INTRODUCTION
The gastrointestinal (GI) tract is an immunologic organ with continuous antigen exposure in the form of food, normal bacteria and pathogens. Despite numerous antigenic challenges, the complicated mucosal immune system maintains GI homeostasis via the concerted actions of the various mucosal immune cells. Dendritic cells (DC), dedicated antigen-presenting cells, modulate the immune balance in the GI tract[1]. DC can take up antigens directly by extending their dendrites into the lumen or indirectly after transport of the antigens by M cells overlying Peyer’s patch[2,3]. Antigen-carrying DC may traffic through the lymphatics to the mesenteric lymph nodes[4], mediating the homing of activated effector/memory T cells and IgA-secreting B cells[5,6] and inducing regulatory T cells to produce interleukin (IL)-10 and transforming growth factor (TGF)-β[7,8]. These roles depend on the regulation of cell surface expression of co-stimulatory molecules and production of inflammatory chemokines and cytokines[9-11].
DC can recognize and present microbial components using pattern receptor system which includes toll-like-receptor (TLR). TLR can interact with microorganism-associated molecules such as peptidoglycan, lipoprotein, and lipopolysaccharide[12-16]. Bifidobacterium and Lactobacillus are major components of the commensal microbes of the GI tract and are frequently used as probiotics[17,18]. Probiotics, defined as live microorganisms which, when consumed in appropriate amounts in food, confer a health benefit on the host[19], exert various host physiological responses such as immunomodulatory effect[20]. Recent experiments reported that DC could be modulated by probiotics. Several Lactobacillus species could regulate DC surface expression and cytokine production[21]. In addition, the probiotics mixture VSL No. 3 upregulated the expression of major histocompatibility complex (MHC) class II and co-stimulation molecules[22].
DC are often located close to epithelial cells, populating the subepithelial dome of Peyer’s patches, immediately adjacent to the follicle-associated epithelium and the lamina propria[23,24]. Intestinal epithelial cells secrete many mediators, including functional peptides such as defensins, mucins, chemokines, and cytokines such as IL 8[25-27]. TLR5 on the epithelium is a key mediator of pro-inflammatory responses to flagella from commensal bacteria[28,29]. Flagella also stimulate the maturation of responsive DC[30].
Interaction between DC and epithelial cells is integral to the intestinal immune system. We hypothesized that epithelial cells stimulated by probiotics could regulate the maturation of DC. Accordingly, the present study investigated the pattern of cytokine production and the surface phenotype of DC in the presence of epithelial cells polarized by heat-killed probiotic bacteria.
MATERIALS AND METHODS
Preparation of probiotic bacteria
Bifidobacterium bifidum BGN4 (BB) was isolated from healthy infant fecal matter and identified in our laboratory[31]. Bifidobacterium lactis AD011 (BL), Lactobacillus casei IBS041 (LC), and Lactobacillus acidophilus AD031 (LA) were provided by the Research Institute of Bifido Co. Ltd. (Hongchun, Gangwondo, South Korea). Four probiotic bacteria were anaerobically propagated in de Man, Rogosa, and Sharpe (Difco, Detroit, MI, United States) broth containing 0.05% L-cysteine (Sigma, St. Louis, MO, United States) at 37 °Cuntil mid-log phase was reached. Subsequently, probiotics were inoculated at 1% and anaerobically cultured in de Man, Rogosa, and Sharpe (Difco) broth containing 0.05% L-cysteine (Sigma) at 37 °C. Lactobacillus species were incubated for 16 h, and Bifidobacteirum species were incubated for 24 h to late log phase. The bacteria were collected by centrifugation at 1000 ×g for 15 min at 4 °C and washed twice with phosphate-buffered saline (PBS). After washing, the bacteria were resuspended in 1 mL of PBS and incubated at 95 °C for 30 min to prepare heat-killed bacteria cells. The killed bacteria were collected by centrifugation at 1000 ×g for 15 min and then lyophilized (Combi-514R, Hanil Science Industrial, Seoul, South Korea).
Generation of CMT-93 monolayers
CMT93 was derived from carcinomas of C57BL mouse large intestine. The cells have an epithelial morphology and forms acini, junctional complexes, and microvilli with attached glycoprotein[32]. CMT-93 cells were maintained in DMEM (Gibco Life Technologies, United Kingdom) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen, Paisley, United Kingdom) and 1% penicillin/streptomycin (Invitrogen), and were incubated at 37 °C in a humidified atmosphere of 5% CO2. Monolayers were grown in 24-well Corning Costar Transwell plates (Corning Inc., United States) with 3 μm pore-size filter inserts. In the normal co-culture system, 5 × 105 cells were seeded into the inserts, and the wells were filled with 1 mL medium. In the inverted co-culture system, inserts were removed and inverted in tissue culture dishes, and the cells of the same volume were seeded to the exposed filter membrane. The culture dishes were filled with enough medium to sink the inserts. The transwell inserts were cultured for 3-4 d until CMT-93 established monolayers. Confluence of the cells was confirmed when the trans-epithelial electrical resistance (TEER; Millicell ERS Ohmmeter, Millipore, Eschborn, Germany) exceeded the cut-off point of 250 Ω/cm2.
JAWS II cell preparation
JAWS II, mouse bone marrow-derived immature DC[33], were maintained in α-MEM (Gibco) supplemented with 5 ng/mL GM-CSF (Sigma, St. Louis, MO, United States), 20% heat-inactivated FBS (Invitrogen), and 1% penicillin/streptomycin (Invitrogen). The mixture was incubated at 37 °C in a humidified atmosphere of 5% CO2. The cells were cultured at a 1/2 subcultivation ratio for 5-6 d in complete medium.
Co-culture experiment model
The co-culture experiment model is shown in Figure 1. JAWS II cells were harvested, washed, and resuspended in RPMI1640 complete medium (Gibco) containing 5 ng/mL GM-CSF (Sigma), 10% heat-inactivated FBS (Invitrogen), and 1% penicillin/streptomycin (Invitrogen). A total of 1 × 106 JAWS II cells were added into lower chambers, and the normal and inverted cultured CMT-93 monolayer inserts were placed in the JAWS II seeded transwell plates. One hundred μg/mL of the experimental bacteria or 10 μg/mL of LPS (Sigma) were added to the CMT-93 monolayer inserts. For comparison, JAWS II cells were also plated at the same concentration in 24 well tissue culture plates (Corning Inc.), and the same amount of the bacteria or LPS were added to the cells. The single- or co-cultured cells were incubated with 1 mL RPMI1640 complete medium at 37 °C in a humidified atmosphere of 5% CO2 for 12 h.
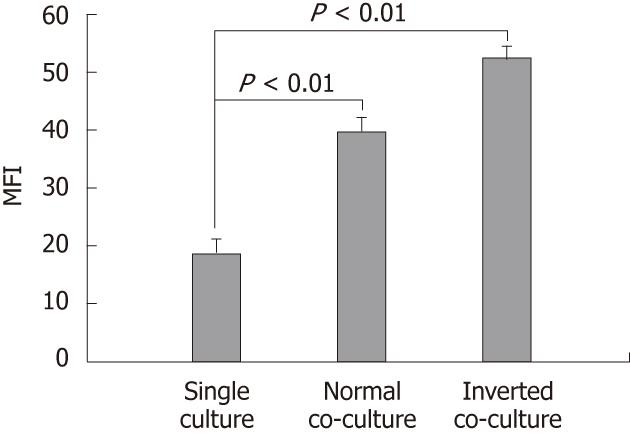
Figure 1 Effect of non-stimulated intestinal epithelial cells on surface phenotype of dendritic cells.
Fluorescence activated cell sorter analysis of dendritic cells (DC) cultured alone or co-cultured with non-stimulated epithelial cell monolayers for 12 h showing DC surface phenotype by staining with I-Ad. Data are shown as the mean fluorescent intensity (MFI) ± SEM of three representative experiments. Significant difference between the single culture and co-culture as determined by Student’s t-test (P < 0.01).
Flow cytometry analysis
Incubated JAWS II cells were harvested and washed three times in cold FACS buffer (Dulbecco’s PBS; Gibco, 2% FBS) and then stained with the appropriate monoclonal antibodies: PE-conjugated anti-I-Ad, anti-CD80, anti-CD86, and anti-CD40 at a final concentration of 10 μg/mL for 30 min at 4 °C in the dark. Isotype control antibodies were hamster IgG2 k, rat IgG2a k, and mouse IgG2b. The stained cells were analyzed immediately by FACSCalibur (Becton Dickinson, San Diego, CA, United States). All of the antibodies used in this flow cytometry analysis were purchased from Pharmingen (San Diego, CA, United States).
Cytokine measurement
JAWS II cell supernatants were harvested from the lower chamber of the Transwell or from the JAWS II cultured-alone plate following incubation, and were assayed for levels of IL-6, IL-10, IL-12p70, tumor necrosis factor (TNF)-α and TGF-β using enzyme-linked immunosorbent assay . Briefly, Nunc-Immuno-Maxisorp plates (Nunc, Roskilde, Denmark) were coated with 2 μg/mL of rat anti-mouse IL-6 and TGF-β capture antibodies in coating buffer (1.6 g/L Na2CO3, 7.1 g/L NaHCO3), pH 9.5, or 2 μg/mL of rat anti-mouse IL-10, IL-12p70, and TNF-α capture antibodies in coating buffer (11.8 g/L Na2HPO4, 16.1 g/L NaH2PO4), pH 6.5, overnight at 4 °C. After washing and blocking, 100 μL of 1:100 diluted (IL-6) or undiluted (IL-10, IL-12p70, TNF-α and TGF-β) supernatant was added to individual wells and incubated overnight at 4 °C. Plates were washed, and biotinylated rat anti-mouse IL-6, IL-10, IL-12p70, TNF-α and TGF-β monoclonal antibodies (2 μg/mL) and HRP-conjugated streptavidin were added to the plates for cytokine detection for 1 h at room temperature. The reactions were developed with the 3,3’,5,5’-tetramethylbenzidine substrate (Fluka, Neu-Ulm, Switzerland) for 30 min at room temperature. The color reactions were stopped with 2 N H2SO4 and analyzed at 450 nm. Equivalent levels of IL-6, IL-10, IL-12p70, TNF-α and TGF-β were measured for comparison with a reference curve generated using standards of these cytokines.
Statistical analyses
Data are presented as the mean ± SE, indicated by bars in the figures. All statistical analyses were performed using SPSS 12.0K for Windows (SPSS Inc., Chicago, IL, United States). Differences between the single culture and co-culture were determined by Student’s t-test, and differences between cytokine levels were analyzed by analysis of variance followed by Duncan’s multiple range test. The P values < 0.05 were considered to be statistically significant.
RESULTS
Development of stable CMT-93 epithelial cell monolayers
To obtain stable CMT-93 intestinal epithelial cell monolayers, we monitored the culture every day for TEER using a Millicell-ERS ohmmeter for a period of 7 d. On day 3, normal insert monolayer integrity was obtained at 300-500 Ω/cm2, and inverted insert monolayer integrity was obtained at 250-350 Ω/cm2. In addition, the generation of epithelial cell monolayers was observed on the surface of the inserts by microscope (data not shown). Monolayers between day 3 and 4 were used for co-culture experiments. After co-culture with DC for 12, the integrity of CMT-93 monolayer was evaluated by TEER. There was no difference between before and after co-culture in terms of the resistances within the margin of error.
Dendritic cells phenotype modulation during co-culturing with epithelial cells
DC surface phenotypes were compared in the presence and absence of epithelial cells. The expression of MHC class II I-Ad on the normal and the inverted co-cultured DCs was upregulated compared with that of the single-cultured DC (single culture, 18.51 ± 2.86; normal co-culture, 39.46 ± 2.53; inverted co-culture, 52.03 ± 2.41; Figure 1). Co-culture with epithelial cells did not alter the DC surface expression of CD80, CD86 and CD40 (data not shown).
Effect of probiotics on the expression of major histocompatibility complex class II and costimulatory molecules
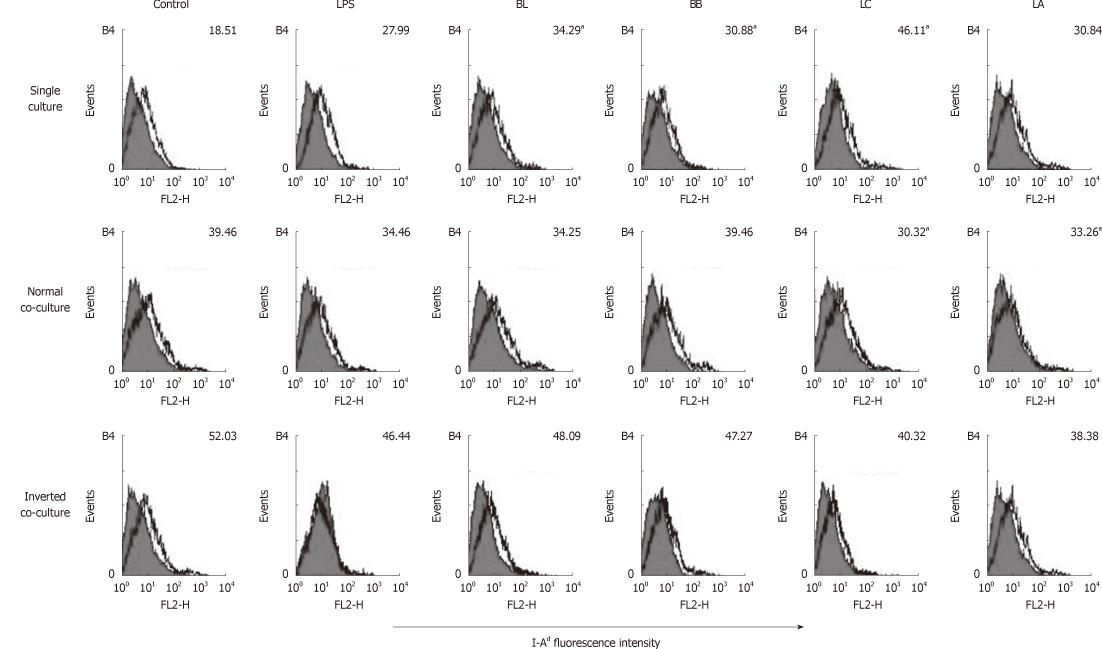
We performed flow cytometery analyses to examine the effects of BL, BB, LC, LA, LPS and control on single- or co-cultured immature DC surface phenotypes. In the DC single culture, the expression of MHC class II I-Ad was significantly increased by stimulation with BL, BB, and LC compared with the control (Figure 2). In the normal co-culture, the expression of I-Ad was significantly decreased by the stimulation of LC and LA compared with the control. In the inverted co-culture, none of the experimental probiotics modulated the expression of I-Ad.
Figure 2 Effect of probiotics on I-Ad of single- or co-cultured dendritic cells.
Fluorescence activated cell sorter analysis of probiotics-treated dendritic cells (DC) cultured in the presence or absence of intestinal monolayers for 12 h. Filled histograms are isotype controls; unfilled histogram show staining for I-Ad. Numbers indicate the mean fluorescent intensity of three representative experiments. aSignificant difference among the control, lipopolysaccharides and probiotics as determined by analysis of variance (P < 0.05). LPS: Lipopolysaccharides; BL: Bifidobacterium lactis AD011, BB: Bifidobacterium bifidum BGN4, LC: Lactobacillus casei IBS041; LA: Lactobacillus acidophilus AD031.
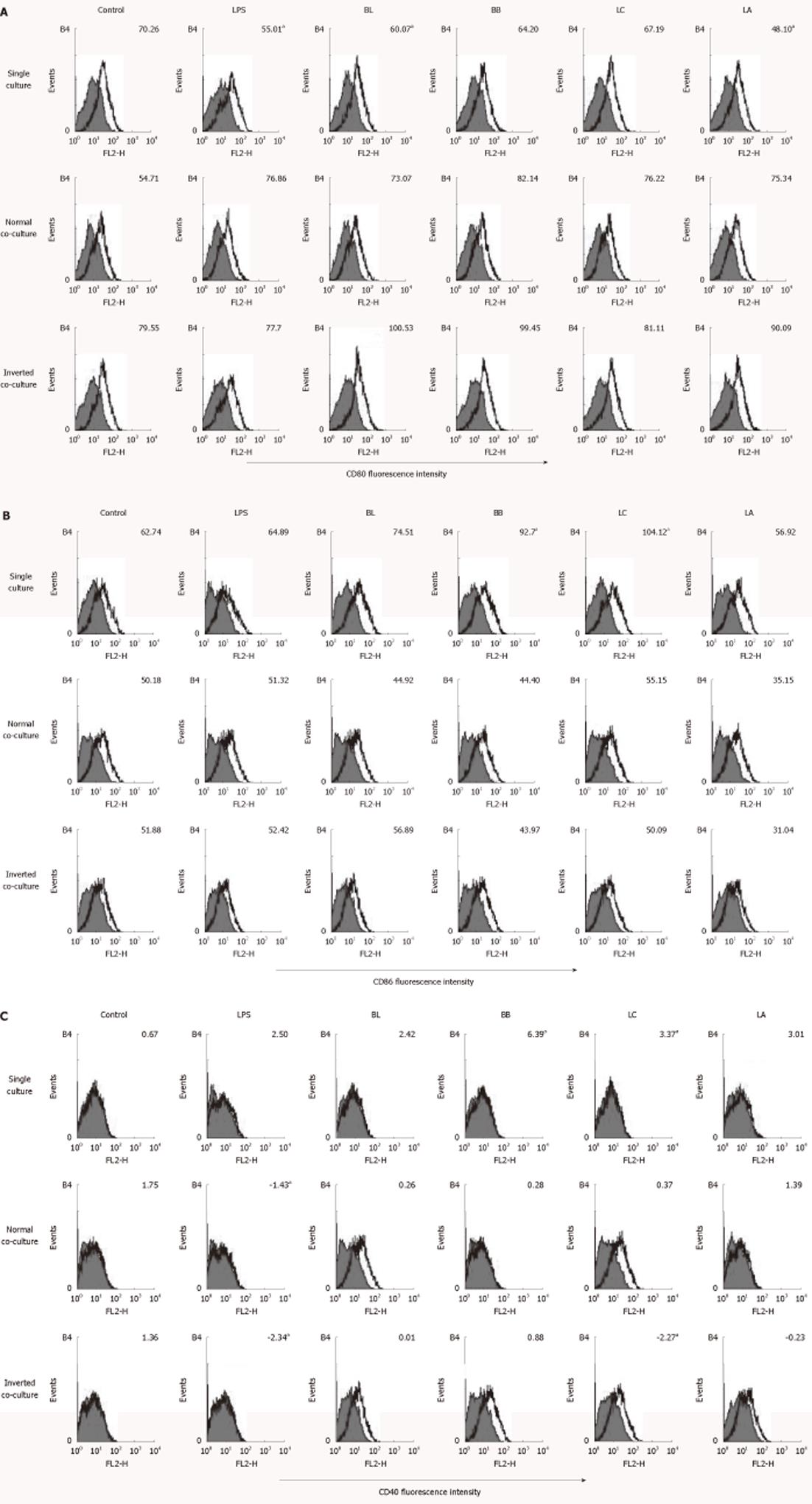
BL and LA significantly downregulated the expression of CD80 in the single-cultured DC. However, none of the experimental probiotics regulated CD80 in the normal and inverted co-cultured DC (Figure 3A).
Figure 3 Effect of probiotics on the CD80, CD86 and CD40 of single- or co-cultured dendritic cells.
Fluorescence activated cell sorter analysis of probiotics-treated dendritic cells (DC) cultured in the presence or absence of intestinal monolayers for 12 h. Filled histograms are isotype controls; unfilled histogram shows staining for CD80 (A), CD86 (B) and CD40 (C). Numbers indicate the mean fluorescent intensity of at least three representative experiments. aSignificant difference among the control, lipopolysaccharides, and probiotics as determined by analysis of variance (P < 0.05). LPS: Lipopolysaccharides; BL: Bifidobacterium lactis AD011, BB: Bifidobacterium bifidum BGN4, LC: Lactobacillus casei IBS041; LA: Lactobacillus acidophilus AD031.
BB and LC upregulated the expressions of CD86 and CD40 in the single-cultured DC, whereas none of the experimental probiotics regulated the expression of CD86 in the normal or inverted co-cultured DC (Figure 3B). LC significantly downregulated the expression of CD40 in the inverted co-cultured DC compared with medium alone (Figure 3C).
Cytokine profiles in dendritic cells supernatant by co-culturing with epithelial cells
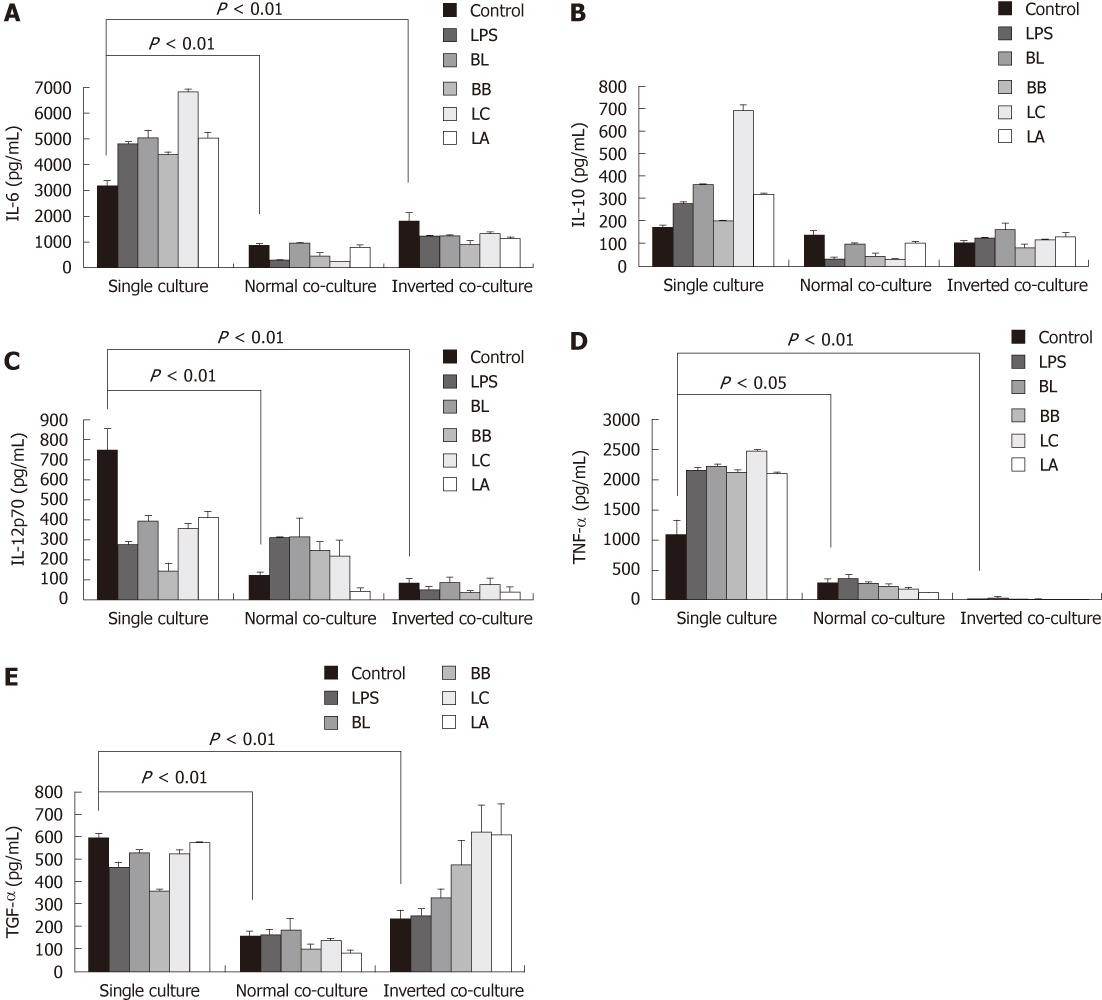
The levels of IL-6, IL-12p70, TNF-α and TGF-β from the co-cultured system were significantly reduced compared with those from the single-cultured DC (Figure 4A and C-E); however, the production of IL-10 showed no decrease in the co-cultured DC.
Figure 4 Effect of probiotics on the production of cytokines from single- or co-cultured dendritic cells.
Supernatants were obtained from probiotic-treated dendritic cells (DC) cultured in the presence or absence of intestinal monolayers for 12 h. Levels of interleutkin (IL)-6 (A), IL-10 (B), IL-12p70 (C), tumor necrosis factor (TNF)-α (D), and transforming growth factor(TGF)-β (E) were determined by enzyme-linked immunosorbent assay. Data are shown as mean ± SE of three representative experiments. Different letters indicate significant differences among the control, lipopolysaccharides (LPS), and probiotics determined by Duncan’s multiple range test (P < 0.05). Significant difference between the single culture and co-culture as determined by Student’s t-test (P < 0.05). BL: Bifidobacterium lactis AD011; BB: Bifidobacterium bifidum BGN4; LC: Lactobacillus casei IBS041; LA: Lactobacillus acidophilus AD031.
Effect of probiotics on the cytokine production in the co-culture system
We quantified the cytokine levels in the single- and co-cultured DC supernatants to investigate the effect of the experimental probiotics on the production of cytokines. LPS was used as a stimulator control to compare with non-treated naïve control. In the single-cultured DC, stimulation with the experimental probiotic bacteria markedly increased the production of IL-6 and TNF-α compared with the control (Figure 4A and D). In the normal co-cultured DC, the levels of IL-6 stimulated by BB and LC and the level of TNF-α stimulated by LA were lower than those of non-stimulated control. In the inverted co-cultured DC, all of the experimental bacteria significantly decreased the production of IL-6 compared with the control, but had no significant effect on the production of TNF-α.
In the single-culture, the level of IL-10 in DC stimulated by BL, LC,and LA was higher than that in the control DC (Figure 4B). The levels of IL-10 from the normal co-cultured DC stimulated by BL, BB and LC were lower. IL-10 from the inverted co-cultured DC stimulated by BL was higher than that from the control.
In the single-cultured DC all of the experimental probiotics decreased the production of IL-12p70. In the normal co-cultured DC only BL increased the production of IL-12p70. In the inverted co-cultured DC the levels of IL-12p70 stimulated by all of the experimental probiotics were similar to that of control.
BL, BB and LC in the single-cultured DC decreased the production of TGF-β. The levels of TGF-β in all of the treated groups in the normal co-culture system were similar to that from the non-stimulated control but in the inverted co-cultured system LC and LA significantly increased the production of TGF-β (Figure 4C and E).
DISCUSSION
The modulatory effect of probiotics on the host immune system was reported in in vivo experiments and clinical trials[34]. However, the exact mechanism of the immunomodulatory effect of probiotics, especially with respect to the interaction between DC and epithelial cells in the presence of probiotics, has not been well elucidated. In the present study, we investigated the effect of heat-killed BL, BB, LC and LA on the modulation of JAWS II (DC) using an in vitro co-culture model. In vitro, live bacteria grown by geometric progression exhausted culture nutrients, produced various acid metabolites, and induced necrosis of the cultured animal cells within a few hours. Therefore, the treatment of the host cells with live bacteria was inappropriate. The adhesive properties of heat killed bacteria might be differentially modified by the heat treatment depending on the specific strain of the experimental bacteria. However, a previous study reported that oral administration of heat-killed and lyophilized BB could suppress the occurrence of allergy by the immune regulatory actions in the mouse allergy model[35], which implied that heat-killed and lyophilized bacteria could maintain their immunomodulatory effects.
To simulate the interaction between DC and epithelial cells in the intestinal environment, we used a transwell co-culture system with CMT93 epithelial cell lines[32]. CMT93 was derived from the same mouse origins as JAWS II, C57BL mouse, and forms junction complexes. A previous study reported that there was a gap junctional communication between murine lymphocytes and CMT93 epithelial cells, and gap junctional communication might regulate cell functions[36]. Nonpathogenic intestinal bacteria can induce DC migration into the epithelial layer and recruit DC uptake of bacteria and apoptotic fragments derived from apoptotic epithelial cells to maintain peripheral self-tolerance[2,37]. In the normal co-culture system, there was an insert membrane and a gap of 1 mm between JAWS II and CMT93. On the other hand, an inverted co-culture model was established by the generation of a CMT93 monolayer on the underside of the inverted insert. A previous study demonstrated that DCs directly interacted with luminal bacteria using CX3CR1-mediated trans-epithelial dendrites[38].
DC interact with microbes and distinguish gram positive, negative, or closely related organisms using TLR and present the processed antigens through MHC class II[12,16,39]. DC then mediate T cell activation, which is regulated by MHC class II molecules, co-stimulatory molecules such as CD80 and CD86, and cytokines[40].
In the single-cultured systems BB and LC upregulated the expression of MHC class II I-Ad, CD86, and CD40, while all of the experimental probiotics attenuated IL-12p70 secretion. IL-12 directed the differentiation of T cells to a Th1 phenotype[41]. Nier reported that Bifidobacterium bifidum enhanced the expression of CD86 and MHC class II in human neonatal DC, which led in turn to the polarization of IFN-γ-producing T cells[42]. Mohamadzadeh et al[43] showed that Lactobacillus gasseri, Lactobacillus johnsonni, and Lactobacillus reuteri upregulated the expression of MHC class II, CD40, CD80 and CD86 in human myeloid DC and increased the level of IL-12p70 which induced the polarization from CD4(+) and CD8(+) T cells to T helper 1 and Tc1 cells. Meanwhile, Drakes et al[22] showed that probiotic products containing Lactobacillus and Bifidobacterium upregulated the expression of MHC class II, CD40, CD80 and CD86, and did not induce the production of IL-12p70 in mouse DC. Additionally, mouse bone marrow-derived DC treated with Lactobacillus reuteri induced Th2 immune response[21]. Taken together, the results of these earlier studies suggested that probiotics upregulated the expression of MHC class II and differently modulated co-stimulatory molecules such as IL-12p70 and T cell polarization, depending on the DC origin and the strain of probiotics.
Interestingly, in the present study the effects of probiotics on cytokine production and the surface phenotype in co-cultured DC with epithelial cells were markedly different from those in single-cultured DC. All of the experimental probiotics induced the production of pro-inflammatory cytokines, IL-6 and TNF-α, in the single cultured DC. TNF-α mediated various immune responses[44], and over-production of TNF-α could play a role in tissue damage and intestinal pathologies[45,46]. In contrast with the results from the single system the experimental probiotics reduced or did not affect the expression of I-Ad, CD86 and CD40 or the production of IL-6, IL-12p70 and TNF-α in inverted co-cultured DC. These findings suggest that epithelial cells are essential components of the immune system to be considered in assessing the effects of probiotics on the regulation of the gastrointestinal immune system. Consequently, previous studies which employed only DC cells without epithelial cells might have provided only partial pictures or sometimes misleading information about the interaction of the probiotics with DC cells.
Previously, Haller et al[47] showed that Lactobacillus johnsonii increased the production of TGF-β in human epithelial cell lines co-cultured with leucocytes. In our study, BL, LC and LA induced IL-10 secretion from single-cultured DC. In an inverted co-culture system, BL increased IL-10 secretion, and LC and LA increased TGF-β secretion. IL-10 was known to activate regulatory T cells[48]. TGF-β which is an important factor in enhancing the differentiation of regulatory Th3 cells was reported to have wide-ranging immunomodulatory properties[49,50]. Th3 cells suppress Th1 and other immune responses and maintain oral tolerance[40,50,51]. Conceivably, enhanced secretion of IL-10 or TGF-β observed in the co-culture systems by BL, LC and LA might contribute to the activation of regulatory T cells in the intestinal tracts. The present study is novel since we assessed the effect of probiotics on immune-modulation in a co-culture model. We suggest that a co-culture model better reflects the environmental status of the in vivo immune system. Our model supports the hypothesis that the interaction of DC and epithelial cells stimulated with probiotics may help maintain intestinal homeostasis by downregulating the production of inflammatory cytokines and expression of MHC class II in DC.
COMMENTS
Background
It is known that dendritic cells (DCs) modulate the immune balance in the intestinal tract by mediating the activation of different subsets of T cells. The functions and differentiation of the DCs may be modulated by probiotics. To better understand the role of the probiotics in the intestinal immune system the interactions of probiotics in the context of epithelial cells and DCs need to be assessed.
Research frontiers
Intestinal epithelial cells secrete various immunological mediators, but the interactions between probiotics, intestinal epithelial cells, and DCs were not fully known. The present study investigated the pattern of cytokine production and the surface phenotype of DCs in the presence of epithelial cells polarized by heat-killed probiotic bacteria.
Innovations and breakthroughs
The present study showed the differential effects of probiotics between the single cultured and the co-cultured DCs. We suggest that a co-culture model better reflects the environmental status of the in vivo immune system. The interaction of DCs and epithelial cells polarized with probiotics may contribute to the homeostasis of the immune system in the intestinal tracts.
Applications
The employment of the co-cuture system may facilitate the development of probiotic bacteria with immunomodulatory effects for people with hypersensitive or imbalanced immune symptoms.
Peer review
The study is well-conducted and results are interesting. However, the results are curiously reported in a confusing manner. Moreover, some controls are needed and the positive control used should be more justified.