Published online Dec 7, 2011. doi: 10.3748/wjg.v17.i45.4960
Revised: September 22, 2011
Accepted: September 29, 2011
Published online: December 7, 2011
AIM: To investigate the effects of moxibustion on down-regulation of the colonic epithelial cell apoptosis and repair of the tight junctions in rats with Crohn’s disease (CD).
METHODS: Sixty male Sprague-Dawley rats were randomly divided into a normal control (NC) group, a model control (MC) group, an herbs-partitioned moxibustion (HPM) group, a mild-warm moxibustion (MWM) group and a salicylazosulphapyridine (SASP) group, with 12 rats in each group. The CD model rats were treated with trinitrobenzene sulphonic acid to induce intestinal inflammation. The rats in the HPM and MWM groups were treated at the Tianshu (ST25) and Qihai (CV6) acupoints once daily for 14 d, and the SASP group was fed SASP twice daily for 14 d. No additional treatment was given to the MC and NC groups. The microstructure of the colonic epithelium was observed under a transmission electron microscope, the transepithelial resistance was measured using a short-circuit current, colonic epithelial cell apoptosis was determined by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labelling assay, and the expression of occludin, claudin-1 and zonula occludens-l (ZO-1) in the colonic epithelial junction was determined by Western blotting and immunofluorescence staining.
RESULTS: Compared with the MC group, the microstructure of the colonic epithelial barrier was significantly improved in rats treated with HPM, MWM or SASP, meanwhile, the current flow was reduced significantly, with values of 168.20 ± 6.14 vs 99.70 ± 3.13, 99.10 ± 4.28 and 120.30 ± 3.65 mA, respectively (P = 0.001). However, the HPM and MWM groups had higher current flow rates than the SASP group (99.70 ± 3.13, 99.10 ± 4.28 vs 120.30 ± 3.65 mA, P = 0.001). The number of the apoptotic colonic epithelial cells in HPM, MWM and SASP groups was largely reduced (61.5 ± 16.91 vs 15.5 ± 8.89, 14.8 ± 6.27 and 24.7 ± 9.68, respectively (P = 0.001); and the expression of occludin, claudin-1 and ZO-1 in the MWM and HPM groups was significantly enhanced (0.48 ± 0.10, 0.64 ± 0.09 vs 0.18 ± 0.05 for occludin, 0.12 ± 0.02, 0.17 ± 0.03 vs 0.05 ± 0.01 for claudin-1, and 0.08 ± 0.01, 0.11 ± 0.01 vs 0.02 ± 0.01 for ZO-1). And in SASP group, the expression of occludin and ZO-1 was also significantly increased (0.27 ± 0.04 vs 0.18 ± 0.05 for occludin and 0.05 ± 0.01 vs 0.02 ± 0.01 for ZO-1), but there was no significant difference for claudin-1. The HPM and MWM groups had higher expression of occludin, claudin-1 and ZO-1 than the SASP group.
CONCLUSION: HPM and MWM treatment can down-regulate apoptosis of colonic epithelial cells, repair tight junctions and enhance colonic epithelial barrier function in rats with CD.
- Citation: Bao CH, Wu LY, Shi Y, Wu HG, Liu HR, Zhang R, Yu LQ, Wang JH. Moxibustion down-regulates colonic epithelial cell apoptosis and repairs tight junctions in rats with Crohn’s disease. World J Gastroenterol 2011; 17(45): 4960-4970
- URL: https://www.wjgnet.com/1007-9327/full/v17/i45/4960.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i45.4960
Crohn’s disease (CD) is a chronic and non-specific inflammatory disease that may affect any part of the gastrointestinal tract from the mouth to the anus, thereby causing a wide variety of symptoms. CD has been mostly seen in Western industrialized countries. However, in recent years, the number of individuals affected by CD in China has significantly increased[1]. The exact cause of Crohn’s disease is still unknown. In Western medical practices, treatment options include steroids, immunosuppressants and 5-aminosalicylic acid. However, these treatments are restricted to controlling symptoms, maintaining remission, and preventing relapse. Currently, there is no known pharmaceutical or surgical cure for Crohn’s disease[2]. Although biologic therapies provide hope for CD patients, the financial cost and the significant side effects limit their application[2].
Moxibustion has been used in China for more than 4000 years to treat various diseases, including gastrointestinal diseases, such as Crohn’s[3], ulcerative colitis[4] and irritable bowel syndrome[5]. Mild-warm moxibustion (MWM) and herbs-partitioned moxibustion (HPM) are critical components of moxibustion therapy. MWM is a type of moxa stick moxibustion that is performed by holding an ignited moxa stick a certain distance above the patient’s skin, keeping the spot warm and making it reddened, but not burnt. HPM is performed by placing an herbal cake (traditional Chinese medicinal formula dispensing) on the patient’s acupoints, followed by the placement and ignition of moxa cones, composed of refined mugwort floss, on the herbal cake to treat diseases. Previously, our group has used moxibustion in the clinic to treat mild or moderate CD, and the efficacy of this treatment can reach nearly 75%[6]. However, the underlying mechanisms of moxibustion treatment of CD remain elusive. Therefore, in this study, we used a rat model with trinitrobenzene sulfonic acid (TNBS)-induced CD and studied the role of moxibustion, as HPM and MWM, in repairing the colonic epithelial barrier using histomorphology, molecular biology, proteinology and electrophysiology.
Several factors have also been shown to contribute to the incidence of CD. For example, defective epithelial barrier function, which can be measured as increased intestinal permeability, has been implicated in CD[7] and can predict relapse during clinical remission[8,9]. However, increased permeability is also present in a subset of unaffected first-degree relatives of patients with CD[10-13] or other functional bowel diseases[14]. It has been demonstrated that damage of the colonic epithelial barrier, as in colonic epithelial death and the connective tissue damage, promotes CD in animal models[15] and humans[16-18]. The mechanisms underlying the loss of barrier function and permeability defects are thought to have a great potential in defining inflammation bowel disease (IBD) pathogenesis and guiding the development of novel treatment for IBD[19].
Therefore, the aim of this study was to investigate whether HPM and MWM can effectively treat CD and to clarify the underlying mechanisms regulating this process. To determine whether HPM and MWM are able to repair the colonic epithelial barrier, we examined the effects of moxibustion on colonic epithelial microstructure and transepithelial resistance, apoptosis of colonic epithelial cells and the expression of the proteins involved in colonic epithelial tight junctions, including occludin, claudin-1 and zonula occludens-l (ZO-1).
Sprague-Dawley (SD) rats (male, Specific Pathogen Free, 150 ± 10 g) were purchased from Shanghai University of Traditional Chinese Medicine. All experimental protocols were approved by the Animal Research Ethics Committee of Shanghai University of Traditional Chinese Medicine, No. 09042.
Sixty male SD rats were randomly divided into a normal control (NC) group, a model control (MC) group, an HPM group, an MWM group and an SASP group, with 12 rats in each group. The CD models were established according to Morris’ method[20]. Prior to model establishment, the rats were fasted and given water for 24 h. The rats were weighed, and 2% sodium pentobarbital (30 mg/kg) was administered through intraperitoneal injection (ip). The rats were anally injected with a TNBS solution mixed in 50% alcohol at a 1:2 ratio using a rubber tube. All groups of rats received the TNBS injection, with the exception of the normal control group, which received normal saline. The rubber tube was put into the anus 6-8 cm deep. Following the injection, the head of the rat was pushed down for about 1 min to prevent loss of the injected solution. The injection was repeated every 7 d for 4 wk. When the experimental CD rat models were completed, one rat was randomly selected from each group to ascertain whether the CD models were successful by hematoxylin eosin staining.
After the experimental CD rat models were confirmed to be successfully established, the rats were exposed to different treatments. In the HPM group, moxa cones (0.5 cm in diameter and 0.6 cm in length) made of refined mugwort floss were placed on an herbal cake [medicinal formula dispensing (radix) Aconiti praeparata, (cortex) Cinnamomi, etc.] at the Tianshu (ST25) and Qihai (CV6) acupoints and ignited. ST25 and CV6 were used to regulate intestinal functions. Two moxa cones were used for each treatment once daily for 14 d. In the MWM group, moxa sticks were ignited 1-2 cm above ST25 and CV6 for 10 min, with the temperature of the local area maintained at 43 °C ± 2 °C. The rats in the SASP group were fed with SASP, which was prepared at the proportion of 1:0.018[21], twice a day for 14 d. The rats in the MC and NC groups did not receive any treatment. After the treatment, five groups of rats were sacrificed simultaneously. The distal colons were dissected at a length of 6 cm.
Samples (rats colonic mucosa) were cut into 1 mm3 strips, fixed for 4 h at 4 °C in 5% glutaraldehyde, fully washed for 3 times in 0.1 mol/L phosphate buffered saline (PBS), postfixed for 2 h at 4 °C in 2% osmium tetroxide, dehydrated in a graded series of ethanols and embedded in Epon 812, cut into ultrathin sections (75 nm) and then stained with uranyl acetate and lead citrate. Sections were viewed in a HITACHI H-600 electron microscope at 80 kV (HITACHI, Tokyo, Japan).
Colonic epithelial cells were isolated and immediately purified according to Evans et al[22]. Transepithelial resistance (Rt) experiments were performed[23] at least 1 wk after seeding the cells on filters, when the Rt of the monolayers reached stable values. The ussing chamber (A and B chamber) (Sigma, United States) used was modified to allow intact inserts to be placed into the chamber. The apical side of the colonic epithelium was directed to the A chamber, and the basolateral side of the colonic epithelium was directed to the B chamber. Both A and B chamber reservoirs contained Krebs-Henseleit solution with 5% oxygenation at 37 °C. Short-circuit current, open-circuit transepithelial voltage, and transepithelial resistance were recorded by an Axon 2B clamp (Axon, United States). The data were analyzed by p Clamp 8.0 software (Axon, United States).
Western blotting analysis was conducted, as described by Zeissig et al[24] We randomly selected six rat colon samples from each group for detection. Rat colon samples were homogenized with a dounce homogeniser in lysis buffer containing 20 mmol/L Tris, pH 7.4, 5 mmol/L magnesium chloride, 1 mmol/L EDTA, 0.3 mmol/L ethyleneglycol tetra-acetic acid, 1 μL/mL aprotinin, 16 μg/mL benzamidine/hydrochloric acid, 10 μg/mL phenanthroline, 10 μg/mL leupeptin, 10 μg/mL pepstatin, 2 mmol/L phenylmethyl-sulphonyl fluoride, 210 μg/mL sodium fluoride, 2.16 mg/mL β-glycerophosphate, 18.4 μg/mL sodium vanadate and 1 μL/mL trypsin inhibitor. The lysates were passed through a needle, and the insoluble material was removed by centrifugation (350 ×g for 5 min at 4 °C). The supernatant was centrifuged at 43 000 ×g for 30 min at 4 °C, and the pellets were resuspended in lysis buffer. The protein concentrations were determined using a Pierce bicinchoninic acid assay. Aliquots of 5 μg of each sample were separated by polyacrylamide gel electrophoresis and were transferred to a nitrocellulose membrane. The blots were blocked for 2 h in 5% non-fat milk in phosphate-buffered saline and overnight in 5% bovine serum albumin in phosphate-buffered saline (at 4 °C) before incubation with primary antibodies for 90 min at room temperature. Primary rabbit monoclonal immunoglobulin (Ig)G antibodies directed against occludin (1:200) (BD, United States), claudin-1 (1:200) (Invitrogen, United States) and ZO-1 (1:200) (Santa Cruz, United States) were used. Peroxidase-conjugated goat anti-rabbit IgG (1:1000) (Bioss, Beijing, China) and chemiluminescence fluid A and B (1:1) (Boster, Wuhan, China) were mixed for 1 h and 1 min, respectively. The reactive bands were detected using chemiluminescent reagents (Pierce Company, Minneapolis, United States).
Rat colon samples were washed with 4 °C normal saline, fixed in 10% formalin for 24 h, embedded in paraffin, cut into sections and heated at 60 °C for 20 min. The sections were soaked in dimethyl benzene twice for 10 min, soaked in 100%, 95% and 70% alcohol for 5 min, and washed with water. A total of 2000 mL of 0.01 mol/L natrium citricum buffer solution (pH 6.0) was added to the pressure kettle, and the colon sections were placed on a staining rack in the pressure kettle. The samples were processed for 1-2 min at 0.142 MPa, removed, and quickly washed with water, followed by an additional wash with PBS for 5 min. Goat serum was added to the samples for 20 min at room temperature, and the superfluous fluid was removed. The samples were incubated with primary monoclonal antibody diluted 1:1000 (occludin Ab, claudin-1 Ab, ZO-1 Ab) in blocking buffer overnight at 4 °C. The samples were incubated at 37 °C for 45 min and were washed 3 times for 2 min with PBS. The samples were incubated with secondary antibody diluted 1:10 000 (FITC-conjugated anti-rabbit) (Abcam, United States) in blocking buffer for 20 min at room temperature. Next, the samples were washed 3 times for 2 min with PBS. The samples were incubated with 5-10 mL DAPI staining solution for 10 min and were washed 4 times for 5 min with PBS. One drop of 50% glycerine was added to the sections, and laser confocal microscopy (Nikon, Japan) was used to detect the expression of occludin, claudin-1 and ZO-1. The protein expression was evaluated in the 5 fields of vision.
Transferase-mediated dUTP-biotin nick end labeling assay (TUNEL) staining was performed using the DeadEnd™ Fluorometric TUNEL System (Promega, Madison, WI). A total of 5 × 107/mL cells were fixed with a 4% formaldehyde solution for 10 min and washed twice for 5 min with PBS. The slides were equilibrated with equilibration buffer and were incubated for 30 min at 37 °C with recombinant terminal deoxynucleotidyl transferase (rTdT) incubation buffer. The negative control sections were incubated with control incubation buffer without the rTdT enzyme. The slides were analyzed using a Leica TCS SP5 confocal microscope (Germany).
Results were expressed as mean ± SD. Statistical analyses were performed using SPSS 13.0 (SPSS Inc. Chicago, Illinois). Differences in mean were compared by one way analysis of variance. Differences were considered statistically significant if P < 0.05.
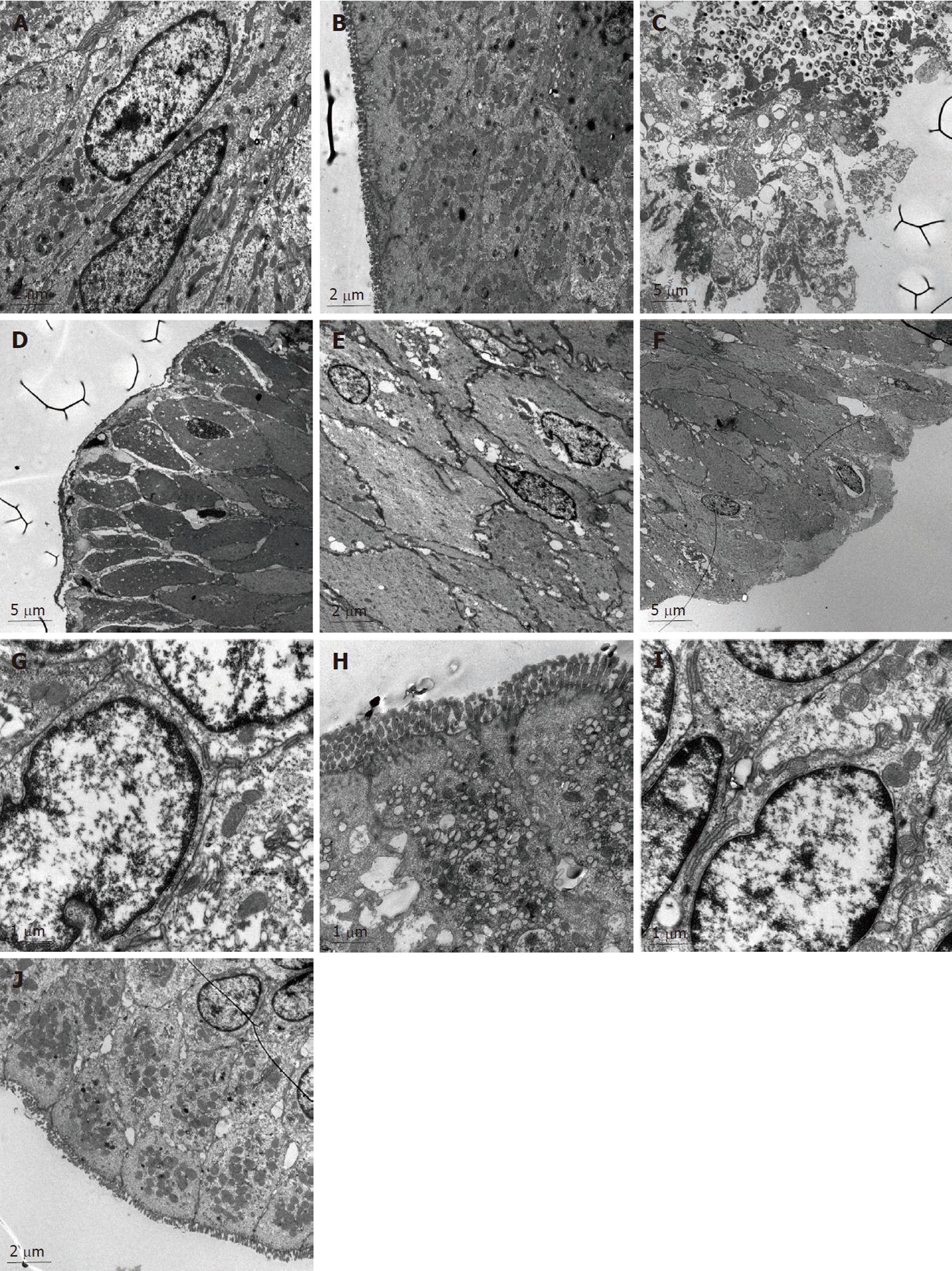
In the NC group (Figure 1A and B), the morphology of the colonic epithelia, the cell membrane and the nuclear membrane were integral. The nucleolus could be seen clearly. In the cytoplasm, both the endoplasmic reticulum and mitochondria could be seen clearly. The junction of the epithelial cells was tight. The junctions among the cells were not broadened. We also observed abundant secretion by the cells, and there were villi present on the cell surfaces.
In the MC group (Figure 1C and D), the colonic epithelial cells were reduced in size, the integrity of the epithelial membrane and the nuclear membrane was compromised, the nuclei were absent, and most of the organelles inside the cytoplasm were absent. The MC group colonic epithelial cells contained bubbles inside the cytoplasm, and the number of mitochondria was significantly reduced. The junctions between the epithelial cells were loose, and a significant broadening of the colonic cells was observed. No villi could be detected on the cell surface.
In the colonic epithelial cells of the SASP group (Figure 1E and F), the integrity of the cell and nuclear membranes were disrupted, the nuclei of the cells were absent, the structure of the organelles inside the cytoplasm could not be seen clearly, and the number of mitochondria was relatively normal. In addition, the size of the colonic epithelial cells was significantly reduced, and bubbles of different sizes were present in the cytoplasm. The junctions between the epithelial cells were not tight, and the intracellular space was enlarged. No villi could be seen on the cell surfaces.
In the colonic epithelial cells of the HPM group (Figure 1G and H), the integrity of the nuclear membranes were almost normal. The nuclei of the cells were clear, the number of the organelles was reduced and the cell membranes were injured. Many mitochondria could be observed, the junctions between the epithelial cells were relatively tight, the intracellular spaces were increased, and some villi could be seen on the cell surfaces.
In the colonic epithelial cells of the MWM group (Figure 1I and J), the integrity of the nuclear membrane was relatively normal. The nuclei of the cells were present, a few bubbles appeared in the cytoplasm, and many mitochondria could be detected. The junctions between the epithelial cells were tight, while some intracellular spaces were tight and some intracellular spaces were broadened. Particle secretion was detected in the intracellular space. There were a few villi on the cell surfaces.
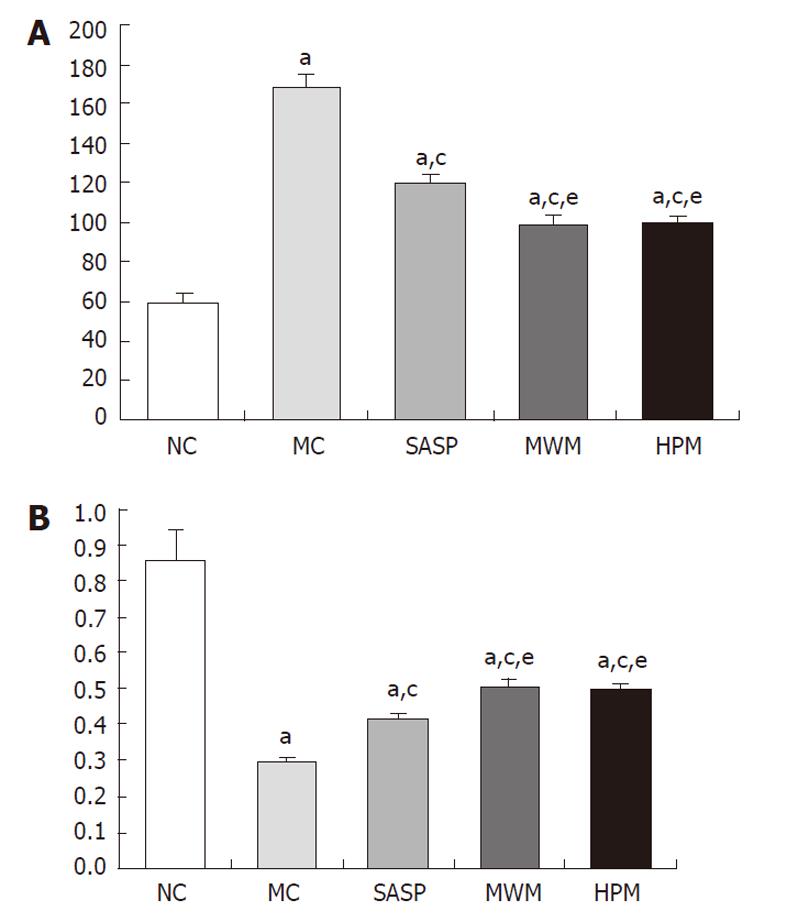
Compared with normal rats, the current flow of the colonic epithelial cells was significantly reduced in the MC group (168.20 ± 6.14 vs 58.60 ± 5.52 mA, respectively, P = 0.001), but the transepithelial resistance was increased (0.30 ± 0.01 vs 0.86 ± 0.08, respectively, P = 0.001) (Table 1). Following the HPM, MWM or SASP treatment, the transepithelial resistance was increased, and the current flow was decreased compared with normal rats. Compared with the MC group, the transepithelial resistance was significantly increased in rats treated with HPM, MWM or SASP, with values of 0.30 ± 0.01 vs 0.50 ± 0.02, 0.51 ± 0.02, 0.42 ± 0.01, respectively (P = 0.001). Meanwhile, the current flow was reduced, with values of 168.20 ± 6.14 vs 99.70 ± 3.13, 99.10 ± 4.28, 120.30 ± 3.65 mA, respectively (P = 0.001). However, we found that the current flow in the HPM and SASP groups was enhanced compared with the SASP group, with values of 0.50 ± 0.02, 0.51 ± 0.02 vs 0.42 ± 0.01 and 99.70 ± 3.13, 99.10 ± 4.28 vs 120.30 ± 3.65 mA, respectively (P = 0.001), but was lower than the flow rate of the normal group. These data suggest that the CD rats treated with HPW, MWM or SASP had colonic epithelial cells with significantly increased transepithelial resistance, which may further cause enhanced repair and/or protection of the colonic epithelial barrier (Figure 2A and B).
Compared with the NC group, the number of apoptotic colonic epithelial cells was significantly increased in the MC group (61.5 ± 16.91 vs 11.5 ± 4.48, respectively, P = 0.001) (Table 2). However, the number of colonic epithelial cells undergoing apoptosis decreased in the MWM, HPM and SASP groups (61.5 ± 16.91 vs 15.5 ± 8.89, 14.8 ± 6.27, 24.7 ± 9.68, respectively, P = 0.001). However, no significant differences in colonic epithelial cell apoptosis were found among the HPM, MWM and SASP groups. In addition, compared with the NC group, the differences in the number of apoptotic colonic epithelial cells were not statistically significant between the MWM and HPM groups; however, there was a difference in apoptosis between the SASP and NC groups (24.7 ± 9.68 vs 11.5 ± 4.48, respectively, P = 0.017). Moreover, the number of apoptotic colonic epithelial cells in the MC group was significantly increased compared with the NC group (0.10 ± 0.03 vs 0.02 ± 0.09, respectively, P = 0.001), and compared with the MC group, the number of apoptotic colonic epithelial cells in the HPM, MWM and SASP groups was also largely reduced (0.03 ± 0.01, 0.03 ± 0.01, 0.05 ± 0.02 vs 0.10 ± 0.03, respectively, P = 0.001). There was a larger number of apoptotic cells in the colonic epithelium in the SASP group than in the MWM or HPM groups (0.05 ± 0.02 vs 0.03 ± 0.01, 0.03 ± 0.01, respectively, P = 0.024 and P = 0.025). These data suggested that the MWM and HPM were able to down-regulate the apoptosis of colonic epithelial cells in rats with CD (Figure 3).
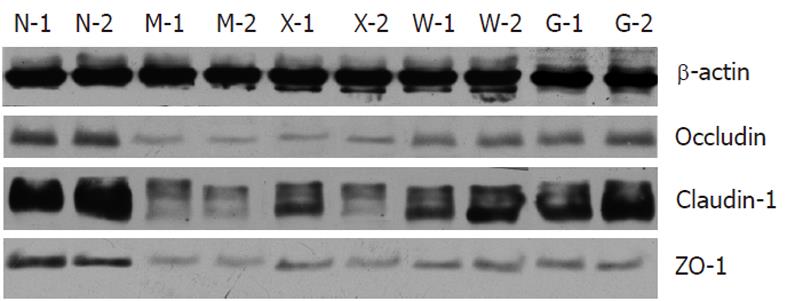
Compared with the NC group, the expressions of occludin, claudin-1 and ZO-1 were significantly reduced in the MC, SASP, MWM and HPM groups (Table 3). Compared with the MC group, the expressions of occludin, claudin-1 and ZO-1 in the MWM and HPM groups were significantly increased (0.48 ± 0.10, 0.64 ± 0.09 vs 0.18 ± 0.05, P = 0.002 and P = 0.001, respectively, for occluding; 0.12 ± 0.02, 0.17 ± 0.03 vs 0.05 ± 0.01, P = 0.001 for claudin-1; and 0.08 ± 0.01, 0.11 ± 0.01 vs 0.02 ± 0.01, P = 0.001 for ZO-1). And in SASP group, the expressions of occludin and ZO-1 were significantly increased (0.27 ± 0.04 vs 0.18 ± 0.05, P = 0.043 for occludin and 0.05 ± 0.01 vs 0.02 ± 0.01, P = 0.001 for ZO-1), but there was no significant difference for claudin-1. Moreover, the HPM and MWM groups had higher expression of occludin, claudin-1 and ZO-1 than the SASP group (0.48 ± 0.10, 0.64 ± 0.09 vs 0.27 ± 0.04, respectively, P = 0.015 and P = 0.001 for occluding; 0.12 ± 0.02, 0.17 ± 0.03 vs 0.08 ± 0.02, P = 0.021 and P = 0.001 for claudin-1; and 0.08 ± 0.01, 0.11 ± 0.01 vs 0.05 ± 0.01, P = 0.002 and P = 0.001 for ZO-1). The expression of ZO-1 between MWM and HPM groups was significantly different (0.08 ± 0.01 vs 0.11 ± 0.01, P = 0.001), but there was no significant difference for occludin and claudin-1 (Figure 4).
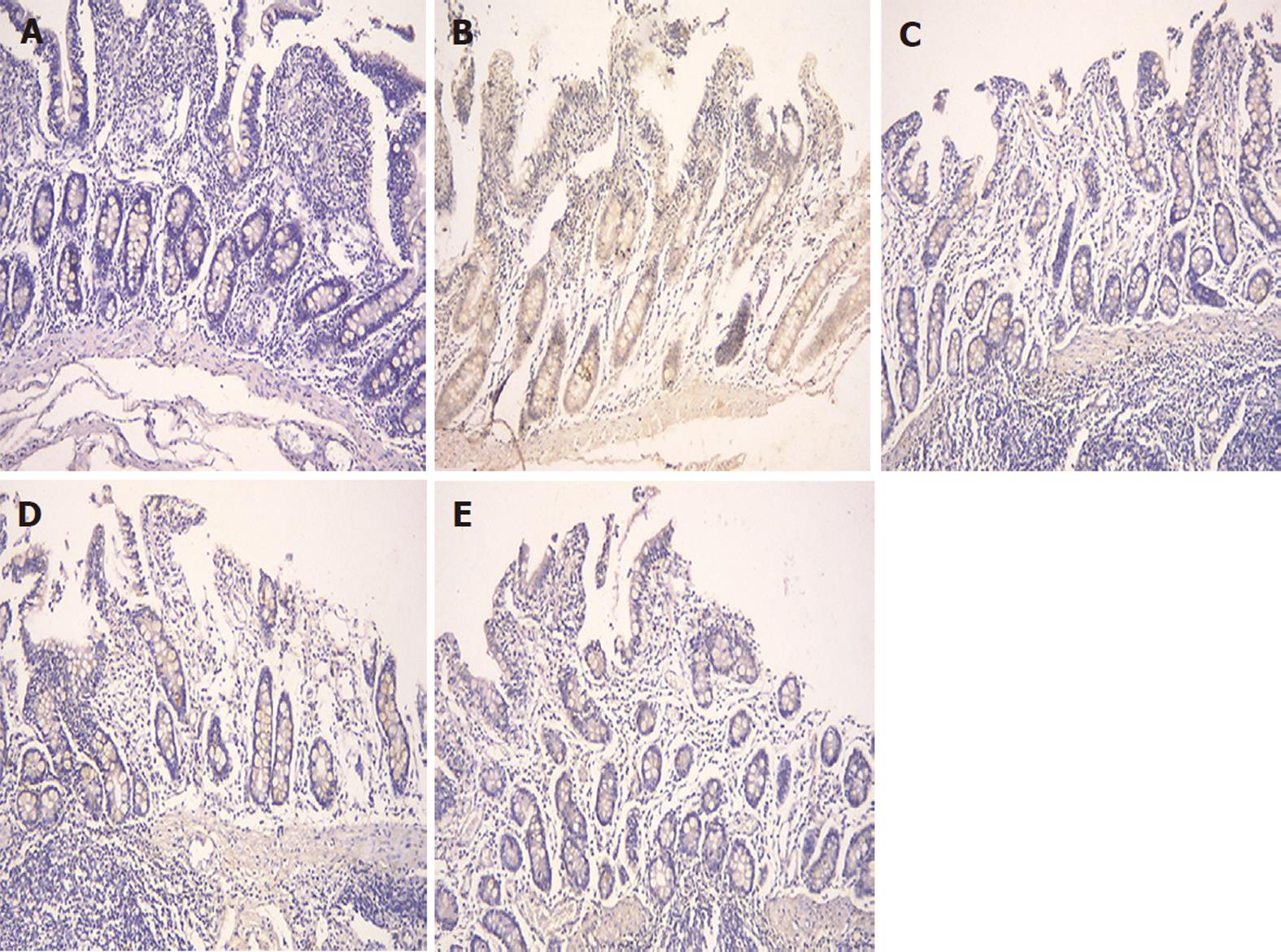
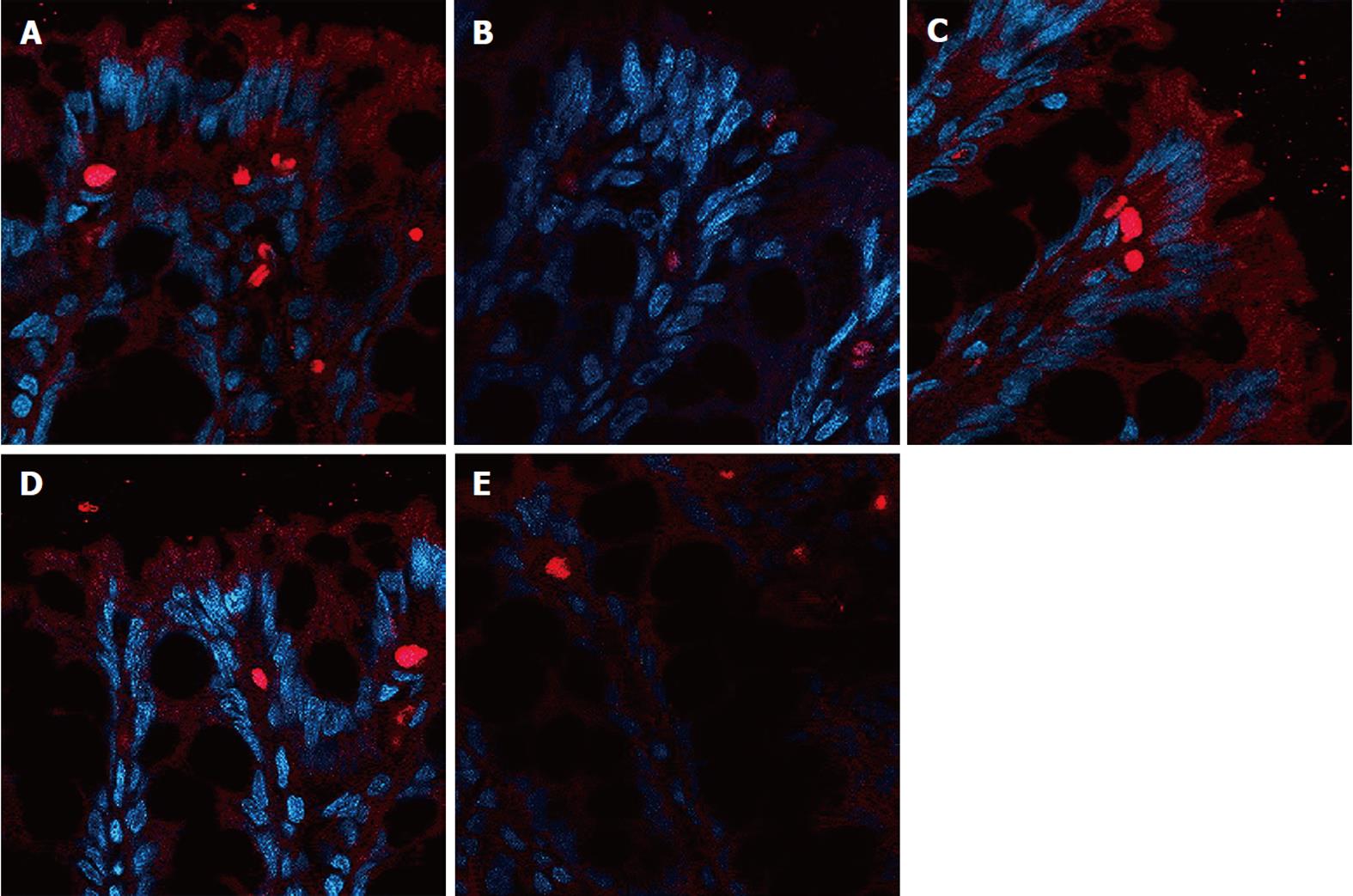
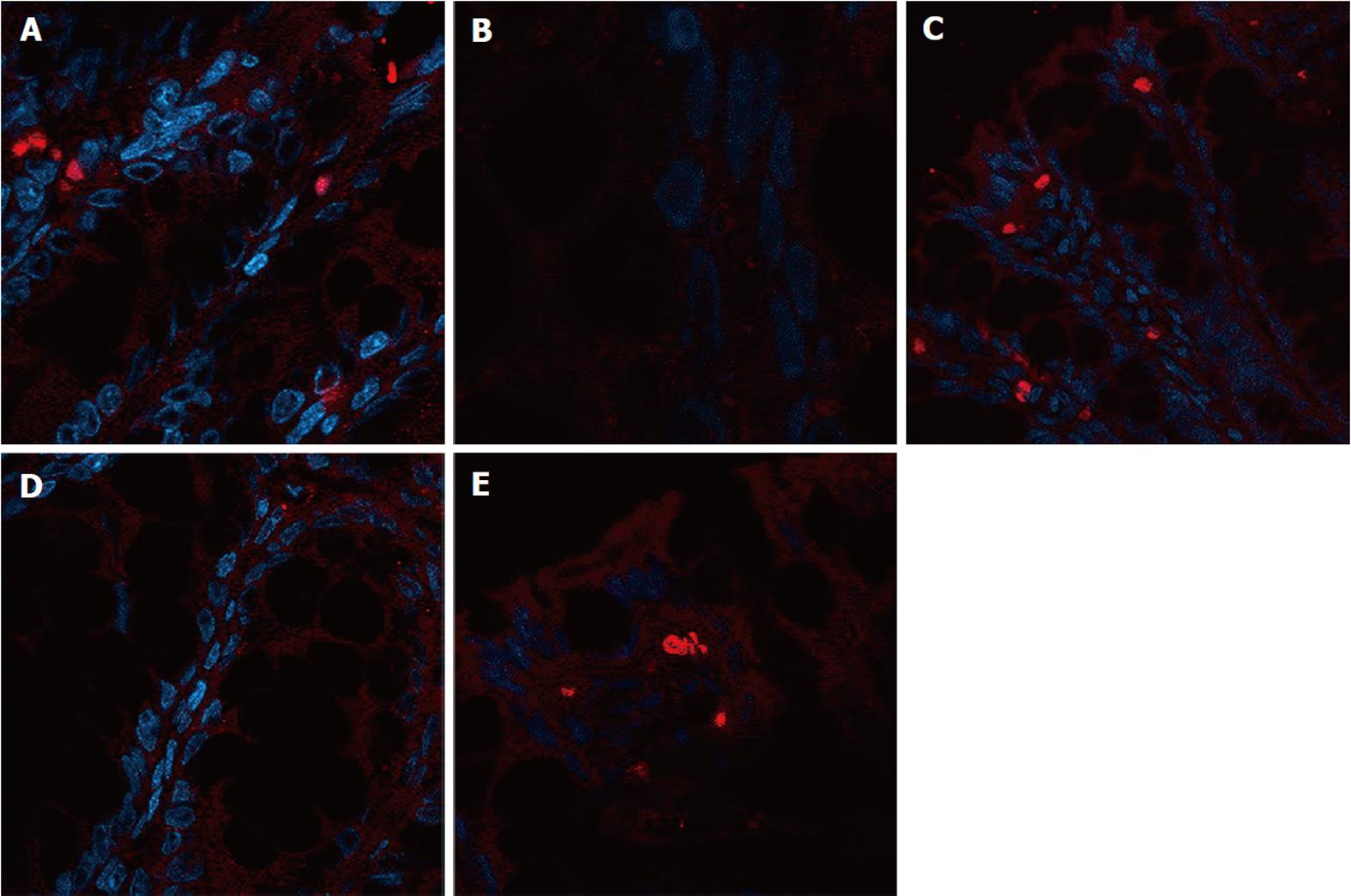
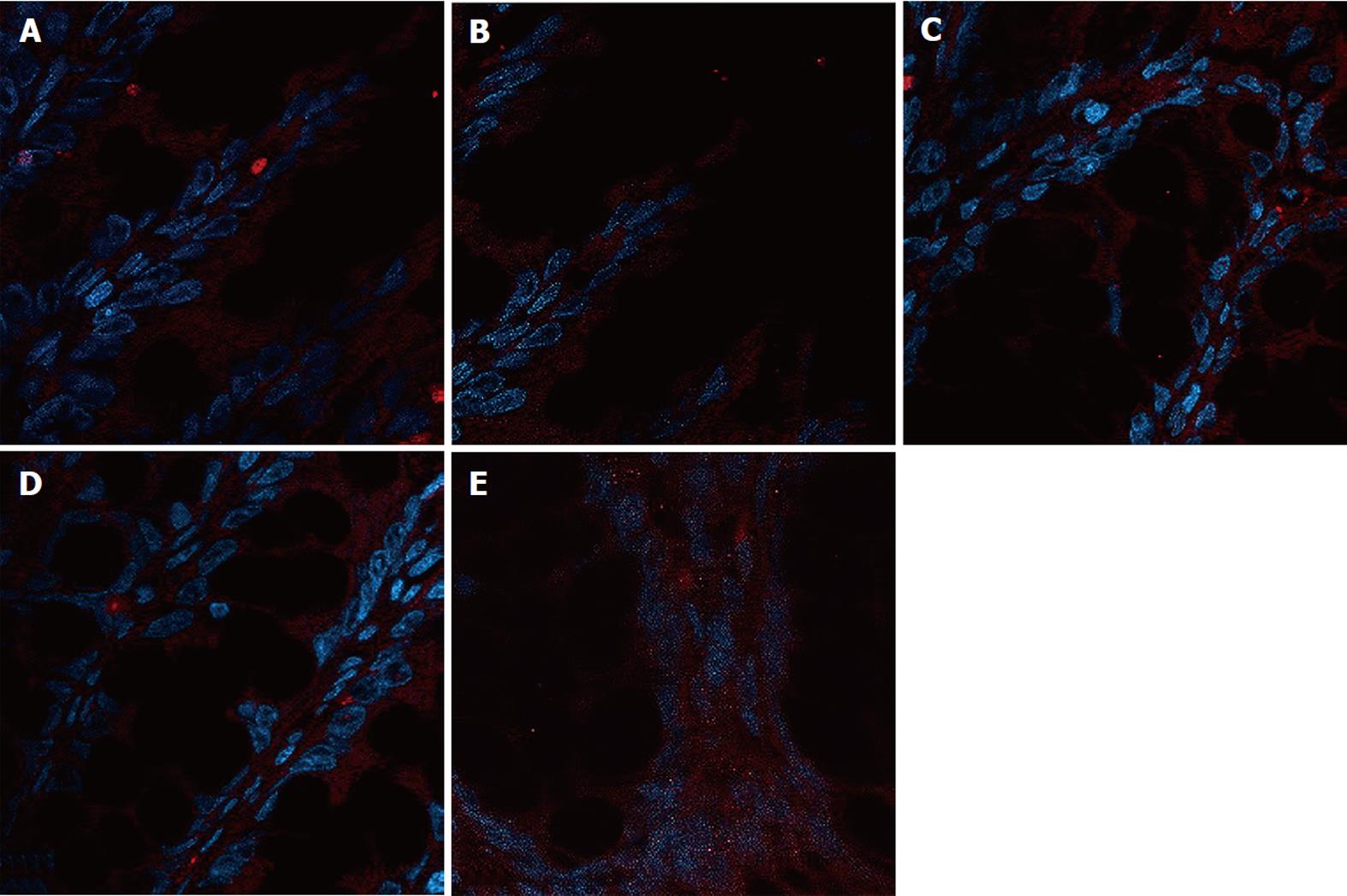
The expression of occludin (Figure 5), claudin-1 (Figure 6) and ZO-1 (Figure 7) was detected in the tight junctions and subjunctional lateral membranes of surface and crypt enterocytes under confocal immunofluorescence microscope, and the strength of the immunofluorescence signals was significantly reduced in these sites in the MC group. However, in the HPM, MWM or SASP groups, the signal significantly increased as HPM > MWM > SASP, which was consistent with the results obtained using Western blotting.
CD is a chronic, recurrent and refractory disease. It primarily causes abdominal pain, diarrhoea with abdominal mass, fistulisation and intestinal obstruction. Patients with CD usually have symptoms, including loss of appetite, sallow complexion, spontaneous perspiration, night sweat, insomnia and loss of energy and weight. The cause of this disease still remains unclear. Although the current pharmaceutical treatments are helpful in temporarily controlling the primary symptoms, they are unable to cure the disease.
Moxibustion has been used to treat various diseases in China and in other countries. Joos et al[3] found that Crohn’s Disease Activity Index can be decreased from 250 ± 51 to 163 ± 56 (P = 0.003) in patients treated with acupuncture and moxibustion. Furthermore, the researchers observed that the general conditions of the patients that received acupuncture and moxibustion were also improved compared with the controls. We have used HPM and MWM to treat patients with mild and moderate CD. We observed that HPM and MWM alleviates abdominal pain and diarrhoea and improves sallow complexion, loss of energy and insomnia in CD patients[6]. So far, no side effects have been observed. However, the mechanisms underlying moxibustion-induced CD alleviation are undefined.
In this study, we observed under transmission electron microscope that there were reduced apoptosis, clearer cell structures and greater numbers of mitochondria in the colonic epithelial cells of rats treated with HPM or MWM compared with the rats in the MC or SASP groups. In addition, compared with the MC group, we also found that apoptosis of rat colonic epithelial cells was significantly reduced in the HPM, MWM and SASP groups. Previously, we found that HPM and MWM could reduce the apoptosis of the colonic epithelial cells in rats with ulcerative colitis, which was mediated by the Bcl-2/Bax and Fas/FasL pathways[25].
The balance between epithelial cell apoptosis and proliferation is pivotal for the maintenance of mucosal integrity in the intestine[26]; Di Sabatino et al[27] found that dysregulation of this balance is caused by an increase in apoptosis, which leads to cellular loss and tissue atrophy and is likely to be involved in the pathogenesis of CD. This increase was not mediated by a Fas-Fas ligand or by abnormal E-cadherin distribution. Increased matrix metalloproteinase-1 release from lamina propria mononuclear cells may be one of the possible mechanisms responsible for the increased apoptosis of enterocytes in CD. Another study showed that an increase of tumor necrosis factor-alpha mucosal release may promote enterocyte apoptosis in CD[28]. Whether similar mechanisms are used by moxibustion to reduce apoptosis in colonic epithelia needs further investigation.
In addition, we investigated the effect of HPM or MWM on the tight junctions of colonic epithelial cells. We observed that the epithelial cell junction was tighter, the intracellular space was relatively broadened and the expression of occludin, claudin-1 and ZO-1 involved in the tight junctions was significantly enhanced in the HPM and MWM groups as compared with the MC and the SASP groups. In addition, we found that the electric current of the cells in the MC group was significantly higher than in the other groups, and the electric current of the epithelial cells in the HPM or MWM groups was lower than in the SASP group.
The tight junction seals the space between adjacent epithelial cells, and in intact gastrointestinal epithelia, tight junction permeability is the rate-limiting step that defines the overall epithelial permeability[14]. Increased permeability of the tight junctions is thought to contribute to CD[24,29]. Occludin, the claudins and ZO-1 are key proteins in the tight junctions[30]. Patients with active or inactive CD have increased intestinal permeability and differential expression of these tight junction proteins[31,32], which may be associated with abnormal expression of the proinflammatory cytokines TNF-α, IFN-γ and IL-13[33-36]; whether moxibustion increases the expression of the proteins involved in tight junctions through similar mechanisms or via other mechanisms requires further investigation.
Overall, we found that HPM or MWM administration of the Tianshu (ST25) and Qihai (CV6) acupoints can reduce colonic epithelial cell apoptosis and repair the intracellular tight junctions in rats with CD, which results in a significant improvement of their barrier function and a decrease in epithelial permeability. Our findings provide the novel mechanisms by which moxibustion can significantly improve CD symptoms. These findings suggest that the moxibustion therapy can be used as an effective treatment for Crohn’s disease.
We want to thank Dr. Xiao-Ping Zhang for his help and technical support in this study.
Crohn’s disease (CD) is a chronic, recurrent and refractory disease. The pathophysiology of CD has not been completely elucidated, making its treatment difficult. Previously, the authors used moxibustion in the clinic to treat mild and moderate CD, and the efficacy of moxibustion has reached nearly 75%. However, the regulatory effect of moxibustion on CD is still unknown.
Defective epithelial barrier function is considered to be a critical factor that promotes CD. In this study, the authors investigated the effect of moxibustion on the colonic epithelial barrier.
Moxibustion at ST25 and CV6 has been found to be effective against CD. Both HPM and MWM are able to prevent the apoptosis of colonic epithelial cells, repair tight junctions, and enhance the function and integrity of the colonic epithelial barrier in rats with CD.
The experimental data can be used in further studies on moxibustion therapy in treatment of CD.
This is an interesting study reporting how moxibustion can repair the colonic epithelial barrier in rats with Crohn’s disease.
| 1. | Zheng JJ, Zhu XS, Huangfu Z, Shi XH, Guo ZR. Prevalence and incidence rates of Crohn's disease in mainland China: a meta-analysis of 55 years of research. J Dig Dis. 2010;11:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 2. | Clark M, Colombel JF, Feagan BC, Fedorak RN, Hanauer SB, Kamm MA, Mayer L, Regueiro C, Rutgeerts P, Sandborn WJ. American gastroenterological association consensus development conference on the use of biologics in the treatment of inflammatory bowel disease, June 21-23, 2006. Gastroenterology. 2007;133:312-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 163] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 3. | Joos S, Brinkhaus B, Maluche C, Maupai N, Kohnen R, Kraehmer N, Hahn EG, Schuppan D. Acupuncture and moxibustion in the treatment of active Crohn's disease: a randomized controlled study. Digestion. 2004;69:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 4. | Zhou EH, Liu HR, Wu HG, Shi Z, Zhang W, Zhu Y, Shi DR, Zhou S. Down-regulation of protein and mRNA expression of IL-8 and ICAM-1 in colon tissue of ulcerative colitis patients by partition-herb moxibustion. Dig Dis Sci. 2009;54:2198-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 5. | Anastasi JK, McMahon DJ, Kim GH. Symptom management for irritable bowel syndrome: a pilot randomized controlled trial of acupuncture/moxibustion. Gastroenterol Nurs. 2009;32:243-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 6. | Shi Yin, Wu HG. The clinical study on herbs-partitioned moxibustion treatment of Crohn’s disease. JiangXi journal of TCM. 2003;38:16-17. |
| 7. | Hollander D. Crohn's disease--a permeability disorder of the tight junction? Gut. 1988;29:1621-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 184] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 8. | Arnott ID, Kingstone K, Ghosh S. Abnormal intestinal permeability predicts relapse in inactive Crohn disease. Scand J Gastroenterol. 2000;35:1163-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 145] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 9. | Wyatt J, Vogelsang H, Hübl W, Waldhöer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993;341:1437-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 462] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Katz KD, Hollander D, Vadheim CM, McElree C, Delahunty T, Dadufalza VD, Krugliak P, Rotter JI. Intestinal permeability in patients with Crohn's disease and their healthy relatives. Gastroenterology. 1989;97:927-931. [PubMed] |
| 11. | Peeters M, Geypens B, Claus D, Nevens H, Ghoos Y, Verbeke G, Baert F, Vermeire S, Vlietinck R, Rutgeerts P. Clustering of increased small intestinal permeability in families with Crohn's disease. Gastroenterology. 1997;113:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 175] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Thjodleifsson B, Sigthorsson G, Cariglia N, Reynisdottir I, Gudbjartsson DF, Kristjansson K, Meddings JB, Gudnason V, Wandall JH, Andersen LP. Subclinical intestinal inflammation: an inherited abnormality in Crohn's disease relatives? Gastroenterology. 2003;124:1728-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Breslin NP, Nash C, Hilsden RJ, Hershfield NB, Price LM, Meddings JB, Sutherland LR. Intestinal permeability is increased in a proportion of spouses of patients with Crohn's disease. Am J Gastroenterol. 2001;96:2934-2938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Barbara G. Mucosal barrier defects in irritable bowel syndrome. Who left the door open? Am J Gastroenterol. 2006;101:1295-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Olson TS, Reuter BK, Scott KG, Morris MA, Wang XM, Hancock LN, Burcin TL, Cohn SM, Ernst PB, Cominelli F. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203:541-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 16. | Ma TY. Intestinal epithelial barrier dysfunction in Crohn's disease. Proc Soc Exp Biol Med. 1997;214:318-327. [PubMed] |
| 17. | Söderholm JD, Streutker C, Yang PC, Paterson C, Singh PK, McKay DM, Sherman PM, Croitoru K, Perdue MH. Increased epithelial uptake of protein antigens in the ileum of Crohn's disease mediated by tumour necrosis factor alpha. Gut. 2004;53:1817-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 18. | Silva MA. Intestinal dendritic cells and epithelial barrier dysfunction in Crohn's disease. Inflamm Bowel Dis. 2009;15:436-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Weber CR, Turner JR. Inflammatory bowel disease: is it really just another break in the wall? Gut. 2007;56:6-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 20. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. [PubMed] |
| 21. | Xu SY, Bian RL, Chen X. Experimental methodology of pharmacology. Beijing: People’s Health Publishing House 1982; 1184. |
| 22. | Evans GS, Flint N, Somers AS, Eyden B, Potten CS. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J Cell Sci. 1992;101:219-231. [PubMed] |
| 23. | Kreusel KM, Fromm M, Schulzke JD, Hegel U. Cl- secretion in epithelial monolayers of mucus-forming human colon cells (HT-29/B6). Am J Physiol. 1991;261:C574-C582. [PubMed] |
| 24. | Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 972] [Article Influence: 51.2] [Reference Citation Analysis (1)] |
| 25. | Wu HG, Gong X, Yao LQ, Zhang W, Shi Y, Liu HR, Gong YJ, Zhou LB, Zhu Y. Mechanisms of acupuncture and moxibustion in regulation of epithelial cell apoptosis in rat ulcerative colitis. World J Gastroenterol. 2004;10:682-688. [PubMed] |
| 26. | Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569-3577. [PubMed] |
| 27. | Di Sabatino A, Ciccocioppo R, Luinetti O, Ricevuti L, Morera R, Cifone MG, Solcia E, Corazza GR. Increased enterocyte apoptosis in inflamed areas of Crohn's disease. Dis Colon Rectum. 2003;46:1498-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 28. | Bachmeyer C, Monge M, Danon O. Phalangeal metastases indicating relapse of non-Hodgkin's lymphoma. J Rheumatol. 2000;27:281-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (3)] |
| 29. | Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermüller N, Otto HF, Autschbach F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281:G216-G228. [PubMed] |
| 30. | Poritz LS, Garver KI, Tilberg AF, Koltun WA. Tumor necrosis factor alpha disrupts tight junction assembly. J Surg Res. 2004;116:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 31. | Oshitani N, Watanabe K, Nakamura S, Fujiwara Y, Higuchi K, Arakawa T. Dislocation of tight junction proteins without F-actin disruption in inactive Crohn's disease. Int J Mol Med. 2005;15:407-410. [PubMed] |
| 32. | Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159:2001-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 401] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 33. | Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999;112:137-146. [PubMed] |
| 34. | Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367-G376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 728] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 35. | Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85:1139-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 387] [Article Influence: 18.4] [Reference Citation Analysis (2)] |
| 36. | Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550-564. [PubMed] |
Peer reviewer: Dr. Marco Scarpa, PhD, Gastroenterology Section, Department of Surgical and Gastroenterological Sciences, University of Padova, via Giustiniani 2, Padova 35128, Italy
S- Editor Lv S L- Editor Ma JY E- Editor Li JY