Published online Mar 14, 2012. doi: 10.3748/wjg.v18.i10.1038
Revised: January 11, 2012
Accepted: February 8, 2012
Published online: March 14, 2012
AIM: To investigate pathological types and influential factors of chronic graft dysfunction (CGD) following liver transplantation (LT) in rats.
METHODS: The whole experiment was divided into three groups: (1) normal group (n = 12): normal BN rats without any drug or operation; (2) syngeneic transplant group (SGT of BN-BN, n = 12): both donors and recipients were BN rats; and (3) allogeneic transplant group (AGT of LEW-BN, n = 12): Donors were Lewis and recipients were BN rats. In the AGT group, all recipients were subcutaneously injected by Cyclosporin A after LT. Survival time was observed for 1 year. All the dying rats were sampled, biliary tract tissues were performed bacterial culture and liver tissues for histological study. Twenty-one day after LT, 8 rats were selected randomly in each group for sampling. Blood samples from caudal veins were collected for measurements of plasma endotoxin, cytokines and metabonomic analysis, and faeces were analyzed for intestinal microflora.
RESULTS: During the surgery of LT, no complications of blood vessels or bile duct happened, and all rats in each group were still alive in the next 2 wk. The long term observation revealed that a total of 8 rats in the SGT and AGT groups died of hepatic graft diseases, 5 rats in which died of chronic bile duct hyperplasia. Compared to the SGT and normal groups, survival ratio of rats significantly decreased in the AGT group (P < 0.01). Moreover, liver necrosis, liver infection, and severe chronic bile duct hyperplasia were observed in the AGT group by H and E stain. On 21 d after LT, compared with the normal group (25.38 ± 7.09 ng/L) and SGT group (33.12 ± 10.26 ng/L), plasma endotoxin in the AGT group was remarkably increased (142.86 ± 30.85 ng/L) (both P < 0.01). Plasma tumor necrosis factor-α and interleukin-6 were also significantly elevated in the AGT group (593.6 ± 171.67 pg/mL, 323.8 ± 68.30 pg/mL) vs the normal (225.5 ± 72.07 pg/mL, 114.6 ± 36.67 pg/mL) and SGT groups (321.3 ± 88.47 pg/mL, 205.2 ± 53.06 pg/mL) (P < 0.01). Furthermore, Bacterial cultures of bile duct tissues revealed that the rats close to death from the SGT and AGT groups were strongly positive, while those from the normal group were negative. The analysis of intestinal microflora was performed. Compared to the normal group (7.98 ± 0.92, 8.90 ± 1.44) and SGT group (8.51 ± 0.46, 9.43 ± 0.69), the numbers of Enterococcus and Enterobacteria in the AGT group (8.76 ± 1.93, 10.18 ± 1.64) were significantly increased (both P < 0.01). Meanwhile, compared to the normal group (9.62 ± 1.60, 9.93 ± 1.10) and SGT group (8.95 ± 0.04, 9.02 ± 1.14), the numbers of Bifidobacterium and Lactobacillus in the AGT group (7.83 ± 0.72, 8.87 ± 0.13) were remarkably reduced (both P < 0.01). In addition, metabonomics analysis showed that metabolic profiles of plasma in rats in the AGT group were severe deviated from the normal and SGT groups.
CONCLUSION: Chronic bile duct hyperplasia is a pathological type of CGD following LT in rats. The mechanism of this kind of CGD is associated with the alterations of inflammation, intestinal barrier function and microflora as well as plasma metabolic profiles.
- Citation: Jiang JW, Ren ZG, Cui GY, Zhang Z, Xie HY, Zhou L. Chronic bile duct hyperplasia is a chronic graft dysfunction following liver transplantation. World J Gastroenterol 2012; 18(10): 1038-1047
- URL: https://www.wjgnet.com/1007-9327/full/v18/i10/1038.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i10.1038
Liver transplantation (LT) has become an established therapy for various end-stage liver diseases for more than three decades[1-3]. Numerous advances in surgical technique, organ preservation, perioperative anesthesia, postoperative care, and clinical immunosuppression, as well as improved recipient selection and donor management have together significantly increased the survival rates of allograft and improved life quality of patients following LT[4]. Currently, approximately 90% of liver transplant patients are alive after 1 year and 75% after 5 years with majority living a full and near-normal life[3,5]. However, although early mortality rates after transplantation have fallen dramatically, long-term graft survival has barely improved over the last two decades[6,7]. That is to say, the incidence of chronic graft dysfunction (CGD) and the mortality of patients after LT have remained constant, and CGD has become the biggest obstacle for long-term function of allograft and better life quality of patients. Thus, it is essential to investigate the causes and relevant mechanisms of CGD to improve long-term outcomes of patients following LT.
In the early day after LT, common causes of hepatic allograft dysfunction include ischemia and reperfusion injury, infection, technical complications such as hepatic artery thrombosis and recipient diseases[3,5]. Thereafter the causes of allograft dysfunction are variable with disease recurrence and chronic rejection as major causes of graft loss[3,8]. Moreover, the biliary tract is still the most common site for postoperative complications. The importance of this condition lies in the fact that the biliary tract complications can be a serious source of morbidity and sometimes mortality[9,10]. These complications not only affect allograft survival but also have a major impact on the life quality for a hepatic allograft recipient.
However, so far little is known on biliary tract variation of CGD and its influential factors in recipients following LT. In this study, we want to explore hepatic graft pathology and the relevant mechanisms of CGD from aspects of inflammation, intestinal barrier function, intestinal microflora and metabonomics following LT.
Specific pathogen-free (SPF) male inbred Lewis and BN rats (weight 220-250 g, 12-15 wk) were purchased from Beijing Vital River Laboratories (Beijing, China). All rats were housed in a SPF lab (Zhejiang Academy of Medical Sciences, China). The rats were caged in 21 °C, 12 h light/dark cycle, and fed with sterilized standard rat chow and water. All animals received humane care and the study was conducted according to the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85-23, revised 1985).
Sixty rats were raised in SPF animal facility. Twelve inbred BN rats were served as normal controls; 12 inbred Lewis and 12 inbred BN rats as donors; 24 inbred BN rats as recipients. All donors and recipients were randomly performed orthotopic LT under strict sterile conditions. The remaining 36 BN rats were divided into three groups: (1) normal group (n = 12): normal BN rats without any drug or operation; (2) syngeneic transplant group (SGT of BN-BN, n = 12): both donors and recipients were BN rats; and (3) allogeneic transplant group (AGT of LEW-BN, n = 12): donors were Lewis and recipients were BN rats. In the AGT group, all recipients were subcutaneously injected by Cyclosporin A at 1 mg/kg daily in the first 30 d, and then at 2 mg/kg daily for the next 100 d after orthotopic LT, All the recipients in the SGT and AGT groups were received alanine (ALA) daily by gastric perfusion for preoperative 3 d and postoperative 130 d. Survival time was observed for 1 year.
All rats were fasted for 12 h before the operation. The initial anesthesia of rats was performed by intraperitoneal injection of Ketamine Hydrochloride (100 mg/kg) and Atropine (1 mg/kg) (Shanghai No. 1 Biochemical and Pharmaceutical, China), and then ether was inhaled to maintain anesthesia. The profiles of rats with orthotopic LT were established according to the previous techniques[11,12], with slight modifications. Briefly, after the liver of the donor was dissociated, the graft was perfused with chilled saline containing 25 U/mL heparin via the portal vein, and then preserved in cold normal saline for no more than 1 h before being placed in the abdomen of recipient. After the anastomosis of suprahepatic vene cava and portal vein was finished, the graft was reperfused. The common bile duct was reconstructed by tying the duct over a stent. All recipients recovered in a short time, and no further treatment was performed.
The survival conditions of rats were monitored continuously. When any rat was dying, the following samples were collected under strict sterile condition; the liver was fixed in 40 g/L neutral formaldehyde for later histological study and the biliary tract tissue for bacterial culture. Moreover, on 21 d after the surgery, biliary tract tissues of 4 rats selected randomly in the normal group were collected for bacterial culture. Eight rats were selected randomly in each group for sampling. Blood samples from the caudal veins were gained for measurements of plasma endotoxin, cytokines and analysis of ultra performance liquid chromatography-mass spectrometry (UPLC-MS), and faeces were collected for the determination of intestinal microflora.
The sample from the left lobe of hepatic graft was fixed in 40 g/L neutral formaldehyde and embedded in paraffin, cut into 3 μm slices, stained with hematoxylin and eosin (HE), and then observed under light microscopy by a pathologist.
The blood sample (100 μL) was placed in the pyrogen-free heparin-containing tube, and then centrifuged at 3000 g for 15 min at 4 °C. Plasma endotoxin of the caudal vein was determined using a quantitative, chromogenic Limulus Amebocyte Lysate assay according to the manufacturer’s instruction (Eihua Medical, Shanghai, China). The value was expressed as nanogram per liter of plasma (ng/L).
The levels of plasma tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were tested with enzyme-linked immunosorbent assay (ELISA) (Groundwork Biotechnology Diagnosticate Ltd, United States) according to the protocol of manufacturer. The result was expressed as ng/L.
Biliary tract tissues from the grafts were weighed and placed in a germ-free glass homogenizer containing a nine-fold amount of anaerobic buffer (phosphate buffered saline with 0.5 g cysteine HCl, 0.5 mL tween 80, and 0.5 g agar). They were homogenized and 50 μL of 10% homogenate were placed on the agar base of Colombian culture medium within 30 min, incubated for 48 h at 37 °C. Bacterial colonies were evaluated qualitatively according to their growth conditions respectively at the end of the culture. In addition, bacterial colonies from biliary tract tissues were identified by the Automatic analyzer of bacteria (Model Viger 60, France) to identify bacterial species according to the report[13].
Intestinal microflora was studied with 4 selected agar media according to the reports[14]. Samples from colorectal contents were placed in sterile tubes, weighed and transferred into other sterile tubes containing appropriate anaerobic buffer (as described above) to approach a 10-fold dilution of samples, and then serial decimal dilutions were taken in the same way from 10-2 to 10-8. Within 30 min of sample collection, bacterial cultures were finished with placing 50 μL dilutions on 4 agar media. According to the instructions, TPY agar medium, LBS agar medium, EC medium, and Eosin-Methylene Blue Agar (EMB) were used for Bifidobacterium, Lactobacillus, Enterococcus and Enterobacter, respectively. Anaerobic bacteria were incubated in Anaerobic Box System including AnaeroPack (MGC, Japan) and GENbox anaer (BioMérieux, France), and aerobic bacteria were incubated aerobically for 48 h at 37 °C, respectively. Bacterial colonies on every plate were counted and calculated at the primal weight of samples. The results were expressed as bacterial colony forming units per gram content (log10 CFU/g).
Blood samples from caudal veins of rats were collected for metabonomics analysis according to the method of UPLC-MS described by Wang et al[15] and Yang et al[16]. Prior to analysis, blood samples were defrosted at room temperature and mixed with acetonitrile (ACN) at the ratio of 1:3 (v/v), the mixture was vortered and centrifuged at 10 000 g for 10 min. The supernatant was transferred to sample bottles for UPLC separation. Chromatographic separations were performed at a 100 mm × 2.1 mm ACQUITY-1.7 lm C18 column (Waters Co., Milford, United States) using an ACQUITY-Ultra Performance Liquid Chromatography system (Waters). Mass spectrometry was performed on a Premier-Q-Tof (Waters MS Technologies, Milford, United States).
The UPLC-MS data were analyzed with the SIMCA-P+ 12 Software (Umetrics, Sweden). An ApexTrack-peak detection algorithm was adopted in MarkerLynx V4.1 software (Waters, United States) to measure peaks and align retention times of the peaks for all chromatograms. The results were transferred into a single data matrix by aligning peaks with the same mass/retention time pair together from each data file in the dataset, along with their relevant intensities. The resulting dataset containing peak numbers (RT-m/z pair), sample names, and ion intensities was analyzed by partial least squares-discriminate analysis (PLS-DA) with the SIMCA-P+ 12 Software.
All the data were presented as mean ± SD. The survival distribution function was evaluated by the Kaplan-Meier survival curve and the others were determined using Students T-test. These analysis were performed with the statistical software SAS 9.1.3 (SAS Institute Inc., North Carolina State University, United States), and UPLC-MS data were analyzed by PLS-DA with the SIMCA-P+ 12 Software (Umetrics, Sweden). A P-value of less than 0.05 was considered statistically significant.
During the surgery of LT, no complication of blood vessels or bile duct happened, and all rats in each group were still alive in the next 2 wk.
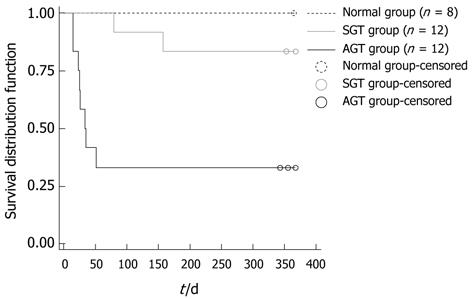
The survival distribution function in each group was shown in Figure 1. A total 8 rats in the normal group were alive for more than one year. Two rats in the SGT group respectively died of hepatic necrosis and chronic bile duct hyperplasia on the 78th and 156th day post-operation. It was worth noting that a total of 8 rats in the AGT group were dead: two died of hepatic necrosis on the 25th and 26th day post-operation, respectively; two died of abdominal infection on the 15th and 23th day post-operation, respectively; the remaining 4 rats died of severe chronic bile duct hyperplasia on the 25th, 34th, 35th and 51th day post-operation, respectively. Compared to the rats in the SGT group and normal group, the survival ratio of rats significantly decreased in the AGT group (aP < 0.01, bP < 0.001, respectively).
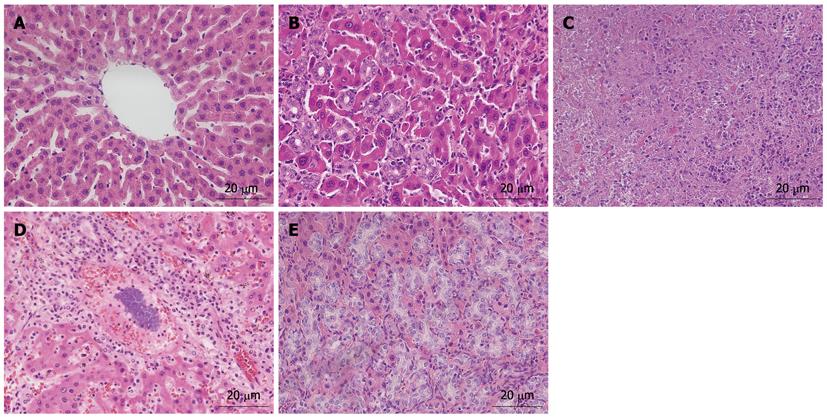
As shown in Figure 2, the different characteristics of hepatic graft histology were observed in the rats of different groups. Under light microscope, rats’ livers in the normal group showed the normal hepatic structure that the hepatocyte cords were presented the radial distribution around the central vein without the infiltration of inflammatory cells (Figure 2A). The rat dead of chronic bile duct hyperplasia in the SGT group showed special pathological features that the proliferative epithelial cells of bile duct invaded into the hepatic parenchyma without lymphocyte infiltration (Figure 2B). However, the dead rats in the AGT group were presented unique pathological structures, including liver necrosis, liver infection, and severe chronic bile duct hyperplasia. Specifically, liver necrosis was presented the extensive destruction of hepatic structure, and the necrosis of lots of hepatocytes (Figure 2C). Liver infection was described as the destruction of hepatic structure, the excessive proliferation of ectogenic bacteria, and the infiltration of lots of inflammatory cells (Figure 2D). And severe chronic bile duct hyperplasia was expressed by some special points that the proliferative epithelial cells of bile duct extensively invaded into the hepatic parenchyma, and bile duct lumen was significantly extended with the destruction of hepatic structure (Figure 2E).
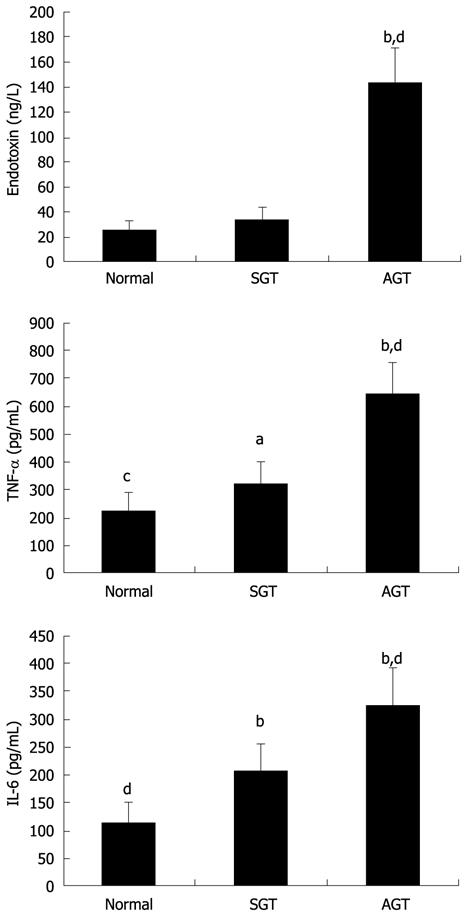
Plasma endotoxin, as a critical medium to aggravate graft injury, is a vital stimulus of CGD following allogeneic LT. As showed in Figure 3, there was no significant difference in plasma endotoxin between the normal group (25.38 ± 7.09 ng/L) and SGT group (33.12 ± 10.26 ng/L). By contrast, plasma level of endotoxin in the AGT group was remarkably increased (142.86 ± 30.85 ng/L) vs the other two groups (P < 0.01).
Both TNF-α and IL-6 are important pro-inflammatory cytokines and can directly or indirectly cause graft injury and CGD of recipients after LT. As shown in Figure 3 (pg/mL), compared to the normal group (225.5 ± 72.07 ng/L, 114.6 ± 36.67 ng/L) and SGT group (321.3 ± 88.47 ng/L, 205.2 ± 53.06 ng/L), the levels of plasma TNF-α and IL-6 were significantly elevated in the AGT group (593.6 ± 171.67 ng/L, 323.8 ± 68.30 ng/L) (P < 0.01). In addition, plasma levels of both TNF-α and IL-6 in the SGT group were also increased vs those in the normal group (aP < 0.05, bP < 0.01, respectively).
Bacterial translocation may participate in some physiological and pathological procedures when recipient suffers from CGD. Samples from biliary duct tissues of hepatic graft were cultured and results were summarized in Table 1. There was rare bacterial colony in the 4 rats from the normal group, while bacterial culture of samples from the SGT and AGT groups were strongly positive. Bacterial identification revealed significantly increased aerobic bacteria, such as Escherichia coli and Enterococcus in the groups of LT. Moreover, Proteus vulgaris, Streptococcus agalactiae and Proteus mirabilis presented remarkably positive in the AGT group, which suggested that more kinds of bacteria translocated to biliary duct tissues when recipients suffered from CGD following allogeneic LT.
| Bacterial species | SGT group (n = 2) | AGT group (n = 8) |
| Escherichia coli | 2/2 | 8/8 |
| Enterococcus | 2/2 | 8/8 |
| Staphylococcus aureu | 1/2 | 0/8 |
| Proteus vulgaris | 0/2 | 8/8 |
| Streptococcus agalactiae | 0/2 | 6/8 |
| Proteus mirabilis | 0/2 | 5/8 |
To determine the alterations of intestinal microflora when recipients suffered from CGD after LT, bacterial species and numbers in colorectal contents were analyzed. As shown in Table 2, compared to the normal group (7.98 ± 0.92, 8.90 ± 1.44) and SGT group (8.51 ± 0.46, 9.43 ± 0.69), the numbers of Enterococcus and Enterobacteria in the AGT group (8.76 ± 1.93, 10.18 ± 1.64) were significantly increased (both aP < 0.01, bP < 0.05, respectively). Meanwhile, compared to the normal group (9.62 ± 1.60, 9.93 ± 1.10) and SGT group (8.95 ± 0.04, 9.02 ± 1.14), the numbers of Bifidobacterium and Lactobacillus in the AGT group (7.83 ± 0.72, 8.87 ± 0.13) were remarkably reduced (both aP < 0.01, bP < 0.05, respectively). There were no statistical differences in bacterial species and counts between the normal group and the SGT group.
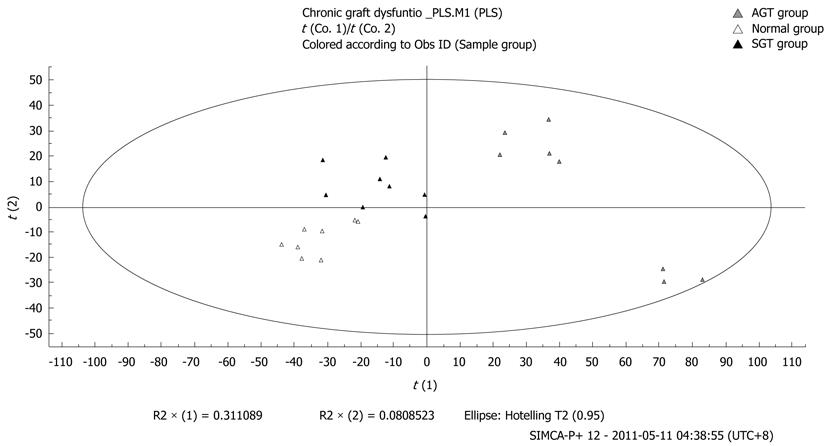
In order to analyze the interactions between metabolic profile and CGD, caudal vein plasma was collected to perform metabonomics analysis by the method of UPLC-MS and PLS-DA. As shown in Figures 4, 5, and 6, the metabolic profiles of plasma in rats from the SGT and AGT groups were deviated from those of the normal group gradually. We also found that metabolic alterations were more severely deviated in the AGT group vs the normal group, which suggested that the degree of metabolic change was positively associated with the severity of CGD in recipients following LT.
Organ transplantation has become the radical method for treatment of many end-stage organic diseases[17,18]. Since the early experiences of the 1960s, liver transplant surgery has evolved over the decades and is now the standard of care in patients with end-stage liver diseases[19-21]. Although there has been consistent improvement in the overall survival rates for transplant recipients, organ shortages and CGD are still two major problems which hinder the development of organ transplantation.
In general, chronic hepatic allograft dysfunction is defined as the declining of hepatic graft function irreversibly and gradually, expressed by increasing or persistent elevations in serum levels of alanine aminotransferase, alkaline phosphatase, or bilirubin (greater than two times the upper limit of normal)[5]. Chronic hepatic allograft dysfunction may result from a variety of causes including rejection[8], vascular stenosis/thrombosis, de novo or recurrent infection, biliary complications[19] including stricture or stenosis, recurrent disease related to autoimmune mechanisms such as that seen in primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune chronic active hepatitis[5]. In addition, drug hepatotoxicity and the development of neoplasms such as a posttransplant lymphoproliferative disorder or the recurrences of hepatocellular carcinoma are important considerations[3].
With the efforts in recent decades, survival after LT is 90% at 1 year and approximately 75% at 5 years[5]. However, biliary tract complications are still the “Achilles heel” of LT[22,23]. Despite great improvements in the surgical techniques and standardization of the method of biliary reconstruction, the biliary tract is still the most common site for postoperative complications. So far little is known about alterations of biliary tract when recipients suffer from CGD following LT. Thus, it has been indispensable to explore changes of biliary tract and relevant mechanisms of CGD to improve survival conditions of recipients following LT for a long time.
In our study, no rats died within 2 wk after LT since applications of immunosuppressants and sterile surgery in SPF-class laboratory. Life span of human is usually 60-90 years, while life expectancy of rats typically is 2-3 years, so we can define CGD of rats as loss of graft function after about one month post-operation. During the whole process of observation, a total of 8 rats in the LT groups died of hepatic graft diseases, such as partial hepatic necrosis and chronic bile duct hyperplasia, and the other 2 rats died of abdominal infection. Notably, 5 rats in these 8 rats that died of hepatic graft diseases died of chronic bile duct hyperplasia, which accounted for 5/8 (62.5%). We concluded that chronic bile duct hyperplasia was a pathological type of CGD and its occurrence frequency was closely associated with the causes of CGD following LT. This finding is consistent with the reports[24] that bile duct hyperplasia extending progressively is a pathology finding in a profile of auxiliary LT with portal vein arterialization in pigs.
In order to explore influential factors and possible mechanisms of chronic bile duct hyperplasia, as a special type of CGD following LT, we further investigated alterations of serum endotoxin and cytokines, bacterial translocation, intestinal microflora, and metabolic profile in the different groups.
Endotoxin, which mainly originated from non-viable intestinal gram-negative bacteria, is a crucial medium to aggravate hepatic graft injury[25]. Plasma level of endotoxin not only can reflect intestinal barrier function[26], but also can predict the defensive ability of body and the injury degree of hepatic graft. Our research found that plasma level of endotoxin in the AGT group remarkably increased compared to the other two groups, which suggested that intestinal barrier function was destroyed when recipients suffered from CGD following LT. Meanwhile, we also speculated that the elevation of plasma endotoxin was closely associated with the occurrence of CGD after LT in rats.
Inflammation plays a vital role in the progression of liver injury[27]. TNF-α and IL-6 are important proinflammatory mediators and can directly, or by inducing inflammatory cascades and enhancing the microvascular dysfunction of liver and intestine, aggravate the injury of hepatic graft[28]. Inflammatory mediators such as TNF-α, interferon-γ (INF-γ) and interleukin (IL) have cytotoxicity, thus being considered as effectors of liver injury. Our results revealed that plasma levels of cytokines gradually increased from the rats in the normal group to those in the syngeneic transplant group, and even to those in the allogeneic transplant group, suggesting that inflammatory reaction was positively associated with the severity of CGD in recipients. Thus, our findings support the concept that inflammation may be a component of the pathogenesis of CGD, which is in line with the report on inflammation and graft deterioration by Dahle et al[29]. However, the exact pathogenetic role of inflammatory cytokines in graft failure is still elusive.
Under certain conditions, the original bacteria in the intestine would cross a relatively complete intestinal epithelium to reach the sites of MLN, abdominal internal and external organs (such as liver, spleen and lung) as well as blood, and may cause the occurrence of infection, which is called “bacterial translocation”[30]. Normally, a small amount of bacteria and endotoxin can go through the intestinal wall, which may be associated with the maintenance of normal intestinal immune response and the activity of reticuloendothelial system[31,32]. In general, three factors mainly attributed to the occurrence of BT: the destruction of intestinal barrier, the imbalance of intestinal microflora, and the reduction of immune defense[33]. There was rare bacterial growth in culture medium of biliary duct in the normal group, while bacterial counts significantly elevated in the AGT group. Bacterial identification mainly designated aerobic bacteria, including Escherichia coli, Enterococcus, Proteus vulgaris, Streptococcus agalactiae and Proteus mirabilis. This result suggested bacterial translocation occurred in recipients after LT under immunosuppression, which might aggravate the severity of CGD in some extent.
The commensal bacteria living in the human intestine play a pivotal role in the maintenance of intestinal homeostasis in their host[34]. The normal formation of intestinal microflora contributes not only to the prevention of enteritis caused by pathogens but also to immunological development and preservation[35]. Under physiological circumstances, intestinal bacteria keep an ecological balance of intestinal microflora. Many researches have indicated that some extrinsic factors, such as administration of abdominal surgery[36], hepatic I/R injury[14], gastrointestinal disorders[37] and LT[25], can cause the imbalance of intestinal microflora. Our results also indicated that the imbalance of intestinal microflora was expressed by an increase in Enterococcus and Enterobacteria, and a reduction in Bifidobacterium and Lactobacillus, when recipients suffered from severe CGD in allogeneic transplant group. Thereafter we may speculate that the occurrence of CGD has some correlations with intestinal microflora.
In addition, we performed metabolomics analysis of plasma in recipients following LT. As a system analysis approach, metabolomics can provide comprehensive information on the dynamic process of postoperative physiopathological development[15]. The systemic detection of chronic diseases can be obtained with metabolomics at an earlier stage compared to the clinical chemistry and histopathological assessment[15]. Our results on metabonomics analysis revealed that the metabolic profile of plasma gradually deviated from the normal parameters, from the rats in the syngeneic transplant group to those in the allogeneic transplant group. To some extent, this finding suggested that the mechanism of CGD might be explained by the alterations of plasma metabolic profile in recipients following LT. However, further exploration need to be taken for the accurate relationship between metabolic changes and CGD of recipients following LT.
In conclusion, according to the observation of one year for recipients following LT in rats, we have found that chronic bile duct hyperplasia is a pathological type of CGD following LT. The mechanism of this kind of CGD is associated with the alterations of inflammation, intestinal barrier function, intestinal microflora, and plasma metabolic profile, which will be the possible therapeutic targets for LT.
We wish to thank Dr. Xin-Hua Chen for her critical assessment of the manuscript, Dr. Wei Xu, Dr. Qing Xie, Ms. De-Ying Chen, and Dr. Jiang-Shan Lian for their help in the technique of UPLC-MS analysis and PLS-DA. We also thank Prof. Da Yu, Mr. Xiao-Ying Sa, Mr. Xian-Fu Ke, Mr. Qi Lou, Mr. Li Jiang, Mr. Yong He, and Ms. Qiao-Juan Shi for their help with the experiment.
Although early mortality rates after liver transplantation (LT) have fallen dramatically, the paradox is that long-term graft survival has barely improved over the last two decades. Chronic graft dysfunction (CGD) has become the biggest obstacle for long-term function of allograft and better life quality of patients. The biliary tract is still the most common site for postoperative complications. These complications not only affect allograft survival, but also have a major impact on the life quality for a hepatic allograft recipient.
So far little is known on biliary tract variation of CGD and its influential factors in recipients following LT. In our experimental study, according to the observation of one year for recipients following LT, we have found that chronic bile duct hyperplasia is a pathological type of CGD following LT. The mechanism of this kind of CGD is associated with the alterations of inflammation, intestinal barrier function, intestinal microflora and plasma metabolic profile.
Chronic hepatic allograft dysfunction may result from a variety of causes including rejection, vascular stenosis/thrombosis, de novo or recurrent infection, recurrent disease related to autoimmune mechanisms such as that seen in primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune chronic active hepatitis. However, So far little is known about alterations of biliary tract when recipients suffer from CGD following LT. Through one-year observation of recipients following LT, we found that chronic bile duct hyperplasia is a pathological type of CGD following LT. Meanwhile, we elaborated the influential factors of this kind of CGD mainly including inflammatory response, intestinal barrier function, intestinal microflora, and plasma metabolic profile. Furthermore, through the technique of ultra performance liquid chromatography-mass spectrometry analysis and partial least squares-discriminate analysis (PLS-DA), we explored the plasma metabonomics alterations during the period of CGD after LT, and indicated the relevance between plasma metabonomics and CGD after LT in rats.
This study provides the experimental data for the research of CGD after organ transplantation in rats, and indicated that chronic bile duct hyperplasia is a kind of CGD following LT in rats. The relative influence factors will be the possible therapeutic targets to prevent or alleviate CGD after LT.
Chronic hepatic allograft dysfunction is defined as the declining of hepatic graft function irreversibly and gradually, expressed by increasing or persistent elevations in serum levels of alanine aminotransferase, alkaline phosphatase, or bilirubin (greater than two times the upper limit of normal). PLS-DA is a statistical method that can grasp the principal contradiction from the complexity, and thereby simplify something complexity, which can generate models that are tightly focused on the effects of interest.
This is a well designed experimental report on chronic allograft dysfunction after LT. The topic is of some interest due to the prolonged survival now being constantly achieved after LT. The authors described chronic bile duct hyperplasia as a type of chronic allograft dysfunction associated to inflammation of the intestinal mucosa
| 1. | Åberg F, Isoniemi H, Höckerstedt K. Long-term results of liver transplantation. Scand J Surg. 2011;100:14-21. [PubMed] |
| 2. | Elliott C, Frith J, Pairman J, Jones DE, Newton JL. Reduction in functional ability is significant postliver transplantation compared with matched liver disease and community dwelling controls. Transpl Int. 2011;24:588-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Desai M, Neuberger J. Chronic liver allograft dysfunction. Transplant Proc. 2009;41:773-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Wang CF, Wang ZY, Li JY. Dual protective role of HO-1 in transplanted liver grafts: a review of experimental and clinical studies. World J Gastroenterol. 2011;17:3101-3108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 5. | Wiesner RH, Menon KV. Late hepatic allograft dysfunction. Liver Transpl. 2001;7:S60-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004;4:1289-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 456] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 7. | Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11:450-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 707] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 8. | Hepatocyte-derived micrornas as serum biomarker of hepatic injury and rejection after liver transplantation. Liver Transpl. 2011;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Sanchez-Urdazpal L, Gores GJ, Ward EM, Maus TP, Buckel EG, Steers JL, Wiesner RH, Krom RA. Diagnostic features and clinical outcome of ischemic-type biliary complications after liver transplantation. Hepatology. 1993;17:605-609. [PubMed] |
| 10. | Colonna JO, Shaked A, Gomes AS, Colquhoun SD, Jurim O, McDiarmid SV, Millis JM, Goldstein LI, Busuttil RW. Biliary strictures complicating liver transplantation. Incidence, pathogenesis, management, and outcome. Ann Surg. 1992;216:344-50; discussion 350-2. [PubMed] |
| 11. | Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 1983;93:64-69. [PubMed] |
| 12. | Ren ZG, Liu H, Jiang JW, Jiang L, Chen H, Xie HY, Zhou L, Zheng SS. Protective effect of probiotics on intestinal barrier function in malnourished rats after liver transplantation. Hepatobiliary Pancreat Dis Int. 2011;10:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Jiang JW, Ren ZG, Chen LY, Jiang L, Xie HY, Zhou L, Zheng SS. Enteral supplementation with glycyl-glutamine improves intestinal barrier function after liver transplantation in rats. Hepatobiliary Pancreat Dis Int. 2011;10:380-385. [PubMed] |
| 14. | Xing HC, Li LJ, Xu KJ, Shen T, Chen YB, Sheng JF, Chen Y, Fu SZ, Chen CL, Wang JG. Protective role of supplement with foreign Bifidobacterium and Lactobacillus in experimental hepatic ischemia-reperfusion injury. J Gastroenterol Hepatol. 2006;21:647-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Systems toxicology study of doxorubicin on rats using ultra performance liquid chromatography coupled with mass spectrometry based metabolomics. Metabolomics. 2009;5:407-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Yang J, Sun X, Feng Z, Hao D, Wang M, Zhao X, Sun C. Metabolomic analysis of the toxic effects of chronic exposure to low-level dichlorvos on rats using ultra-performance liquid chromatography-mass spectrometry. Toxicol Lett. 2011;206:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Snydman DR, Limaye AP, Potena L, Zamora MR. Update and review: state-of-the-art management of cytomegalovirus infection and disease following thoracic organ transplantation. Transplant Proc. 2011;43:S1-S17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Gordon EJ, Daud A, Caicedo JC, Cameron KA, Jay C, Fryer J, Beauvais N, Skaro A, Baker T. Informed consent and decision-making about adult-to-adult living donor liver transplantation: a systematic review of empirical research. Transplantation. 2011;92:1285-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 282] [Article Influence: 15.7] [Reference Citation Analysis (3)] |
| 20. | Vivarelli M, Risaliti A. Liver transplantation for hepatocellular carcinoma on cirrhosis: strategies to avoid tumor recurrence. World J Gastroenterol. 2011;17:4741-4746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Varma V, Webb K, Mirza DF. Liver transplantation for alcoholic liver disease. World J Gastroenterol. 2010;16:4377-4393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Calne RY, McMaster P, Portmann B, Wall WJ, Williams R. Observations on preservation, bile drainage and rejection in 64 human orthotopic liver allografts. Ann Surg. 1977;186:282-290. [PubMed] |
| 23. | Starzl TE, Putnam CW, Hansbrough JF, Porter KA, Reid HA. Biliary complications after liver transplantation: with special reference to the biliary cast syndrome and techniques of secondary duct repair. Surgery. 1977;81:212-221. [PubMed] |
| 24. | Fernández OM, Ríos A, Sánchez A, Palenciano CG, Martínez L, Conesa C, Montoya M, Pons JA, Ramírez P, Parrilla P. Pathology findings in a model of auxiliary liver transplantation with portal vein arterialization in pigs. Transplant Proc. 2005;37:3939-3942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Li Y, Chen Y, Zhang J, Zhu JF, Liu ZJ, Liang SY, Sun K, Liao WY, Gong JP. Protective effect of glutamine-enriched early enteral nutrition on intestinal mucosal barrier injury after liver transplantation in rats. Am J Surg. 2010;199:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Liu C, Li A, Weng YB, Duan ML, Wang BE, Zhang SW. Changes in intestinal mucosal immune barrier in rats with endotoxemia. World J Gastroenterol. 2009;15:5843-5850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Mukhopadhyay P, Rajesh M, Horváth B, Bátkai S, Park O, Tanchian G, Gao RY, Patel V, Wink DA, Liaudet L. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic Biol Med. 2011;50:1368-1381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 28. | Bezinover D, Kadry Z, McCullough P, McQuillan PM, Uemura T, Welker K, Mastro AM, Janicki PK. Release of cytokines and hemodynamic instability during the reperfusion of a liver graft. Liver Transpl. 2011;17:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Dahle DO, Mjøen G, Oqvist B, Scharnagl H, Weihrauch G, Grammer T, März W, Abedini S, Norby GE, Holme I. Inflammation-associated graft loss in renal transplant recipients. Nephrol Dial Transplant. 2011;26:3756-3761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1625] [Cited by in RCA: 1810] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 31. | Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1864] [Cited by in RCA: 1973] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 32. | Wang YB, Liu J, Yang ZX. Effects of intestinal mucosal blood flow and motility on intestinal mucosa. World J Gastroenterol. 2011;17:657-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Levitsky J. Probiotics: application of "healthy" bacteria to liver transplant recipients. Hepatology. 2006;44:507-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3394] [Cited by in RCA: 3628] [Article Influence: 172.8] [Reference Citation Analysis (5)] |
| 35. | Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004;28:405-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 776] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 36. | Rayes N, Seehofer D, Theruvath T, Schiller RA, Langrehr JM, Jonas S, Bengmark S, Neuhaus P. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation--a randomized, double-blind trial. Am J Transplant. 2005;5:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 317] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 37. | Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 722] [Article Influence: 32.8] [Reference Citation Analysis (1)] |
Peer reviewer: Salvatore Gruttadauria, Professor, Abdominal Transplant Surgery, Mediterranean Institute for Transplantation and Advanced Specialized Therapies (IsMeTT), Via E. Tricomi, Palermo 90127, Italy
S- Editor Gou SX L- Editor Webster JR E- Editor Zheng XM