) | |
| Diffuse | 9 (7.1) | 5 (55.6) | 4 (44.4) | NS |
| Rab27B clinicopathological features | ||||
| All patients | 148 | |||
| Gender | ||||
| Male | 106 (75.2) | 61 (57.5) | 45 (42.5) | |
| Female | 35 (24.8) | 20 (57.1) | 15 (42.9) | NS |
| Age at diagnosis | ||||
| < 60 | 114 (80.9) | 65 (57) | 49 (43) | |
| ≥ 60 | 27 (19.1) | 16 (59.3) | 11 (40.7) | NS |
| Carcinoma and adjacent tissue | ||||
| Carcinoma tissue | 141 (51) | 81 (57.4) | 60 (42.6) | |
| Adjacent tissue | 136 (49) | 119 (87.5) | 17 (12.5) | < 0.001 |
| Hepatic and cirrhosis tissue | ||||
| Normal hepatic tissue | 40 (44) | 29 (72.5) | 11 (27.5) | |
| Hepatitis and cirrhosis tissue | 50 (56) | 38 (76) | 12 (24) | NS |
| Degree of differentiation | ||||
| Well | 39 (27.5) | 26 (66.7) | 13 (33.3) | |
| Moderate | 89 (63.4) | 49 (53.9) | 41 (46.1) | |
| Poor | 13 (9.2) | 8 (61.5) | 5 (38.5) | NS |
| TNM classification | ||||
| Stage I/II | 103 (73) | 51 (49.5) | 52 (50.5) | |
| Stage III/IV | 38 (27) | 30 (78.9) | 8 (21.1) | 0.002 |
| HBV | ||||
| Negative | 27 (19.9) | 17 (63) | 10 (37) | |
| Positive | 109 (80.1) | 59 (54.1) | 50 (45.9) | NS |
| AFP | ||||
| Negative | 43 (33.6) | 23 (53.5) | 20 (46.5) | |
| Positive | 85 (66.4) | 49 (57.6) | 36 (42.4) | NS |
| Type of hepatoma | ||||
| Nodular | 84 (66.7) | 40 (47.6) | 44 (52.4) | |
| Massive | 33 (26.2) | 15 (45.5) | 18 (54.5) | |
| Diffuse | 9 (7.1) | 6 (66.7) | 3 (33.3) | NS |
The human HCC cell lines MHCC97L and MHCC97M3 were a kind gift from Professor Ye SL (FuDan University, Shanghai). Cell lines BEL-7402, Huh-7, SMMC-7721 and HL-7702 were routinely cultured in our laboratory. HL-7702 was cultured in Roswell Park Memorial Institute medium (RPMI 1640, Gibco, Grand Island, NY, United States), supplemented with 20% fetal bovine serum (FBS; Gibco). BEL-7402 and SMMC-7721 cell lines were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum. The remaining cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with 5% FBS. All media contained 100 units/mL penicillin and 100 μg/mL streptomycin. All cell lines were maintained at 37 °C in 5% CO2.
Antigen retrieval was performed for 2.5 min in citrated buffers using a pressure cooker. A mouse monoclonal antibody specific to human Rab27A (diluted 1:50; cat. ab55667, Abcam, United Kingdom) was incubated on the sections for 36 h at 4 °C, followed by incubation with a rabbit anti-Rab27B polyclonal antibody (diluted 1:100; cat. 13412-1-AP, Proteintech Group, United States) overnight at 4 °C. The rest of the immunohistochemistry (IHC) procedure has been previously described[30]. Fewer than 5% specimens were missing from the tissue microarrays. Phosphate buffered saline was substituted for the primary antibodies for a general negative control. All sections were examined microscopically in a blinded fashion and scored by 2 independent pathologists. Rab27A and Rab27B IHC signal was scored on the following scale, taking into account both the proportion of cells stained and the intensity of staining in those cells: score 0: Weak or absent cytoplasmic staining, with less than 5% of cancer cells showing Rab27A or Rab27B localized on the plasma membrane; score 1: Cytoplasmic staining and between 5% and 30% of cancer cells showing Rab27A or Rab27B localized prominently on the plasma membrane; score 2: Cytoplasmic staining and more than 30% of the cancer cells containing Rab27A or Rab27B localized prominently on the membrane.
Total RNA was extracted from cell lines using TRIzol (Qiagen, United States). The prepared RNA (5 μg) was mixed with oligo-dT primers and reverse-transcribed with MMLV reverse transcriptase (Promega, United States) for 60 min at 37 °C, followed by polymerase chain reaction (PCR) amplification with specific primers for Rab27A (F,5’-GAAGCCATAGCACTCGCAGAG-3’,R,5’-ATGACCATTTGATCGCACCA-3’) or Rab27B (F,5’-TGCGGGACAAGAGCGGTTCCG-3’,R,5’-GCCAGTTCCCGAGCTTGCCGTT-3’). PCR amplification was performed in 20 μL using a thermocycler (Biometra, Germany) with the following PCR program: pre-denaturation for 3 min at 94 °C, denaturation for 45 s at 94 °C, annealing for 45 s at 56 °C, extension for 45 s at 72 °C, and a final elongation at 72 °C for 10 min. β-Actin served as an internal positive control. PCR was performed for 24-30 cycles (β-actin 24 cycles; Rab27A 28 cycles; Rab27B 30 cycles). PCR products were analyzed by electrophoresis on a 1.5% agarose gel, and band intensity was measured directly on an Alphaimager 2200 system (Alpha Innotech, San Leandro, CA). Real-time PCR reactions were carried out using Applied Biosystem’s 7500 QPCR System (ABI, Foster, CA). Results were normalized to individual β-actin expression, and data were analyzed according to the relative standard curve. Melting curves for each PCR reaction were generated to ensure the purity of the amplified products.
Equal amounts of protein were electrophoresed on a 12% sodium dodecylsulfate polyacrylamide gel electrophoresis gel and transferred to a polyvinylidene difluoride membrane using standard techniques. Immunoreactivity was tested with anti-Rab27A (diluted 1:100; cat. ab55667, Abcam, United Kingdom) or anti-Rab27B (diluted 1:1200; cat. 13412-1-AP, Proteintech Group, United States) antibodies. Nonspecific binding was blocked by a 5% fat-free milk solution. Rab27A and Rab27B proteins were detected by an enhanced chemiluminescence system (Amersham Pharmacia Biotech).
Statistical analyses were performed using the SPSS software package (version 16.0; SPSS Inc., United States). The χ2 test was used to evaluate relationships between clinicopathological variables and Rab27A and Rab27B expression. Kaplan-Meier survival analysis with the logrank test was used to evaluate the prognosis of patients according to their levels of Rab27A and Rab27B expression. Multivariate analysis was performed with the Cox proportional hazards regression model to assess the effects of different variables on patient survival. Differences were considered significant at P < 0.05.
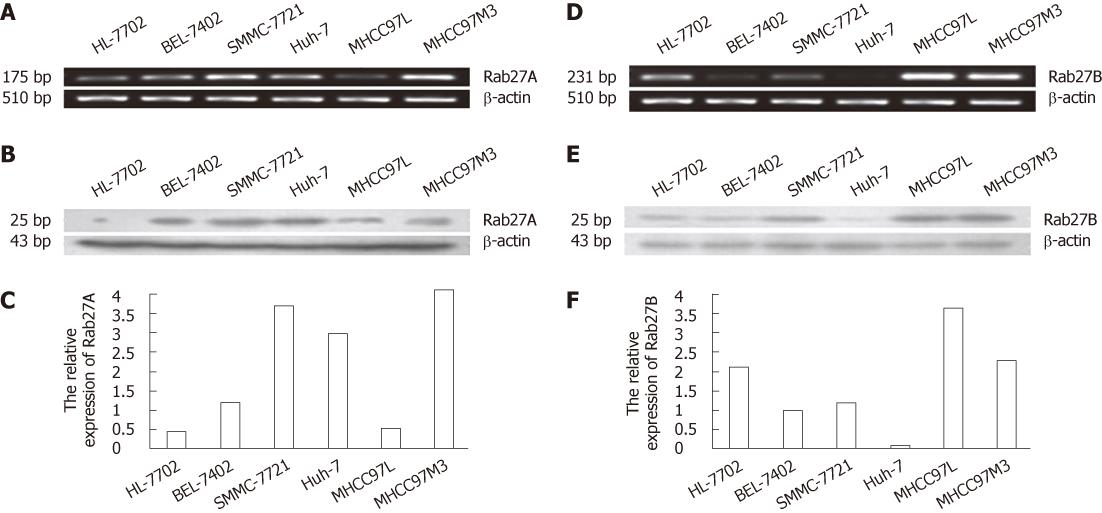
Rab27A and Rab27B mRNA and protein expression were examined in 5 HCC cell lines and the human hepatocyte line HL-7702. As shown in Figure 1, Rab27A mRNA and protein were detected in all 6 cell lines, though at very low intensities in some cell lines, whereas Rab27B was more differentially expressed. Rab27A protein and mRNA were highly expressed in 67% (4/6) of all cell lines and 80% (4/5) of HCC cell lines; Rab27B protein and mRNA were highly expressed in 50% (3/6) of all cell lines and 40% (2/5) of HCC cell lines. Interestingly, Rab27A expression was weaker in the low metastatic cell line MHCC97L than in the high metastatic cell line MHCC97H, whereas Rab27B expression was higher in MHCC97L than in MHCC97H. In addition, Rab27A had only negligible expression in the immortal human hepatocyte line HL-7702, while Rab27B was moderately expressed.
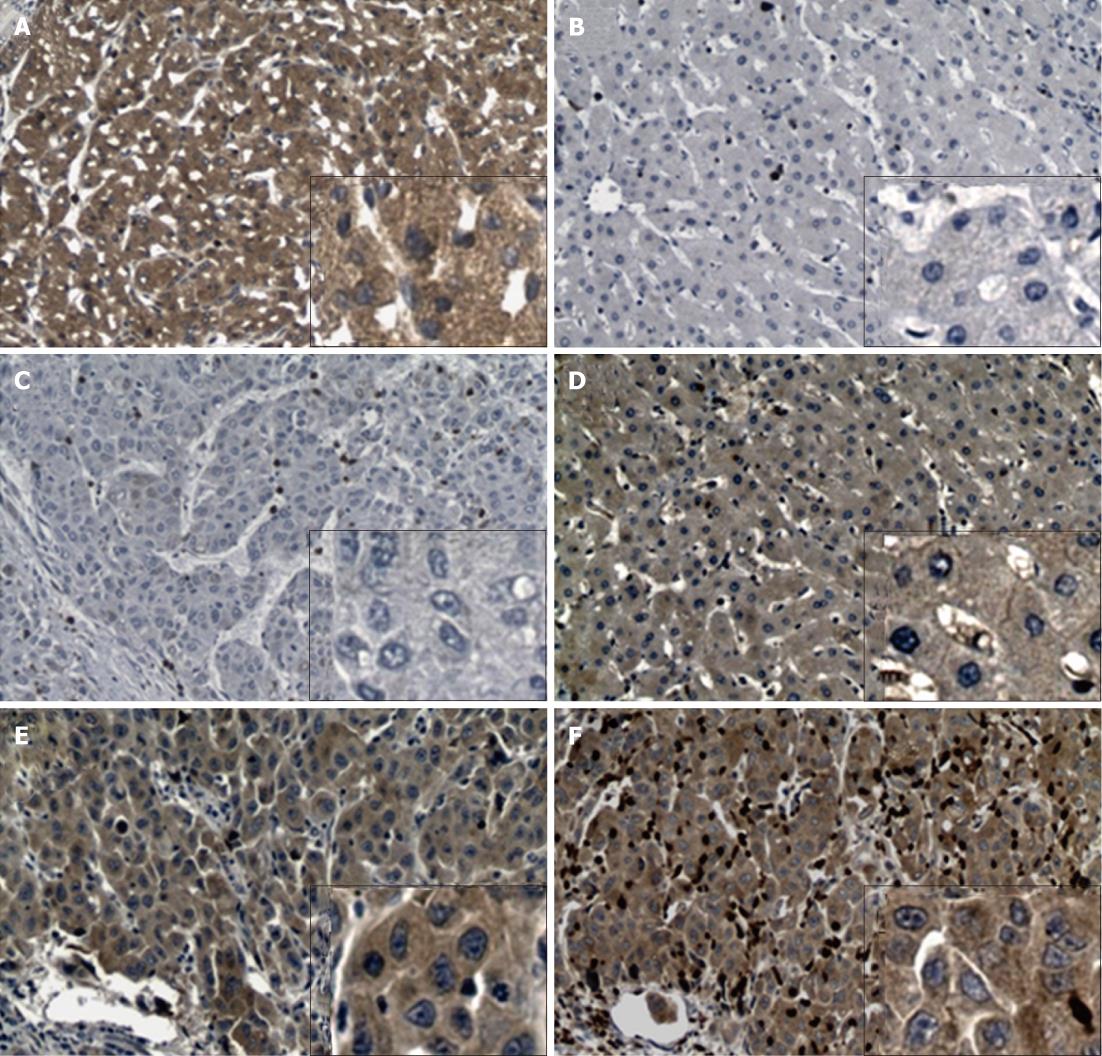
To determine Rab27A and Rab27B expression in HCC specimens, IHC was performed on tumor tissue, tumor-adjacent normal tissue, unrelated normal hepatic tissue, and hepatitis or cirrhosis tissues. In primary HCC tumors, Rab27B and Rab27A were detected in 57.4% (81/141) and 46.2% (66/143) of specimens, respectively (Table 1, Figure 2). In adjacent tissue, Rab27A expression was less apparent, with significantly less positivity (24.3%, 33/136) than that in HCC specimens (P < 0.001); however, Rab27B showed strong immunopositivity (87.5%, 119/136), with significantly higher expression than that in HCC specimens (P < 0.001). In addition, we found no differences in immunostaining for Rab27A and Rab27B between normal (n = 40) and hepatitis or cirrhosis tissues (n = 50), excluding the possibility that alterations in Rab27A and Rab27B expression in primary HCC tumors were caused by hepatitis or liver cirrhosis (Table 1).
Analysis of the clinicopathological characteristics of the 148 HCC specimens revealed significant correlations between Rab27A and Rab27B expression and tumor TNM classification (P = 0.046 and P = 0.027, respectively; Table 1), as well as strong Rab27A expression with tumor differentiation (P = 0.008, Table 1). Moreover, there was a statistically significant correlation between the expression of Rab27A and Rab27B in HCC (P = 0.017; r = 0.192). However, we found no relationship between Rab27A or Rab27B positivity and hepatitis B virus (HBV) status, alpha fetoprotein level, or type of hepatoma. Further subdivision of the HCC specimens using Rab27A and Rab27B expression as covariables (i.e., Rab27A-/Rab27B-, Rab27A-/Rab27B+, Rab27A+/Rab27B-, and Rab27A+/Rab27B+ specimens) showed no significant differences between the 4 groups in relation to clinicopathological features (data not shown).
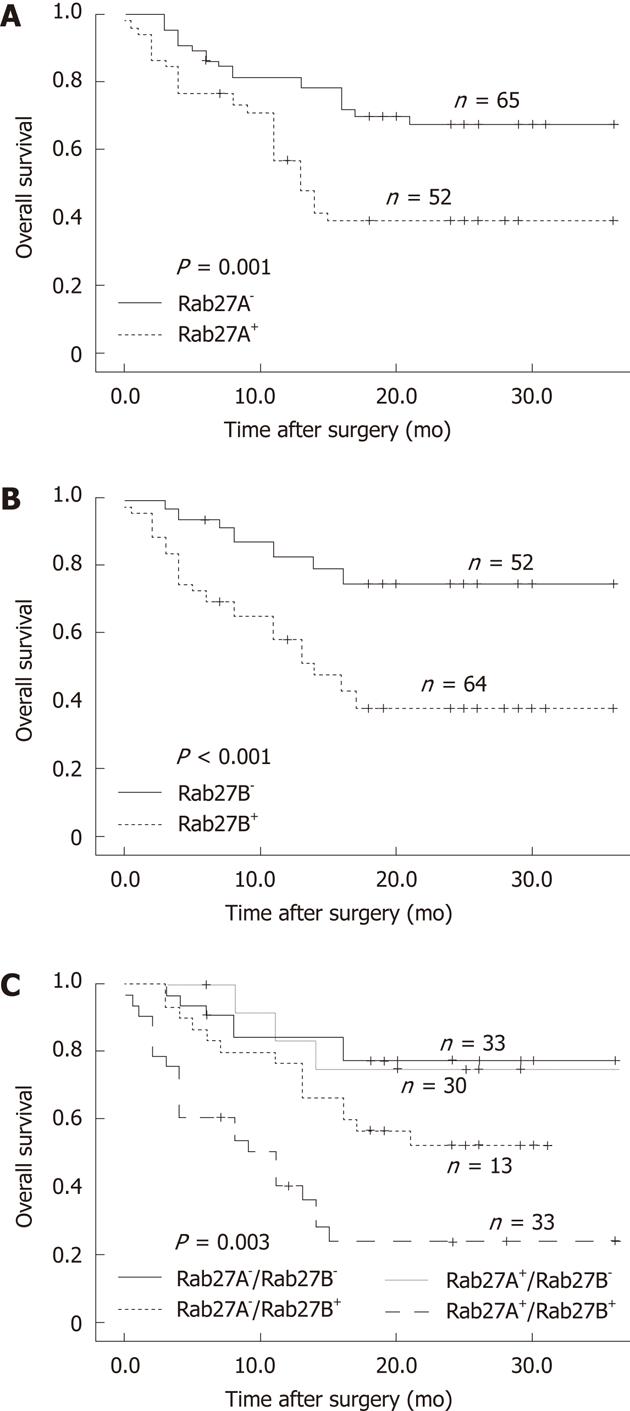
Survival data were available for 120 patients. The overall 1-year survival rate was 71%. Patients with Rab27A+ or Rab27B+ tumors had significantly reduced overall survival compared with that of patients with Rab27A- or Rab27B- tumors (P = 0.015 and P = 0.005, respectively; Figure 3A and B). Subsequently, we analyzed the survival curves of the 4 groups according to the expression status of Rab27A and Rab27B. Patients with Rab27A-/Rab27B- tumors had the longest survival, with the Rab27A+/Rab27B- group showing a similar survival curve; the Rab27A-/Rab27B+ group had lower survival, while the Rab27A+/Rab27B+ group had the poorest survival (P = 0.003, Figure 3C).
To determine relative risk, we analyzed the data with a Cox proportional hazards model using tumor differentiation grade, TNM stage, HBV status, hepatoma type, and Rab27A and Rab27B expression status as covariates. The positive expression of Rab27B as well as TNM III-IV were independent poor prognosis factors associated with a 3.36- and 3.37-fold higher relative risk of death, respectively (95% CI, 1.33-8.5 and 1.391-8.186; P value, 0.01 and 0.007, respectively).
To the best of our knowledge, this is the first study investigating the expression of Rab27A and Rab27B in a large series of HCC patients. Utilizing RT-PCR, real-time PCR, Western blotting, and IHC, we found that Rab27A and Rab27B were strongly associated with tumor TNM stage and may serve as useful biomarkers to monitor the clinical course of patients with HCC. Although the expression of Rab27A and Rab27B was closely related, Rab27B seemed to play a key role in determining prognosis.
Our results revealed that Rab27A and Rab27B were differentially expressed in HCC cell lines and tissue specimens; this indicates that Rab27A and Rab27B may be related to malignant transformation in this carcinoma. In the present study, we found that Rab27A and Rab27B were expressed in the cytoplasm and plasma membrane of hepatic cells. Rab27A was expressed at higher levels in primary HCC than in matched adjacent tissue, whereas Rab27B expression was lower in primary HCC than in matched adjacent tissue. In accordance with these data, Rab27A and Rab27B mRNA and protein were differentially expressed in the 6 human cell lines. The results from the tissue specimens were mirrored in the immortal human hepatocyte line HL-7702, low metastatic cell line MHCC97L, and high metastatic cell line MHCC97H. In addition, Rab27A mRNA and protein were expressed in all 6 cell lines, albeit at different intensities, whereas Rab27B was differentially expressed; this finding is consistent with the findings of previous studies that Rab27A is more widely expressed than Rab27B.
We further analyzed the association between Rab27A and Rab27B expression and clinicopathologic variables utilizing IHC. In HCC specimens, positive expression of Rab27A and Rab27B was strongly correlated with stage III/IV, while negative expression was correlated with stage I/II. Furthermore, strong expression of Rab27A (score 2) also correlated with tumor differentiation. Our study is the first to identify that Rab27A and Rab27B strongly correlate with tumor progression, and provides evidence that Rab27A might function in cell differentiation. Previous research demonstrated that when Rab27A effectors are bound by another protein, Rab27B plays an important role in the regulation of many secretory mechanisms[31]. Another study showed that Rab27A and Rab27B play both redundant and distinct roles in regulating the secretion and released quantity of platelet dense granules[9]. In agreement with these studies, our results provide evidence that Rab27A and Rab27B may have redundant and distinct functions. In addition, numerous studies have demonstrated that tumor cells use exosomes (endosome-derived membrane vesicles) to communicate with surrounding tissues and immune cells, creating a suitably immunosuppressive microenvironment for tumor growth, invasion, and metastasis[32-35]. We hypothesize that Rab27A and Rab27B are associated with tumor progression because of their functions as transport vesicles.
Our study also showed that patients with Rab27A+ or Rab27B+ tumors had significantly reduced overall survival compared with that of patients with Rab27A- or Rab27B- tumors. Moreover, patients with Rab27A-/Rab27B- tumors had the longest survival, while those with Rab27A+/Rab27B+ tumors had the poorest survival. An analysis of the tumor variables as risk factors showed that Rab27B+ and TNM III-IV were independent poor prognosis factors, conferring a 3.36-fold and 3.37-fold greater relative risk of death. These results provide further evidence that Rab27A and Rab27B can serve as useful biomarkers for determining prognosis as well as monitoring the clinical course of patients with HCC. Furthermore, Rab27B is more useful than Rab27A as an independent prognosis factor.
Although studies on Rab27 in breast cancer have provided contradictory results, previous findings are partially consistent with our results. Wang et al[27] showed that overexpression of Rab27A is associated with invasive and metastatic potential in human breast cancer cells both in vitro and in vivo. However, they found no Rab27B expression in breast cancer. This discrepancy may be because they utilized cell lines and samples from nude mice, rather than human breast cancer tissue. Another study on Rab27 and breast cancer showed that Rab27B regulates invasive growth and metastasis in ER-positive breast cancer cell lines and that increased expression is associated with poor prognosis in humans[28]. However, they found no expression of Rab27A in normal tissue (n = 5) or primary breast carcinoma (n = 20), though this may have been due to the small sample size. Additionally, the results of our previous study on gastric cancer (GC) and colorectal cancer (CRC) were not consistent with our HCC results. Expression of Rab27A and Rab27B was much lower in primary GC and CRC than in adjacent mucosal tissue. Among these patients, those with Rab27A+/Rab27B+ tumors had the longest survival, while the Rab27A-/Rab27B- group had the poorest survival; a Cox proportional hazards model showed that Rab27A+/Rab27B+ expression was a protective prognosis factor in both GC and CRC (unpublished data). These findings show that Rab27A and Rab27B may have different molecular mechanisms in HCC compared with those in GC and CRC.
In conclusion, our study demonstrated that Rab27A and, to a greater degree, Rab27B were correlated with tumor progression and may serve as valuable prognostic indicators for HCC patients. However, further investigation is required to determine the molecular mechanism of Rab27A and Rab27B in HCC.
Primary hepatocellular carcinoma (HCC) carries a high mortality rate and is one of the top 10 most frequent tumor types globally. The identification of novel biomarkers correlating with HCC progression is crucial to monitor clinical disease course and provide corresponding therapy strategies. A large body of evidence indicates that vesicle trafficking and exocytosis are important in tumorigenesis, with many reports implicating the Rab family of proteins.
Several members of the Rab family, such as Rab25, Rab23 and Rab11, have been well studied in cancer. However, research on Rab27 in cancer is both limited and contradictory, and has focused exclusively on breast cancer. Due to the high mortality rate and lack of efficacious therapy strategies, the identification of Rab27 expression on primary HCC as a novel biomarker of the disease may allow for the development of improved therapy strategies.
This is the first study to investigate the expression of Rab27A and Rab27B in a large series of HCC patients. It was found that Rab27A and Rab27B expression were closely linked and both Rab27A and Rab27B were strongly associated with tumor progression, which can be valuable prognostic indicators for HCC patients.
Rab27A and Rab27B were closely related with tumor progression and may serve as useful biomarkers to monitor the clinical course of patients with HCC. The relationship between the expression of Rab27A and Rab27B and tumor progression may be due to their functions as transport vesicles, which may provide potential therapeutic targets for HCC.
Rabs are a ubiquitously expressed family of small monomeric Ras-like GTPases. They function as molecular switches, oscillating between GTP- or GDP-bound conformations, which enables them to reversibly recruit GTP-dependent effectors and elicit their regulatory functions at multiple stages of vesicular transport; Rab27A is expressed in a wide variety of secretory cell types, most of which function specifically in regulated exocytic pathways. Loss-of-function mutations in the human Rab27A gene result in Griscelli syndrome; Rab27A and Rab27B constitute the Rab27 subfamily and share 71% identity. Rab27B expression is much more restricted than that of Rab27A and no human disease or animal strain with mutations in the Rab27B gene has been identified.
In the present study, the authors found that Rab27A and Rab27B were expressed differently in HCC. Rab27A was expressed at higher levels in primary HCC, whereas Rab27B expression was lower in primary HCC. In agreement with these findings, Rab27A and Rab27B mRNA and protein were also differentially expressed in 6 human cell lines. Survival analysis further showed that Rab27 expression may be used as an indicator for prognosis in HCC patients, providing important information for the treatment of patients.
| 1. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4301] [Article Influence: 226.4] [Reference Citation Analysis (2)] |
| 2. | Chan AM, Weber T. A putative link between exocytosis and tumor development. Cancer Cell. 2002;2:427-428. [PubMed] |
| 3. | Palmer RE, Lee SB, Wong JC, Reynolds PA, Zhang H, Truong V, Oliner JD, Gerald WL, Haber DA. Induction of BAIAP3 by the EWS-WT1 chimeric fusion implicates regulated exocytosis in tumorigenesis. Cancer Cell. 2002;2:497-505. [PubMed] |
| 4. | Wright PK. Targeting vesicle trafficking: an important approach to cancer chemotherapy. Recent Pat Anticancer Drug Discov. 2008;3:137-147. [PubMed] |
| 5. | Chavrier P, Goud B. The role of ARF and Rab GTPases in membrane transport. Curr Opin Cell Biol. 1999;11:466-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 389] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 6. | Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821-11827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 808] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 7. | Pereira-Leal JB, Seabra MC. Evolution of the Rab family of small GTP-binding proteins. J Mol Biol. 2001;313:889-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 631] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 8. | Gomi H, Mori K, Itohara S, Izumi T. Rab27b is expressed in a wide range of exocytic cells and involved in the delivery of secretory granules near the plasma membrane. Mol Biol Cell. 2007;18:4377-4386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Tolmachova T, Anders R, Stinchcombe J, Bossi G, Griffiths GM, Huxley C, Seabra MC. A general role for Rab27a in secretory cells. Mol Biol Cell. 2004;15:332-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Tolmachova T, Ramalho JS, Anant JS, Schultz RA, Huxley CM, Seabra MC. Cloning, mapping and characterization of the human RAB27A gene. Gene. 1999;239:109-116. [PubMed] |
| 11. | Ménasché G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000;25:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 737] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 12. | Griscelli C, Durandy A, Guy-Grand D, Daguillard F, Herzog C, Prunieras M. A syndrome associating partial albinism and immunodeficiency. Am J Med. 1978;65:691-702. [PubMed] |
| 13. | Barral DC, Ramalho JS, Anders R, Hume AN, Knapton HJ, Tolmachova T, Collinson LM, Goulding D, Authi KS, Seabra MC. Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome. J Clin Invest. 2002;110:247-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Chen X, Li C, Izumi T, Ernst SA, Andrews PC, Williams JA. Rab27b localizes to zymogen granules and regulates pancreatic acinar exocytosis. Biochem Biophys Res Commun. 2004;323:1157-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Chen Y, Guo X, Deng FM, Liang FX, Sun W, Ren M, Izumi T, Sabatini DD, Sun TT, Kreibich G. Rab27b is associated with fusiform vesicles and may be involved in targeting uroplakins to urothelial apical membranes. Proc Natl Acad Sci U S A. 2003;100:14012-14017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Zhao S, Torii S, Yokota-Hashimoto H, Takeuchi T, Izumi T. Involvement of Rab27b in the regulated secretion of pituitary hormones. Endocrinology. 2002;143:1817-1824. [PubMed] |
| 17. | Cheng KW, Lahad JP, Gray JW, Mills GB. Emerging role of RAB GTPases in cancer and human disease. Cancer Res. 2005;65:2516-2519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Fan Y, Xin XY, Chen BL, Ma X. Knockdown of RAB25 expression by RNAi inhibits growth of human epithelial ovarian cancer cells in vitro and in vivo. Pathology. 2006;38:561-567. [PubMed] |
| 19. | Cheng KW, Lahad JP, Kuo WL, Lapuk A, Yamada K, Auersperg N, Liu J, Smith-McCune K, Lu KH, Fishman D. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med. 2004;10:1251-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 456] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 20. | Croizet-Berger K, Daumerie C, Couvreur M, Courtoy PJ, van den Hove MF. The endocytic catalysts, Rab5a and Rab7, are tandem regulators of thyroid hormone production. Proc Natl Acad Sci U S A. 2002;99:8277-8282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Mor O, Nativ O, Stein A, Novak L, Lehavi D, Shiboleth Y, Rozen A, Berent E, Brodsky L, Feinstein E. Molecular analysis of transitional cell carcinoma using cDNA microarray. Oncogene. 2003;22:7702-7710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Goldenring JR, Shen KR, Vaughan HD, Modlin IM. Identification of a small GTP-binding protein, Rab25, expressed in the gastrointestinal mucosa, kidney, and lung. J Biol Chem. 1993;268:18419-18422. [PubMed] |
| 23. | Hou Q, Wu YH, Grabsch H, Zhu Y, Leong SH, Ganesan K, Cross D, Tan LK, Tao J, Gopalakrishnan V. Integrative genomics identifies RAB23 as an invasion mediator gene in diffuse-type gastric cancer. Cancer Res. 2008;68:4623-4630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Subramani D, Alahari SK. Integrin-mediated function of Rab GTPases in cancer progression. Mol Cancer. 2010;9:312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Montel V, Huang TY, Mose E, Pestonjamasp K, Tarin D. Expression profiling of primary tumors and matched lymphatic and lung metastases in a xenogeneic breast cancer model. Am J Pathol. 2005;166:1565-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Steeg PS. New insights into the tumor metastatic process revealed by gene expression profiling. Am J Pathol. 2005;166:1291-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Wang JS, Wang FB, Zhang QG, Shen ZZ, Shao ZM. Enhanced expression of Rab27A gene by breast cancer cells promoting invasiveness and the metastasis potential by secretion of insulin-like growth factor-II. Mol Cancer Res. 2008;6:372-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Hendrix A, Maynard D, Pauwels P, Braems G, Denys H, Van den Broecke R, Lambert J, Van Belle S, Cocquyt V, Gespach C. Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis. J Natl Cancer Inst. 2010;102:866-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 29. | Tang Z, Zhao M, Ji J, Yang G, Hu F, He J, Shen H, Gao Z, Zhao A, Li J. Overexpression of gastrin and c-met protein involved in human gastric carcinomas and intestinal metaplasia. Oncol Rep. 2004;11:333-339. [PubMed] |
| 30. | Wang HY, Zhang JY, Cui JT, Tan XH, Li WM, Gu J, Lu YY. Expression status of S100A14 and S100A4 correlates with metastatic potential and clinical outcome in colorectal cancer after surgery. Oncol Rep. 2010;23:45-52. [PubMed] |
| 31. | Izumi T. Physiological roles of Rab27 effectors in regulated exocytosis. Endocr J. 2007;54:649-657. [PubMed] |
| 32. | Mignot G, Chalmin F, Ladoire S, Rébé C, Ghiringhelli F. Tumor exosome-mediated MDSC activation. Am J Pathol. 2011;178:1403-144; author reply 1403-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol. 2012;40:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 34. | Hegmans JP, Bard MP, Hemmes A, Luider TM, Kleijmeer MJ, Prins JB, Zitvogel L, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am J Pathol. 2004;164:1807-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 273] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 35. | Ochieng J, Pratap S, Khatua AK, Sakwe AM. Anchorage-independent growth of breast carcinoma cells is mediated by serum exosomes. Exp Cell Res. 2009;315:1875-1888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
Peer reviewers: Fernando J Corrales, Associate Professor of Biochemistry, Division of Hepatology and Gene Therapy, Proteomics Laboratory, CIMA, University of Navarra, Avd. Pío VII, 55, 31008 Pamplona, Spain; Thomas Kietzmann, Professor, Department of Biochemistry, University of Oulu, FI-90014 Oulu, Finland
S- Editor Gou SX L- Editor A E- Editor Xiong L