Published online Nov 28, 2011. doi: 10.3748/wjg.v17.i44.4867
Revised: March 28, 2011
Accepted: April 5, 2011
Published online: November 28, 2011
AIM: To characterize the implications of vascular endothelial growth factor (VEGF)-A in stromal cells and colorectal cancer and the expression of VEGF-A splice variants.
METHODS: VEGF-A expression in tumor and stromal cells from 165 consecutive patients with colorectal cancer was examined by immunohistochemistry. The association between VEGF-A expression status and clinicopathological factors was investigated. Twenty fresh-frozen samples were obtained for laser capture microdissection to analyze the splice variants of VEGF-A.
RESULTS: VEGF-A was expressed in 53.9% and 42.4% of tumor and stromal cells, respectively. VEGF-A expression in tumor cells (t-VEGF-A) was associated with advanced clinical stage (stage 0, 1/9; stage 1, 2/16; stage 2, 32/55; stage 3, 38/66; stage 4, 16/19, P < 0.0001). VEGF-A expression in stromal cells (s-VEGF-A) increased in the earlier clinical stage (stage 0, 7/9; stage 1, 6/16; stage 2, 33/55; stage 3, 22/66; stage 4, 5/19; P = 0.004). Multivariate analyses for risk factors of recurrence showed that only s-VEGF-A expression was an independent risk factor for recurrence (relative risk 0.309, 95% confidence interval 0.141-0.676, P = 0.0033). The five-year disease-free survival (DFS) rates of t-VEGF-A-positive and -negative cases were 51.4% and 62.9%, respectively. There was no significant difference in t-VEGF-A expression status. The five-year DFS rates of s-VEGF-A-positive and -negative cases were 73.8% and 39.9%, respectively. s-VEGF-A-positive cases had significantly better survival than s-VEGF-A-negative cases (P = 0.0005). Splice variant analysis revealed that t-VEGF-A was mainly composed of VEGF165 and that s-VEGF-A included both VEGF165 and VEGF165b. In cases with no venous invasion (v0), the level of VEGF165b mRNA was significantly higher (v0 204.5 ± 122.7, v1 32.5 ± 36.7, v2 2.1 ± 1.7, P = 0.03). The microvessel density tended to be lower in cases with higher VEGF165b mRNA levels.
CONCLUSION: s-VEGF-A appears be a good prognostic factor for colorectal cancer and includes VEGF165 and VEGF165b.
- Citation: Tayama M, Furuhata T, Inafuku Y, Okita K, Nishidate T, Mizuguchi T, Kimura Y, Hirata K. Vascular endothelial growth factor 165b expression in stromal cells and colorectal cancer. World J Gastroenterol 2011; 17(44): 4867-4874
- URL: https://www.wjgnet.com/1007-9327/full/v17/i44/4867.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i44.4867
The growth and metastasis of cancer depend on angiogenesis, and vascular endothelial growth factor (VEGF)-A. VEGF-A is known to be one of the most important angiogenic factors. VEGF-A protein was discovered by Ferrara in 1989 as a specific growth factor and a blood vascular permeability factor for endothelial cells[1-2]. As a result of alternative splicing, 6 VEGF isoforms of 121, 145, 165, 183, 189 and 206 amino acids are produced from a single gene[3-7]. Most studies suggest that VEGF165 is the most abundant and biologically active isoform[3,8]. The biological effects of VEGF165 are mediated by tyrosine kinase receptors, i.e., VEGF receptor (VEGFR) 1 (Flt-1), VEGFR2 (KDR/Flk-1), and VEGFR3 (Flt-4)[9-11]. In colorectal cancer, VEGF-A is highly expressed in the case of hematogenous metastasis; therefore, VEGF-A is assumed to have value as a prognostic factor. VEGF-A and its receptor system are deeply involved in tumor angiogenesis. Thus, they are important molecular targets in the therapeutic strategy against colorectal cancer. It has been reported that the combined chemotherapy and an anti-VEGF antibody improves the response ratio of the tumor and extends the length of survival[12-15]. Tumor cells are the predominant source of VEGF; however, stromal cells surrounding the tumor have also been shown to produce VEGF[16]. Researches on the invasive and metastatic mechanisms mainly focused on the characteristics of the cancer cell itself, and there are few reports concerning the stromal cells[17-19]. Over the past decade, the role of stromal cells has gradually become a matter of interest to many researchers. The median survival in stromal VEGF-A-positive patients was 9.7 years vs 4.3 years in stromal VEGF-A-negative patients with stage II and III colorectal cancers[20]. However, the reason why VEGF-A expression in stromal cells resulted in a better prognosis has not been clarified.
VEGF165b was recently isolated from kidney epithelial cells as an angiogenesis inhibitor[21]. This variant is identical to VEGF165 except for the last six amino acids encoded by alternative splicing. VEGF165b also binds to both the VEGF receptor 1 (VEGF-R1) and the VEGF receptor 2 (VEGF-R2) with a similar affinity to that of VEGF165. VEGF165b was shown to bind to VEGF-R2, but not to stimulate phosphorylation, and to inhibit VEGF165-mediated phosphorylation in human umbilical vein endothelial cells[22-25].
We examined the association between VEGF-A expression status and clinicopathological characteristics in order to determine how VEGF-A in stromal cells affects tumor progression. We also analyzed the expression of VEGF-165 and VEGF165b using fresh-frozen specimens.
Tumor specimens were obtained from 165 consecutive patients with colorectal cancer who underwent resection at the First Department of Surgery, Sapporo Medical University from 1997 through 2001. Of these 165 patients, 146 at stages 0-III received curative resection. None of the patients received radiation or chemotherapy before surgery. The pathological stages, depth, histology, venous invasion, and lymphatic invasion of the primary tumor are shown in Table 1. Venous invasion and lymphatic invasion were both classified into four grades according to the Japanese Classification of Colorectal Carcinoma. v0 and ly0 represent no invasion, v1 and ly1, slight invasion, v2 and ly2, moderate invasion, and v3 and ly3, high invasion. immunohistochemical (IHC) analysis was performed in these 165 cases. We also obtained 20 fresh-frozen samples from patients with colorectal cancer in 2006-2007 to analyze the expression of VEGF165 and VEGF165b mRNAs.
| n | % | |
| Gender | ||
| Female | 75 | 45.5 |
| Male | 90 | 54.5 |
| Primary tumor location | ||
| Ascending colon | 29 | 17.6 |
| Transverse colon | 18 | 10.9 |
| Descending colon | 6 | 3.6 |
| Sigmoid colon | 30 | 18.2 |
| Rectum | 82 | 49.7 |
| TNM stage | ||
| 0 | 9 | 5.5 |
| I | 16 | 9.7 |
| II | 55 | 33.3 |
| III | 66 | 40.0 |
| IV | 19 | 11.5 |
| T factor | ||
| Tis | 9 | 5.5 |
| T1 | 7 | 4.2 |
| T2 | 25 | 15.2 |
| T3 | 111 | 67.3 |
| T4 | 13 | 7.9 |
| Histological differentiation | ||
| Well | 47 | 28.5 |
| Moderate | 95 | 57.6 |
| Poor | 8 | 4.8 |
| Mucinous | 10 | 6.1 |
| Other | 5 | 3.0 |
| Venous invasion | ||
| v0 | 46 | 27.9 |
| v1 | 73 | 44.2 |
| v2 | 32 | 19.4 |
| v3 | 14 | 8.5 |
| Lymphatic invasion | ||
| ly0 | 53 | 32.1 |
| ly1 | 79 | 47.9 |
| ly2 | 28 | 17.0 |
| ly3 | 5 | 3.0 |
| Recurrence except stage IV cases | ||
| No | 95 | 65.1 |
| Yes | 51 | 34.9 |
For IHC staining, paraffin-embedded tissues were cut at 4 μm. Slides were deparaffinized in xylene for 3 min three times, 3 min in absolute alcohol, 3 min in 90% ethanol, 3 min in 70% ethanol, and finally, 3 min in phosphate-buffered saline (PBS) for three times. After being deparaffinized, sections were incubated in 3% H2O2-methanol for 20 min to inactivate endogenous peroxidase. Deparaffinized and rehydrated sections were heated in DAKO Target Retrieval Solution (DAKO Japan, Tokyo, Japan) for 15 min in an autoclave at 105 °C. Nonspecific binding was blocked with 10% goat serum for 15 min at room temperature followed by incubation with the primary antibody in a moist chamber at 4 °C overnight. After rinsing in PBS for 3 min three times, the sections were incubated with a biotinylated secondary antibody, ENVISION + Mouse/HRP (Dako Japan, Tokyo, Japan), for 30 min. Sections were stained using aminoethylcarbazole (Dako Japan, Tokyo, Japan). Slides were mounted prior to observation under conventional light microscope.
The primary antibodies were mouse monoclonal antibodies against VEGF-A, anti-human VEGF (N5) (IBL, Takasaki, Japan), CD34, anti-human CD34 (QBEnd10) and mouse monoclonal antibody Dako N1632 (Dako, Japan, Tokyo, Japan).
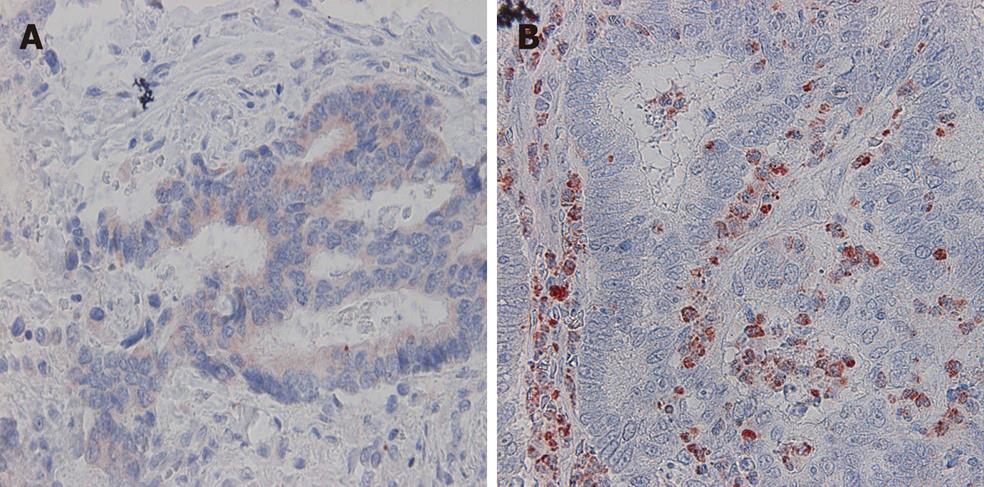
VEGF-A expression was examined under light microscope, and both the tumor and the stromal cells were separately classified into stained cells and unstained cells. Three sections of tumor cells and stromal cells were counted respectively at × 400 magnification for marginal cancer tissue to determine whether the cells were positive for VEGF-A, and the percentage of stained cells was averaged. Specimens were regarded as VEGF negative if less than 5% of the cells were stained and as VEGF positive if more than 5% were stained. These criteria were used in many previous reports[26-27]. Microvessel density (MVD) was assessed using light microscopy in invasive tumors containing the highest number of capillaries and small venules per unit area. Any single endothelial cell or cell cluster stained with CD34 was counted as a single vessel at × 400 magnification for marginal cancer tissues[28]. Three sections were counted in one case, and the number of vessels was averaged.
Laser capture microdissection (LCM) is a method for obtaining pure populations of cells from heterogeneous samples. Using this technique, colorectal tumor tissues were separated into tumor and stromal tissues. The frozen tissues were sectioned at a thickness of 8 μm using a cryostat and mounted on nonadhesive glass slides. Tissue sections were rehydrated using 70% ethanol for 3 min and rinsed twice in distilled water (Invitrogen Corp., Carlsbad, CA). They were then stained using hematoxylin for 30 s and rinsed in distilled water, followed by dehydration with 95% and 100% ethanol for 10 s in each case. Counterstaining was performed three times with eosin. Dehydration with xylene was conducted twice for 1 min each time, followed by air drying for 20 min. The PixCell LM200 system (Arcturus Engineering, Mountain View, CA) was used to microdissect the tumor cells and the stromal cells from the colorectal tissue sections. Ten sections were used to obtain sufficient RNA for reverse transcription polymerase chain reaction (RT-PCR), and each section needed at least 10 000 pulses. Processing of the total RNA began immediately following LCM. Extraction and isolation were performed using a QIAGEN RNeasy Mini Kit (QIAGEN, Valencia, CA).
We constructed the following primers to amplify fragments of human VEGF165 and VEGF165b specifically. The forward primer was located in exon 7a (TGTTTG TACAAGATCCGCAGACGTG). One reverse primer complementary to exon 8 (TCACCGCCTCGG CTTGTCACATCTGCAAGTACGTT) detected VEGF165 but not VEGF165b, and the other reverse primer complementary to exon 9 (GTTCTGTATCAGTCTTTCCTGGTGAGAGATCTGCA) detected VEGF165b but not VEGF165. Denaturing was conducted at 96 °C for 30 s, with annealing at 55 °C for 30 s and extension at 72 °C for 60 s in reactions cycled 30 times. PCR products were run on 3% agarose gels containing 0.5 μg/mL ethidium bromide and visualized under a UV transilluminator. This reaction consistently resulted in amplicons of 121 bp consistent with VEGF165b and 119 bp consistent with VEGF165. To confirm the amplification of VEGF165 and VEGF165b, we performed sequence analysis of these PCR products.
Real time PCR was performed on a LightCycler (Roche, Basel, Switzerland) for the semi-quantitation of VEGF165 and VEGF165b mRNA levels. The primer sequences were the same as those of the primers used for RT-PCR. The calculated amounts of VEGF165 and VEGF165b mRNAs were normalized to the endogenous reference control gene, human glyceraldehyde-3-phosphate dehydrogenase (h-GAPDH). All data were presented as the ratio of the target gene/GAPDH expression.
The χ2 test and Mann-Whitney U test were used to examine the association between the expression status of VEGF and clinicopathological characteristics. To analyze the risk factors for recurrence, logistic regression analysis was conducted. Survival curves were computed according to the Kaplan-Meier method. The log-rank test was used to compare the survival curves. P < 0.05 was considered statistically significant.
VEGF-A expression in tumor cells was positive in 53.9% (89/165) of the cases (Figure 1A). VEGF-A immunoreactivity was observed mainly in the cytoplasm of tumor cells. VEGF-A expression in stromal cells was observed in 42.4% (73/165) of the cases (Figure 1B).
A summary of the correlation between VEGF-A expression and clinicopathological characteristics is shown in Table 2. Tumor VEGF-A (t-VEGF-A) expression rates in tumors were 11.1% (1/9) in stage 0, 12.5% (2/16) in stage I, 58.2% (32/55) in stage II, 57.6% (38/66) in stage III, and 84.2% (16/19) in stage IV. t-VEGF-A expression was associated with the clinical stage (P < 0.0001). VEGF-A (s-VEGF-A) expression rates in stromal cells were 77.8% (7/9) in stage 0, 37.5% (6/16) in stage I, 60.0% (33/55) in stage II, 33.3% (22/66) in stage III, and 26.3% (5/19) in stage IV. The s-VEGF-A expression rate increased in the earlier clinical stage (P = 0.004). The t-VEGF-A expression rate increased with the depth of invasion (P = 0.0002). Conversely, the s-VEGF-A expression rate decreased with the depth of invasion (P = 0.01). There was no significant association between VEGF-A expression and the histological type. t-VEGF-A expression became significantly higher with the grade of venous and lymphatic invasion, while s-VEGF-A expression became significantly lower with the grade of venous and lymphatic invasion.
| n | Tumor VEGF positive cases | Stromal VEGF positive cases | |
| TNM stage | |||
| 0 | 9 | 1 (11.1) | 7 (77.8) |
| I | 16 | 2 (12.5) | 6 (37.5) |
| II | 55 | 32 (58.2) | 33 (60.0) |
| III | 66 | 38 (57.6) | 22 (33.3) |
| IV | 19 | 16 (84.2) | 5 (26.3) |
| Total | 165 | 89 (53.9) | 73 (44.2) |
| P < 0.0001 | P = 0.004 | ||
| T factor | |||
| Tis | 9 | 1 (11.1) | 7 (77.8) |
| T1 | 7 | 0 (0.0) | 6 (85.7) |
| T2 | 25 | 9 (36.0) | 11 (44.0) |
| T3 | 111 | 70 (63.1) | 45 (40.5) |
| T4 | 13 | 9 (69.2) | 4 (30.8) |
| Total | 165 | 89 (53.9) | 73 (44.2) |
| P = 0.0002 | P = 0.01 | ||
| Histological differentiation | |||
| Well | 47 | 15 (31.9) | 23 (48.9) |
| Moderate | 95 | 63 (66.3) | 41 (43.2) |
| Poor | 8 | 3 (37.5) | 4 (50.0) |
| Mucinous | 10 | 5 (50.0) | 2 (20.0) |
| Other | 5 | 1 (20.0) | 3 (60.0) |
| NS | NS | ||
| Venous invasion | |||
| v0 | 46 | 14 (30.4) | 27 (58.7) |
| v1 | 73 | 43 (58.9) | 29 (39.7) |
| v2 | 32 | 23 (71.9) | 15 (46.9) |
| v3 | 14 | 9 (64.3) | 2 (14.3) |
| P = 0.001 | P = 0.015 | ||
| Lymphatic invasion | |||
| ly0 | 53 | 16 (30.1) | 26 (49.1) |
| ly1 | 79 | 48 (60.8) | 39 (49.4) |
| ly2 | 28 | 20 (71.4) | 7 (25.0) |
| ly3 | 5 | 5 (100.0) | 1 (20.0) |
| P < 0.0001 | P = 0.04 |
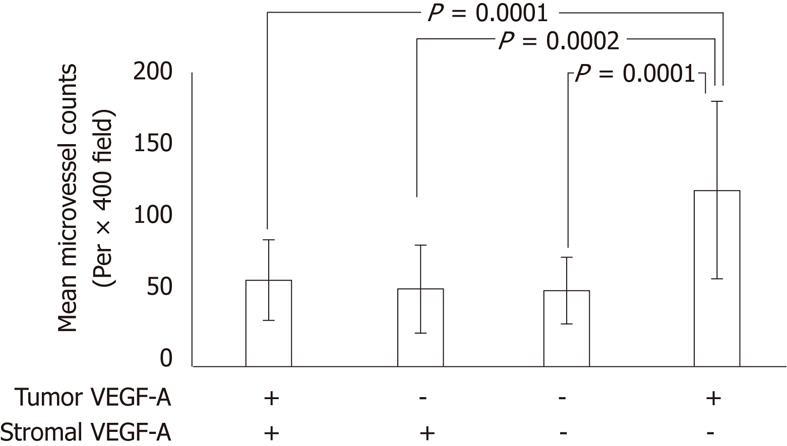
MVD was calculated by counting CD34-positive vascular endothelial cells. The association between VEGF-A expression status and MVD is shown in Figure 2. The MVDs of t-VEGF-A and s-VEGF-A expression (+, +), (-, +), (-, -), and (+, -) were 58.5, 52.4, 51.2 and 119.0, respectively. In s-VEGF-A-positive cases, the low MVD score was almost the same regardless of t-VEGF-A expression. t-VEGF-A-positive and s-VEGF-A-negative cases had significantly higher MVD scores.
Risk factors for recurrence in the 146 cases excluding stage IV cases were examined using logistic regression analysis. In univariate analysis, clinical stage, venous invasion, lymphatic invasion, t-VEGF-A positivity and s-VEGF-A negativity were risk factors for recurrence (Table 3). Multivariate analyses of these risk factors were performed, which showed that only s-VEGF-A expression was an independent risk factor for recurrence (P = 0.0033) (Table 3).
| Factor | n (Recurrence) | Univariate analysis | Multivariate analysis | ||||
| Relative risk | 95% CI | P value | Relative risk | 95% CI | P value | ||
| Clinical stage | |||||||
| 0 | 9(1) | 2.120 | 1.302-3.451 | 0.0250 | 1.718 | 0.980-3.010 | 0.0586 |
| I | 16(3) | ||||||
| II | 55(15) | ||||||
| III | 66(32) | ||||||
| Venous invasion | |||||||
| v0 | 46(12) | 1.500 | 1.050-2.143 | 0.0260 | 0.812 | 0.504-1.307 | 0.3907 |
| v1 | 63(27) | ||||||
| v2 | 27(6) | ||||||
| v3 | 10(6) | ||||||
| Lympathic invasion | |||||||
| ly0 | 52(13) | 2.094 | 1.354-3.238 | 0.0010 | 1.27 | 0.714-2.261 | 0.4155 |
| ly1 | 68(24) | ||||||
| ly2 | 23(12) | ||||||
| ly3 | 3(2) | ||||||
| s-VEGF-A positive | 68(14) | 0.269 | 0.135-0.535 | 0.0002 | 0.309 | 0.141-0.676 | 0.0033 |
| t-VEGF-A positive | 73(31) | 2.340 | 1.218-4.495 | 0.0110 | 1.918 | 0.768-3.718 | 0.1918 |
| Total | 146(51) | ||||||
Survival analysis was performed for stage II and III patients (n = 121). The five-year disease-free survival (DFS) rates of t-VEGF-A-positive (n = 70) and -negative cases (n = 51) were 51.4% and 62.9%, respectively. There was no significant difference in t-VEGF-A expression status (Figure 3A). The five-year DFS rates of s-VEGF-A-positive (n = 55) and -negative (n = 66) cases were 73.8% and 39.9%, respectively. s-VEGF-A-positive cases had significantly better survival than negative cases (P = 0.0005) (Figure 3B).
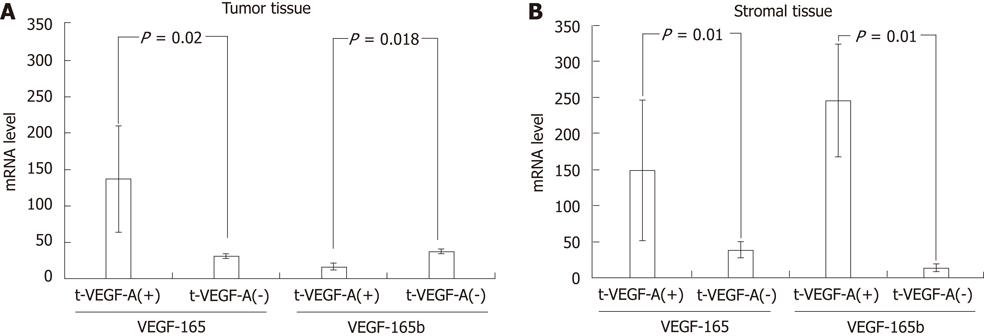
Expression analysis of VEGF165 and VEGF165b was performed using specimens of 20 cases obtained by LCM. RT-PCR was performed using specific primer sets (exon7/exon8 and exon7/exon9) to investigate the expression of VEGF165 and VEGF165b. Sequence analysis revealed that the PCR products were VEGA165 and VEGF165b (data not shown)[26]. IHC analysis was performed in the same 20 cases. Expression of s-VEGF-A and t-VEGF-A was positive in 40% (8/20) and 70% (14/20), respectively. mRNA levels of VEGF165 and VEGF165b were semi-quantified by real time PCR for each VEGF-A expression status determined by IHC. In tumor tissues, only VEGF165 was expressed in t-VEGF-A-positive cases (P = 0.02) (Figure 4A). In stromal tissues, both VEGF165 and VEGF165b were expressed in s-VEGF-A-positive cases (Figure 4B).
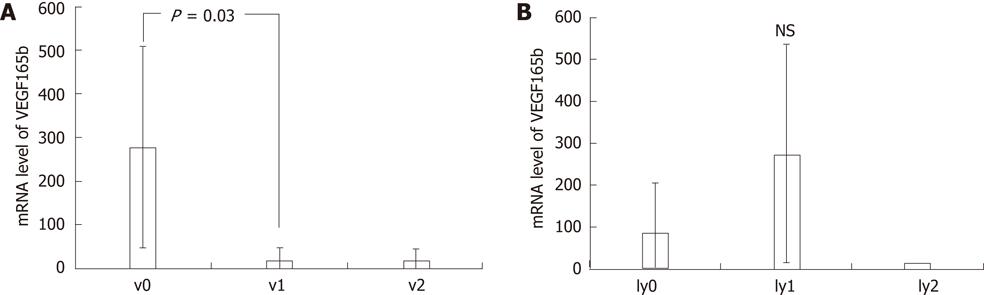
The VEGF165b mRNA level in v0 cases was significantly higher than in v1 cases (Figure 5A). There were no significant differences of VEGF165bmRNA levels among various degrees of lymphatic invasion (Figure 5B).
In cases with lower VEGF165b mRNA levels (numbers 1-8), MVD depended on the VEGF165 mRNA level, while in cases with higher VEGF165b mRNA levels (numbers 14-20), MVD did not reach a high score regardless of the VEGF165 mRNA level (Figure 6).
Neoangiogenesis plays an important role in the progression and metastasis of colorectal cancer, and VEGF-A, among many molecules, is known to be of paramount importance because VEGF-A secreted from tumor cells chiefly binds to VEGFR-2 and induces angiogenesis. In colorectal cancer, it is well known that VEGF-A is highly expressed in cases with hematogenous metastasis[29,30]. Therefore, it is assumed that VEGF-A is one of the biomarkers for prognosis[31]. VEGF-A expression in tumor cells was examined to evaluate the degree of risk in many studies. However, there have been few reports focusing on stromal cells surrounding tumor cells. Concerning VEGF-A expression in stromal cells, stromal VEGF-A positivity generally results in a better prognosis than VEGF-A negativity[20].
In this report, IHC staining was performed in 165 consecutive patients with colorectal cancer to detect VEGF-A expression in tumor and stromal cells. Our results showed that s-VEGF-A expression might be a factor indicating a better prognosis. These results were consistent with a previous report[20] and implied that the functions of VEGF-A expressed in stromal cells might be different from those in tumor cells. Since VEGF has 6 splicing isoforms[2-6], we focused on one of them, VEGF165b, which was reported to inhibit neoangiogenesis. Our report demonstrated that s-VEGF-A, including VEGF165 and VEGF165b expressed in stromal cells, might inhibit angiogenesis and reduce MVD. However, we could not conclude that VEGF165b expression improved the prognosis of colorectal cancer patients because the association between VEGF165b expression and the prognosis has not been investigated in a large series.
In this study, we clarified that s-VEGF-A, including VEGF165b, had a function to inhibit neoangiogenesis. However, it remains unexplained what kinds of cells secrete VEGF165b and what factors induce VEGF165b expression. A previous report showed that a subset of macrophages expressed VEGF-A resulting from CD68 (a macrophage-specific immunostain) macroIHC staining[32]. In our series, 76% of CD68-positive cases were s-VEGF-A positive and most of the s-VEGF-A(+) cells were identical to CD68(+) cells under light microscope (data not shown). CD68(+) stromal cells, and tumor-associated macrophages (TAMs) have been reported to have dual potential to improve and worsen the prognosis[33]. We speculate that CD68(+) stromal cells may secrete VEGF165b and inhibit the angiogenesis induced by VEGF165 from tumor cells to interfere with tumor progression. In the future, we will study TAMs in colorectal cancer, especially those expressing VEGF165b, which may be a key to developing a novel therapeutic strategy.
In summary, the s-VEGF-A appears be a good prognostic factor for colorectal cancer and includes VEGF165 and VEGF165b.
Neoangiogenesis plays an important role in the progression and metastasis of colorectal cancer and vascular endothelial growth factor (VEGF)-A, among many molecules, is known to be highly important because VEGF-A secreted from tumor cells chiefly binds to VEGFR-2 and induces angiogenesis. In colorectal cancer, it is well known that VEGF-A is highly expressed in cases with hematogenous metastasis. Therefore, VEGF-A is assumed to have value as a prognostic factor. VEGF-A and its receptor system are deeply involved in tumor angiogenesis. Thus, they are important molecular targets in the therapeutic strategy against colorectal cancer.
It has been reported that combined chemotherapy and an anti-VEGF-A antibody improves the response ratio of the tumor and extends the length of survival. Tumor cells are the predominant source of VEGF-A; however, stromal cells surrounding the tumor have also been shown to produce VEGF-A. In many reports, VEGF-A expression in tumor cells was examined to evaluate the degree of risk. However, there have been few reports focusing on stromal cells surrounding tumor cells.
In this report, immunohistochemical staining was performed in 165 consecutive patients with colorectal cancer to detect VEGF-A expression in tumor and stromal cells. The results showed that s-VEGF-A expression might be a factor indicating a better prognosis. These results implied that the functions of VEGF-A expressed in stromal cells might be different from those in tumor cells. This report demonstrated that s-VEGF-A, including VEGF165 and VEGF165b, expressed in stromal cells, might inhibit angiogenesis and reduce microvessel density.
The authors clarified that s-VEGF-A, including VEGF165b, had a function to inhibit neoangiogenesis. However, it remains unexplained what kinds of cells secrete VEGF165b and what factors induce VEGF165b expression. Studies of TAMs in colorectal cancer, especially those expressing VEGF165b, may be a key to developing a novel therapeutic strategy.
This is an excellent manuscript, with a well done methodological approach, and showing a correlation with stromal VGEF expression and colorectal cancer prognosis.
| 1. | Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1485] [Cited by in RCA: 1494] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 2. | Tischer E, Gospodarowicz D, Mitchell R, Silva M, Schilling J, Lau K, Crisp T, Fiddes JC, Abraham JA. Vascular endothelial growth factor: a new member of the platelet-derived growth factor gene family. Biochem Biophys Res Commun. 1989;165:1198-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 209] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5:1806-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 897] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 4. | Poltorak Z, Cohen T, Sivan R, Kandelis Y, Spira G, Vlodavsky I, Keshet E, Neufeld G. VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J Biol Chem. 1997;272:7151-7158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 337] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 5. | Lei J, Jiang A, Pei D. Identification and characterization of a new splicing variant of vascular endothelial growth factor: VEGF183. Biochim Biophys Acta. 1998;1443:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Petrova TV, Makinen T, Alitalo K. Signaling via vascular endothelial growth factor receptors. Exp Cell Res. 1999;253:117-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 195] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Kaipainen A, Korhonen J, Pajusola K, Aprelikova O, Persico MG, Terman BI, Alitalo K. The related FLT4, FLT1, and KDR receptor tyrosine kinases show distinct expression patterns in human fetal endothelial cells. J Exp Med. 1993;178:2077-2088. [PubMed] |
| 8. | Ferrara N. Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Mol Biol Cell. 2010;21:687-690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 9. | Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marmé D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336-3343. [PubMed] |
| 10. | Meyer M, Clauss M, Lepple-Wienhues A, Waltenberger J, Augustin HG, Ziche M, Lanz C, Büttner M, Rziha HJ, Dehio C. A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. EMBO J. 1999;18:363-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 336] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 11. | Yin Y, Cao LY, Wu WQ, Li H, Jiang Y, Zhang HF. Blocking effects of siRNA on VEGF expression in human colorectal cancer cells. World J Gastroenterol. 2010;16:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1209] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 13. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [PubMed] |
| 14. | Emmanouilides C, Sfakiotaki G, Androulakis N, Kalbakis K, Christophylakis C, Kalykaki A, Vamvakas L, Kotsakis A, Agelaki S, Diamandidou E. Front-line bevacizumab in combination with oxaliplatin, leucovorin and 5-fluorouracil (FOLFOX) in patients with metastatic colorectal cancer: a multicenter phase II study. BMC Cancer. 2007;7:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Prat A, Casado E, Cortés J. New approaches in angiogenic targeting for colorectal cancer. World J Gastroenterol. 2007;13:5857-5866. [PubMed] |
| 16. | Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 697] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 17. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11513] [Article Influence: 479.7] [Reference Citation Analysis (2)] |
| 18. | Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625-4629. [PubMed] |
| 19. | Tan SY, Fan Y, Luo HS, Shen ZX, Guo Y, Zhao LJ. Prognostic significance of cell infiltrations of immunosurveillance in colorectal cancer. World J Gastroenterol. 2005;11:1210-1214. [PubMed] |
| 20. | Khorana AA, Ryan CK, Cox C, Eberly S, Sahasrabudhe DM. Vascular endothelial growth factor, CD68, and epidermal growth factor receptor expression and survival in patients with Stage II and Stage III colon carcinoma: a role for the host response in prognosis. Cancer. 2003;97:960-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123-4131. [PubMed] |
| 22. | Ladomery MR, Harper SJ, Bates DO. Alternative splicing in angiogenesis: the vascular endothelial growth factor paradigm. Cancer Lett. 2007;249:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Nowak DG, Amin EM, Rennel ES, Hoareau-Aveilla C, Gammons M, Damodoran G, Hagiwara M, Harper SJ, Woolard J, Ladomery MR. Regulation of vascular endothelial growth factor (VEGF) splicing from pro-angiogenic to anti-angiogenic isoforms: a novel therapeutic strategy for angiogenesis. J Biol Chem. 2010;285:5532-5540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 180] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 24. | Rennel ES, Hamdollah-Zadeh MA, Wheatley ER, Magnussen A, Schüler Y, Kelly SP, Finucane C, Ellison D, Cebe-Suarez S, Ballmer-Hofer K. Recombinant human VEGF165b protein is an effective anti-cancer agent in mice. Eur J Cancer. 2008;44:1883-1894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Varey AH, Rennel ES, Qiu Y, Bevan HS, Perrin RM, Raffy S, Dixon AR, Paraskeva C, Zaccheo O, Hassan AB. VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer. 2008;98:1366-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 26. | Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, Cui TG, Sugiono M, Waine E, Perrin R. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822-7835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 342] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 27. | Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003;28:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 446] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 28. | Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417-2426. [PubMed] |
| 29. | Kuramochi H, Hayashi K, Uchida K, Miyakura S, Shimizu D, Vallböhmer D, Park S, Danenberg KD, Takasaki K, Danenberg PV. Vascular endothelial growth factor messenger RNA expression level is preserved in liver metastases compared with corresponding primary colorectal cancer. Clin Cancer Res. 2006;12:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964-3968. [PubMed] |
| 31. | Lee JC, Chow NH, Wang ST, Huang SM. Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer. 2000;36:748-753. [PubMed] |
| 32. | Micklem K, Rigney E, Cordell J, Simmons D, Stross P, Turley H, Seed B, Mason D. A human macrophage-associated antigen (CD68) detected by six different monoclonal antibodies. Br J Haematol. 1989;73:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 747] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
Peer reviewer: Josep M Pique, MD, Department of Gastroenterology, Hospital Clínic of Barcelona, Barcelona 08036, Spain
S- Editor Wu X L- Editor Ma JY E- Editor Xiong L