Published online Dec 21, 2011. doi: 10.3748/wjg.v17.i47.5150
Revised: June 20, 2011
Accepted: June 27, 2011
Published online: December 21, 2011
Islet transplantation is characterized by the transplantation of isolated islets from donor pancreata into a diabetic recipient. Although it is a viable choice in the treatment of insulin dependent diabetes mellitus, most patients (approximately 90%) require insulin five years after transplantation. Recently, the co-transplantation of mesenchymal stem cells (MSCs) and islets in animal studies has revealed the effectiveness of MSCs co-transplantation for improving islet function. The mechanisms underlying the beneficial impact of MSCs include immunomodulation and the promotion of angiogenesis. In this review, we discuss MSCs and how they support improved graft survival and function.
- Citation: Sakata N, Goto M, Yoshimatsu G, Egawa S, Unno M. Utility of co-transplanting mesenchymal stem cells in islet transplantation. World J Gastroenterol 2011; 17(47): 5150-5155
- URL: https://www.wjgnet.com/1007-9327/full/v17/i47/5150.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i47.5150
According to the International Diabetes Federation (IDF) database, the number of patients with diabetes mellitus (DM) worldwide is 285 million, indicating that 6.4% of the global population have DM. Furthermore, the IDF predict that the number will increase to 438 million by 2030. DM is a serious disease; approximately 4 million people die each year from DM. In addition, DM is a major cause of serious complications such as blindness, renal failure, and ischemic heart disease. Type 1 diabetes is characterized by the irreversible autoimmune destruction of pancreatic β cells and is usually diagnosed in children and young adults[1]. Islet transplantation consists of the transplantation of pancreatic islets that have been isolated from a donor pancreas[2]. The therapeutic effect was regarded as insufficient for a long time; however, since the use of the “Edmonton Protocol”[2], a markedly improved islet transplant protocol developed at Alberta University, islet transplantation has been performed widely for 10 years. However, according to a recent report, although approximately 70% of patients did not need daily insulin one year after transplantation, approximately 90% of patients required insulin after five years[3]. Therefore, studies aimed at improving the outcome of islet transplantation are still required.
One of the reasons for failure of insulin independence is islet graft loss due to a variety of causes including instant blood-mediated inflammatory reaction[4], acute rejection[5], islet toxicity by immunosuppressive agents[6], and ischemia caused by poor vascularity at transplantation[7] and the embolization effect of the islets[8]. Moreover, islet transplantation also faces the problem of a limited supply of suitable donor human pancreata[9]. Thus, to promote islet transplantation in the future, there is a need to establish a novel donor source and develop more effective treatments to prolong the function of transplanted islets.
Mesenchymal stem cells (MSCs) are multipotent cells capable of self-renewal and differentiation into a various cell lineages. They are derived from many organs such as bone marrow, adipose tissue, skin, fetal liver, and umbilical cord blood[10,11]. Although the number of MSCs in the bone marrow is very small compared with other component cells (only 0.01%-0.001%)[12], MSCs regulate the maintenance and proliferation of hematopoietic stem cells (HSCs) in the bone marrow[13]. MSCs are able to differentiate into various cells derived from ectoderm (epithelial cells and neurons), mesoderm (connective stroma, cartilage, fat and bone cells), and endoderm (muscle cells, gut epithelial cells, and lung cells)[13]. A number of groups have demonstrated that insulin-producing cells could also be differentiated from MSCs[14-18]. The regeneration of insulin-producing cells is an important theme in research aiming to improve the outcome of cell replacement therapy and has been the focus of some groups. However, in an alternative approach, many studies have examined the effect of transplanting islets with MSCs and have demonstrated improved islet function when co-transplanted with MSCs[7,19-22]. The beneficial effects of MSCs in the context of islet transplantation could be attributed to immunomodulation and angiogenesis.
The pancreata used for clinical islet transplantation are allogeneic and recipients therefore require immunosuppressive drugs to prevent rejection. Recently, Solari et al[23] demonstrated that MSCs could promote the prolonged survival of allograft islets in a rat model with limited treatment with immunosuppressive agents. Allogeneic islets transplanted to diabetic rats with syngeneic MSCs survived for over one month whilst islets transplanted alone survived for only seven days. They also demonstrated prolonged graft survival of allogeneic islets transplanted with allogeneic MSCs compared to allogeneic islets transplanted alone. T cell production of interferon (IFN)-γ and tumor necrosis factor (TNF)-α was decreased in allogeneic islets transplanted with syngeneic MSCs. Furthermore, in vitro studies of cocultured MSCs and islets generated interleukin (IL)-10 which inhibited CD4+ T cells. Melzi et al[20] performed allogeneic islet and allogeneic neural stem cell (NSC) transplantation in a murine model and showed significantly longer graft survival in allogeneic islet/NSC transplanted mice in the absence of immunosuppression, compared to mice transplanted with islets alone (> 100 d survival). Intriguingly, they detected expansion of regulatory T cells in the spleen of co-transplanted mice. These results indicate that MSCs exert an immunomodulatory role and can actively limit the rejection of co-transplanted islets.
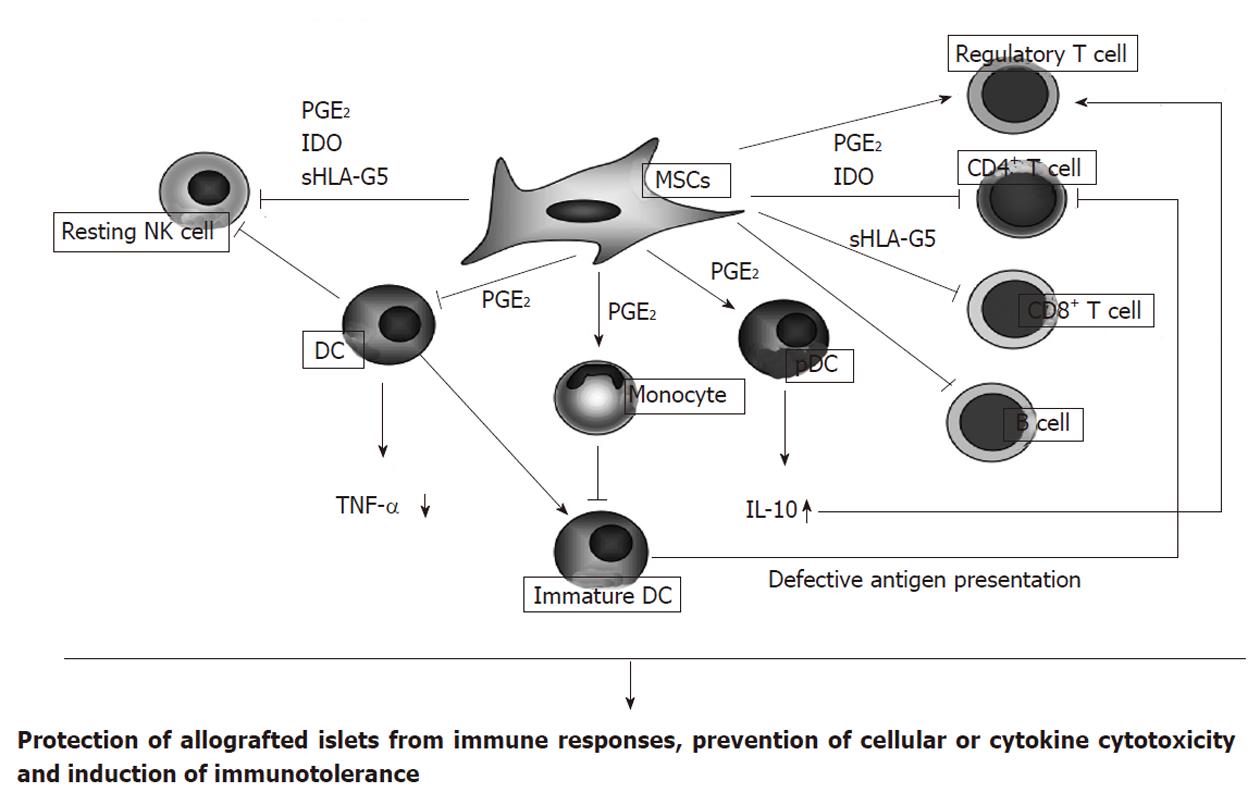
The mechanism underlying the immunomodulatory effect of MSCs is likely to be multifactorial and result from the communication between various immune cells and cytokine generation (Figure 1). For example, MSCs can inhibit the proliferation and cytotoxicity of resting natural killer (NK) cells, which are key effector cells of the innate immune system and play an important role in antiviral and anti-tumor immune responses[24]. Spaggiari et al[25] demonstrated that the cytokine-induced proliferation of freshly isolated NK cells was inhibited by the presence of MSCs. MSCs also inhibited NK cell activation, cytotoxic activity, and IFN-γ production[26]. These effects are mediated by prostaglandin E2 (PGE2) and indoleamine 2,3-dioxygenase (IDO)[13,26].
Another important effect of MSCs is to inhibit the differentiation of monocytes to dendritic cells (DCs) that, following DC maturation, present antigens to naïve T cells[27,28]. MSCs also inhibit TNF-α production by DCs and upregulate IL-10 production by plasmacytoid DCs (pDCs)[29] - effects modulated by PGE2. These effects of MSCs upon DC function undoubtedly contribute to their anti-inflammatory and immunoregulatory effects.
MSCs may also directly inhibit CD4+ T cells, CD8+ T cells, and B cells, immune cells involved in rejection of allogeneic cells, by releasing soluble mediators, including PGE2, IDO, or soluble human leukocyte antigen (sHLA)-G5. Inhibition of CD4+ T cells impairs B cell proliferation and antibody production[13]. CD8+ cytotoxic T cells are involved in killing virus-infected or allogeneic cells, and MSCs are capable of inhibiting the induction of CD8+ T cell responses and preventing cytotoxicity[30]. MSCs inhibit B cell proliferation and antibody secretion, as well as their differentiation to plasma cells[31]. On the other hand, MSCs may induce the generation of regulatory T cells, which suppress immune cell activation, and help to maintain homeostasis and promote self tolerance by inducing production of IL-10 from pDCs and by releasing HLA-G5[29,32]. In summary, MSCs can promote immunological tolerance and facilitate the survival and function of allogeneic islets. It is likely, however, that the immunomodulatory roles of MSCs have not been fully clarified.
Pancreatic islets have a rich vascular supply in the pancreas, with some reports indicating that islets receive 5%-10% of pancreatic blood flow, despite the islet mass only comprising 1%-2% of the total pancreas[33,34]. However, isolated islets are avascular, as the process of islet isolation destroys the vascular network between the islet and surrounding tissue[35]. As a result, islets undergo prolonged ischemia during the reconstruction of the vascular network, which may take approximately 14 d[36] and many islets become damaged. It is thus apparent that strategies to limit islet ischemia are necessary to improve the outcome of islet transplantation.
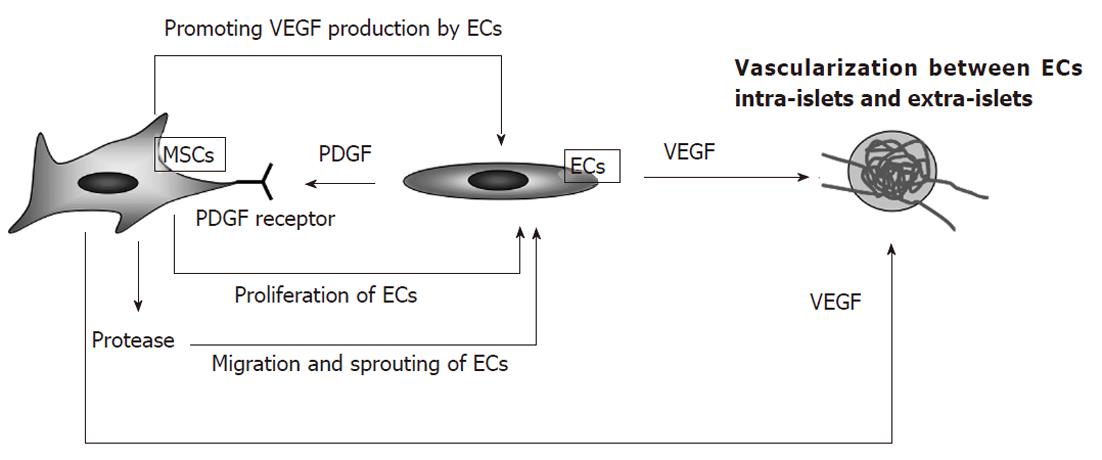
Some studies suggest that angiogenic factors, such as vascular endothelial growth factor-A (VEGF-A) and angiopoietin-1, are required to generate a vascular network around transplanted islets[37,38]. Recently, the pro-angiogenic effects of MSCs have been examined (Figure 2). The process of revascularization consists of proteolytic digestion of the vascular wall and subsequent migration, proliferation, and differentiation of endothelial cells (ECs)[39]. MSCs express platelet-derived growth factor (PDGF) receptors and respond to PDGF production by ECs during revascularization[40]. MSCs promote EC migration by producing proteases that facilitate immature EC sprouting[41] and upregulating the expression of angiopoietin and VEGF in ECs, as these factors promote angiogenesis and stability of the developing vasculature[42]. The roles of MSCs in angiogenesis have been explored in experimental models of ischemia. Martens et al[43] demonstrated that MSCs produced VEGF and induced neovascularization in the ischemic myocardium. Jiang et al[44] showed that transplantation of MSCs into ischemic limbs promoted angiogenesis. Johansson et al[45] explored the enhancement of angiogenesis by MSCs in the context of islets. They first examined cultures of human islets and ECs in the presence or absence of MSCs. The study indicated that the inclusion of MSCs promoted EC proliferation and migration of ECs to the surface of islets to form a “coat”[45]. Islets with this surrounding “coat” of endothelial cells survived for a long time in culture and exhibited improved insulin release[45]. The coated islets had many sprouts and were connected to other endogenous islets by vessel-like structures[45]. These findings indicated that MSCs act to promote EC proliferation in both donor and recipient sides, together with sprout formation and growth of ECs into the islet. Thus, MSCs may exert a potent angiogenic function and contribute to islet engraftment by promoting islet vascularization.
Recently, Ito et al[21] co-transplanted rat islets and MSCs into diabetic severe combined immunodeficiency mice and evaluated the rate of normoglycemia and extent of islet vascularization. All the diabetic mice that received 500 islets with 107 MSCs exhibited normoglycemia, compared to 30% of mice transplanted with islets alone. Neovascular density was also increased in the islet/MSCs co-transplanted group and was associated with strong expression of VEGF and endothelial von Willebrand factor. Sakata et al[7] and Figliuzzi et al[22] also evaluated the impact of MSCs upon transplanted islets, and found a similar improvement of islet function and vascularization. The beneficial impact of vascularization is in accord with our previous work indicating that hyperbaric oxygen therapy prevented cellular apoptosis of islets[46]. These data indicate that promoting islet vascularization by co-transplanting MSCs acts to limit the duration and severity of islet ischemia, thereby limiting islet cell apoptosis and promoting islet integrity and function.
It is likely that MSCs may exert beneficial effects in addition to those previously outlined. For example, Olerud et al[19] demonstrated that neural crest stem cells, a kind of MSCs, augment islet cell proliferation and improve islet function. In addition, Melzi et al[20] showed that transplanted bone marrow cells stimulated pancreatic β-cell proliferation after streptozotocin-induced pancreatic injury.
In conclusion, MSCs may exert beneficial immunomodulatory and pro-angiogenic effects when co-transplanted with islets. Immunomodulatory effects include the functional inhibition of immunocompetent cells, such as NK cells, DCs, cytotoxic T cells, and B cells. MSCs may also induce the generation of regulatory T cells that promote immunological tolerance. The pro-angiogenic effects of MSCs result from the release of angiogenic factors and promotion of the vascular network linking islets to the surrounding tissue. This pro-angiogenic role limits the duration and severity of islet ischemia and improves islet function. We therefore believe that the co-transplantation of MSCs represents a viable method for improving islet transplantation. Recently, a clinical trial of combined islet and hematopoietic stem cell allotransplantation was performed[47]. The data did not support the effectiveness of HSCs co-transplantation in prevention of graft rejection and in avoiding side effects of immunosuppression. Moreover, Melzi et al[20] reported the risk of inducing cancer because of NSC co-transplantation, as well as proving the effectiveness of this strategy to prevent islet graft rejection. Thus, further studies examining the effect of MSCs in a clinical setting should be undertaken.
| 1. | Vanikar AV, Dave SD, Thakkar UG, Trivedi HL. Cotransplantation of adipose tissue-derived insulin-secreting mesenchymal stem cells and hematopoietic stem cells: a novel therapy for insulin-dependent diabetes mellitus. Stem Cells Int. 2010;2010:582382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230-238. [PubMed] |
| 3. | Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1275] [Cited by in RCA: 1206] [Article Influence: 57.4] [Reference Citation Analysis (1)] |
| 4. | Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51:1779-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 212] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Chen X, Zhang X, Larson CS, Baker MS, Kaufman DB. In vivo bioluminescence imaging of transplanted islets and early detection of graft rejection. Transplantation. 2006;81:1421-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Desai NM, Goss JA, Deng S, Wolf BA, Markmann E, Palanjian M, Shock AP, Feliciano S, Brunicardi FC, Barker CF. Elevated portal vein drug levels of sirolimus and tacrolimus in islet transplant recipients: local immunosuppression or islet toxicity? Transplantation. 2003;76:1623-1625. [PubMed] |
| 7. | Sakata N, Chan NK, Chrisler J, Obenaus A, Hathout E. Bone marrow cell cotransplantation with islets improves their vascularization and function. Transplantation. 2010;89:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Sakata N, Hayes P, Tan A, Chan NK, Mace J, Peverini R, Sowers L, Pearce WJ, Chinnock R, Obenaus A. MRI assessment of ischemic liver after intraportal islet transplantation. Transplantation. 2009;87:825-830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Sakata N, Gu Y, Qi M, Yamamoto C, Hiura A, Sumi S, Sunamura M, Matsuno S, Inoue K. Effect of rat-to-mouse bioartificial pancreas xenotransplantation on diabetic renal damage and survival. Pancreas. 2006;32:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Shenaq DS, Rastegar F, Petkovic D, Zhang BQ, He BC, Chen L, Zuo GW, Luo Q, Shi Q, Wagner ER. Mesenchymal Progenitor Cells and Their Orthopedic Applications: Forging a Path towards Clinical Trials. Stem Cells Int. 2010;2010:519028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Sato K, Ozaki K, Mori M, Muroi K, Ozawa K. Mesenchymal stromal cells for graft-versus-host disease : basic aspects and clinical outcomes. J Clin Exp Hematop. 2010;50:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15357] [Article Influence: 568.8] [Reference Citation Analysis (1)] |
| 13. | Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2768] [Article Influence: 162.8] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Shen W, Hua J, Lei A, Lv C, Wang H, Yang C, Gao Z, Dou Z. Pancreatic islet-like clusters from bone marrow mesenchymal stem cells of human first-trimester abortus can cure streptozocin-induced mouse diabetes. Rejuvenation Res. 2010;13:695-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Kadam S, Muthyala S, Nair P, Bhonde R. Human placenta-derived mesenchymal stem cells and islet-like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Rev Diabet Stud. 2010;7:168-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Anzalone R, Lo Iacono M, Loria T, Di Stefano A, Giannuzzi P, Farina F, La Rocca G. Wharton's jelly mesenchymal stem cells as candidates for beta cells regeneration: extending the differentiative and immunomodulatory benefits of adult mesenchymal stem cells for the treatment of type 1 diabetes. Stem Cell Rev. 2011;7:342-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Li HY, Chen YJ, Chen SJ, Kao CL, Tseng LM, Lo WL, Chang CM, Yang DM, Ku HH, Twu NF. Induction of insulin-producing cells derived from endometrial mesenchymal stem-like cells. J Pharmacol Exp Ther. 2010;335:817-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Wang HS, Shyu JF, Shen WS, Hsu HC, Chi TC, Chen CP, Huang SW, Shyr YM, Tang KT, Chen TH. Transplantation of insulin-producing cells derived from umbilical cord stromal mesenchymal stem cells to treat NOD mice. Cell Transplant. 2011;20:455-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Olerud J, Kanaykina N, Vasylovska S, King D, Sandberg M, Jansson L, Kozlova EN. Neural crest stem cells increase beta cell proliferation and improve islet function in co-transplanted murine pancreatic islets. Diabetologia. 2009;52:2594-2601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Melzi R, Antonioli B, Mercalli A, Battaglia M, Valle A, Pluchino S, Galli R, Sordi V, Bosi E, Martino G. Co-graft of allogeneic immune regulatory neural stem cells (NPC) and pancreatic islets mediates tolerance, while inducing NPC-derived tumors in mice. PLoS One. 2010;5:e10357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Ito T, Itakura S, Todorov I, Rawson J, Asari S, Shintaku J, Nair I, Ferreri K, Kandeel F, Mullen Y. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation. 2010;89:1438-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 22. | Figliuzzi M, Cornolti R, Perico N, Rota C, Morigi M, Remuzzi G, Remuzzi A, Benigni A. Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant Proc. 2009;41:1797-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Solari MG, Srinivasan S, Boumaza I, Unadkat J, Harb G, Garcia-Ocana A, Feili-Hariri M. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. J Autoimmun. 2009;32:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 514] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 25. | Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 782] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 26. | Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 826] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 27. | Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120-4126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 992] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 28. | Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080-2087. [PubMed] |
| 29. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3302] [Article Influence: 150.1] [Reference Citation Analysis (1)] |
| 30. | Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208-1213. [PubMed] |
| 31. | Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1263] [Cited by in RCA: 1276] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 32. | Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 778] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 33. | Stagner JI, Mokshagundam S, Samols E. Hormone secretion from transplanted islets is dependent upon changes in islet revascularization and islet architecture. Transplant Proc. 1995;27:3251-3254. [PubMed] |
| 34. | Lifson N, Lassa CV, Dixit PK. Relation between blood flow and morphology in islet organ of rat pancreas. Am J Physiol. 1985;249:E43-E48. [PubMed] |
| 35. | Emamaullee JA, Rajotte RV, Liston P, Korneluk RG, Lakey JR, Shapiro AM, Elliott JF. XIAP overexpression in human islets prevents early posttransplant apoptosis and reduces the islet mass needed to treat diabetes. Diabetes. 2005;54:2541-2548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Menger MD, Yamauchi J, Vollmar B. Revascularization and microcirculation of freely grafted islets of Langerhans. World J Surg. 2001;25:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 300] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 38. | Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J. Pancreatic islet production of vascular endothelial growth factor--a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974-2985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 355] [Article Influence: 17.8] [Reference Citation Analysis (5)] |
| 39. | Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49:507-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 706] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 40. | Ball SG, Shuttleworth CA, Kielty CM. Mesenchymal stem cells and neovascularization: role of platelet-derived growth factor receptors. J Cell Mol Med. 2007;11:1012-1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Ghajar CM, Blevins KS, Hughes CC, George SC, Putnam AJ. Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng. 2006;12:2875-2888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 180] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 42. | Zacharek A, Chen J, Cui X, Li A, Li Y, Roberts C, Feng Y, Gao Q, Chopp M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27:1684-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 231] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 43. | Martens TP, See F, Schuster MD, Sondermeijer HP, Hefti MM, Zannettino A, Gronthos S, Seki T, Itescu S. Mesenchymal lineage precursor cells induce vascular network formation in ischemic myocardium. Nat Clin Pract Cardiovasc Med. 2006;3 Suppl 1:S18-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Jiang M, Wang B, Wang C, He B, Fan H, Guo TB, Shao Q, Gao L, Liu Y. Angiogenesis by transplantation of HIF-1 alpha modified EPCs into ischemic limbs. J Cell Biochem. 2008;103:321-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Johansson U, Rasmusson I, Niclou SP, Forslund N, Gustavsson L, Nilsson B, Korsgren O, Magnusson PU. Formation of composite endothelial cell-mesenchymal stem cell islets: a novel approach to promote islet revascularization. Diabetes. 2008;57:2393-2401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 46. | Sakata N, Chan NK, Ostrowski RP, Chrisler J, Hayes P, Kim S, Obenaus A, Zhang JH, Hathout E. Hyperbaric oxygen therapy improves early posttransplant islet function. Pediatr Diabetes. 2010;11:471-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Mineo D, Ricordi C, Xu X, Pileggi A, Garcia-Morales R, Khan A, Baidal DA, Han D, Monroy K, Miller J. Combined islet and hematopoietic stem cell allotransplantation: a clinical pilot trial to induce chimerism and graft tolerance. Am J Transplant. 2008;8:1262-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Peer reviewer: Ming Li, Associate Professor, Tulane University Health Sciences Center, 1430 Tulane Ave Sl-83, New Orleans, LA 70112, United States
S- Editor Tian L L- Editor Stewart GJ E- Editor Li JY