Published online Nov 14, 2011. doi: 10.3748/wjg.v17.i42.4704
Revised: July 28, 2011
Accepted: August 4, 2011
Published online: November 14, 2011
AIM: To describe the socio-demographic features, etiology, and risk factors for Budd-Chiari syndrome (BCS) in Egyptian patients.
METHODS: Ninety-four Egyptian patients with confirmed primary Budd-Chiari syndrome were presented to the Budd-Chiari Study Group (BCSG) and admitted to the Tropical Medicine Department of Ain Shams University Hospital (Cairo, Egypt). Complete clinical evaluation and laboratory investigations, including a thrombophilia workup and full radiological assessment, were performed to determine underlying disease etiologies.
RESULTS: BCS was chronic in 79.8% of patients, acute or subacute in 19.1%, and fulminant in 1.1%. Factor V Leiden mutation (FVLM) was the most common etiological cause of disease (53.1%), followed by mutation of the gene encoding methylene tetrahydrofolate reductase (MTHFR) (51.6%). Current or recent hormonal treatment was documented in 15.5% of females, and BCS associated with pregnancy was present in 17.2% of females. Etiology could not be determined in 8.5% of patients. Males had significantly higher rates of MTHFR gene mutation and Behçet’s disease, and females had significantly higher rates of secondary antiphospholipid antibody syndrome. A highly significant positive relationship was evident between the presence of Behçet’s disease and inferior vena caval occlusion, either alone or combined with occlusion of the hepatic veins (P < 0.0001).
CONCLUSION: FVLM is the most common disease etiology and MTHFR the second most common in Egyptian BCS patients. BCS etiology tends to vary with geographic region.
- Citation: Sakr M, Barakat E, Abdelhakam S, Dabbous H, Yousuf S, Shaker M, Eldorry A. Epidemiological aspects of Budd-Chiari in Egyptian patients: A single-center study. World J Gastroenterol 2011; 17(42): 4704-4710
- URL: https://www.wjgnet.com/1007-9327/full/v17/i42/4704.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i42.4704
Budd-Chiari syndrome (BCS) is a rare, but potentially life-threatening, hepatic disorder caused by obstruction of hepatic venous outflow at any level from the hepatic venules to the right atrium[1]. The exact prevalence of BCS is unknown, but has been estimated as 1 per 100 000 of the general population worldwide[2], with a higher prevalence being evident in developing countries such as China, India, Nepal, and South Africa[3]. BCS affects all races, usually during the third or fourth decade of life, and is more common in females[4]. The etiology of BCS can be classified as either primary, attributable to intrinsic intraluminal thrombosis or development of venous webs; or secondary, caused by intraluminal invasion by a parasite or a malignant tumor, or extraluminal compression by an abscess, cyst, or solid tumor[5].
At least one hereditary or acquired procoagulative disorder is present in 74% of BCS patients; intravascular thrombosis, mostly encountered in patients with primary myeloproliferative disorders (MPD), is the most common etiological factor[4]. Polycythemia vera is present in 10%-40% of BCS patients, whereas essential thrombocythemia and myelofibrosis are less common[6]. Hepatic vein thrombosis occurs in up to 12% of patients with paroxysmal nocturnal hemoglobinuria (PNH); this is the leading cause of mortality from BCS[7]. As many as 30% of BCS patients carry a Factor V Leiden mutation (FVLM); such a mutation is present in the majority of pregnancy- or oral contraceptive-related instances of hepatic vein thrombosis[8]. Patients with BCS may show nonspecifically decreased levels of Protein C, Protein S, and antithrombin, attributable to impaired hepatic synthesis, but levels less than 20% of normal suggest the presence of an inherited deficiency[9].
BCS is less common in western countries, but primary membranous obstruction of the inferior vena cava (IVC) is the most common cause of BCS in South Africa and Asia[10]. No underlying etiology can be identified in about 5% of BCS patients. Recent research has suggested that endothelial dysfunction and decreased fibrinolytic activity contribute to idiopathic instances of BCS[1].
The aim of the present epidemiological study was to describe the socio-demographic features, etiology, and risk factors for BCS in Egyptian patients.
The present descriptive study enrolled 94 consecutive Egyptian patients between April 2009 and February 2011. Each patient was confirmed to have primary BCS, were introduced to the Budd-Chiari Study Group (BCSG), and then admitted to the Tropical Medicine Department of Ain Shams University Hospital (Cairo, Egypt). All patients provided written informed consent to participate in the study. Complete histories and clinical examinations were recorded for all patients.
Laboratory investigations included a complete blood count, a liver profile, and a coagulation profile. A thrombophilia workup, performed to determine the underlying etiology of BCS, included measurement of anti-cardiolipin antibodies, lupus anticoagulant, antinuclear antibodies, protein C, protein S, and antithrombin III; and flow cytometry quantitating CD55 and CD59 levels to diagnose PNH. The possible presence of a FVLM was assessed in 64 patients, whereas the statuses of pro-thrombin and methylene tetrahydrofolate reductase (MTHFR) gene mutations were evaluated in 60 patients. JAK2 mutational status was assessed, and/or a bone marrow biopsy exploring the possible presence of a myeloproliferative disorder was performed, in 62 patients.
Radiological assessment using abdominal Duplex ultrasonography (US) was performed to assess the patency of all of the hepatic veins (HVs), the portal vein, and the IVC. Abdominal magnetic resonance (MR) imaging, MR venography, or multislice computed tomography, was performed to confirm all diagnoses and to assess vascular anatomy.
Analysis of variance was used to compare the mean values of laboratory parameters. Multiple comparisons were performed using the least significant difference post-hoc test and results are presented as means and standard deviations (SDs). Non-parametric data were analyzed using the Kruskal-Wallis test and are presented as medians with interquartile ranges (IQRs). The chi-squared test and Fisher’s exact test were used to test for differences among variables; the results are presented as percentages with corresponding P values. The unpaired Student’s t test was used to test for differences in mean values of laboratory parameters between males and females, and the results are presented as means with SDs. Non-parametric data were analyzed using the Mann-Whitney U test and data are presented as medians with IQRs. Spearman’s correlation coefficient was used to test the strength of associations between variables. All data were analyzed using SPSS version 15. A P value less than 0.05 was considered significant (S); a P value less than 0.01 was highly significant (HS); and a P value less than 0.001 was very highly significant (VHS).
We enrolled 94 Egyptian patients with BCS. There were 58 females (61.7%, mean age: 28.88 ± 9.08 years) and 36 males (38.3%, mean age: 28.64 ± 8.35 years). A total of 34 patients (36.2%) were from Cairo, 39 (41.5%) from the Delta, and 21 (22.3%) from Upper Egypt. A total of 75 patients (79.8%) had chronic BCS, 18 (19.1%) acute or subacute BCS, and 1 (1.1%) fulminant BCS. By the Child-Pugh classification, 30 patients (32%) were class A, 33 (35%) class B, and 31 (33%) class C.
Table 1 summarizes the clinical manifestations of our 94 patients. The most common symptoms were abdominal enlargement (89.4%) and abdominal pain (83%), and the most common clinical signs were ascites (85.1%), hepatomegaly (83%), and splenomegaly (51.1%).
| Symptom | n (%) |
| Abdominal enlargement | 84 (89.4) |
| Abdominal pain | 78 (83) |
| History of previous thrombosis | 26 (27.7) |
| Recurrent abortion (females; 58) | 14 (24.1) |
| Gastrointestinal bleeding | 15 (15.9) |
| Recurrent oral and/or genital ulcers | 13 (13.8) |
| Signs | |
| Ascites | 80 (85.1) |
| Hepatomegaly | 78 (83) |
| Splenomegaly | 48 (51.1) |
| Lower limb edema | 46 (48.9) |
| Dilated abdominal veins | 39 (41.5) |
| Jaundice | 36 (38.3) |
| Abdominal tenderness | 34 (36.2) |
| Encephalopathy | 29 (30.9) |
Table 2 summarizes the disease etiologies of our 94 patients. The most common etiologies were FVLM mutation (53.1%) and MTHFR mutation (51.6%). A total of 15.5% of female patients were currently, or had recently, received hormonal treatment (oral or injectable) whereas 17.2% had BCS associated with pregnancy. The etiology of BCS was undefined in eight patients (8.5%). Forty-six patients (48.9%) demonstrated a single etiological factor, 29 (30.9%) two such factors, 8 (8.5%) three, and 3 (3.2%) four. There was no statistically significant relationship between disease pattern (acute, subacute, fulminant, or chronic) and etiology.
| Etiology | n (%) | |
| FVLM (64 tested) | Homozygous | 10 (15.6) |
| Heterozygous | 24 (37.5) | |
| MTHFR (60 tested) | Homozygous | 8 (13.3) |
| Heterozygous | 23 (38.3) | |
| PGM (60 tested) | Homozygous | 1 (1.7) |
| Heterozygous | 2 (3.3) | |
| JAK2 (MPD) (62 tested) | + | 18 (29) |
| Primary APA | + | 16 (17) |
| Secondary APA | + | 11 (11.7) |
| Behçet’s disease | + | 12 (12.8) |
| Protein C deficiency | + | 4 (4.3) |
| Antithrombin III deficiency | + | 4 (4.3) |
| Protein S deficiency | + | 1 (1.1) |
| PNH | + | 2 (2.1) |
| Hormonal therapy (58 females) | + | 9 (15.5) |
| Pregnancy-related (58 females) | + | 10 (17.2) |
Table 3 shows the relationship between gender and BCS etiology. Males had significantly higher rates of MTHFR gene mutation and Behçet’s disease, whereas females had a significantly higher rate of secondary anti-phospholipid syndrome (APA).
| Etiology | Gender n (%) | χ² | P value | Sig | ||
| Male | Female | |||||
| PC deficiency | + | 1 (2.90) | 3 (5.20) | 1 | NS | |
| PS deficiency | + | 1 (2.90) | 0 (0.00) | 0.38 | NS | |
| AT III deficiency | + | 2 (5.70) | 2 (3.40) | 0.63 | NS | |
| FVLM | Homozygous | 5 (20.80) | 5 (12.50) | 1.77 | 0.18 | NS |
| Heterozygous | 6 (25.00) | 18 (45.00) | ||||
| PGM | Homozygous | 0 (0.00) | 1 (2.60) | 1.54 | 0.21 | NS |
| Heterozygous | 0 (0.00) | 2 (5.10) | ||||
| MTHFR | Homozygous | 6 (28.60) | 2 (5.10) | 8.41 | 0.01 | HS |
| Heterozygous | 9 (42.90) | 14 (35.90) | ||||
| JAK2 (MPD) | + | 6 (28.60) | 12 (29.30) | 0.003 | 0.95 | NS |
| Primary APA | + | 5 (13.90) | 11 (19.00) | 0.4 | 0.52 | NS |
| Secondary APA | + | 1 (2.80) | 10 (17.20) | 0.05 S | ||
| Behçet’s disease | + | 11 (30.60) | 1 (1.70) | < 0.001 VHS | ||
| PNH | + | 1 (2.80) | 1 (1.70) | 1 NS | ||
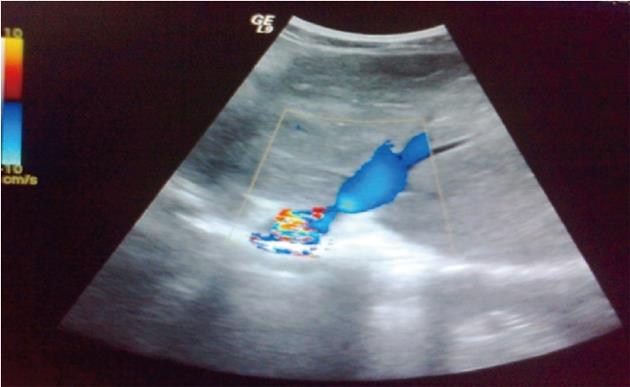
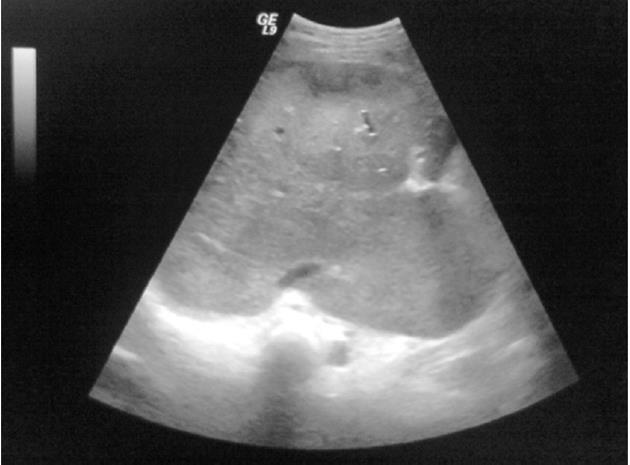
Table 4 summarizes the Duplex US findings. A total of 74.5% of all patients had HV occlusion, 3.2% IVC occlusion, and 17% both HV and IVC occlusion. PV thrombosis was present in 5.3% of patients. Figure 1 shows a representative color Doppler US of a patient with a dilated congested left HV and significant stenosis at the junction thereof with the IVC. Figure 2 is a representative B-mode sonograph showing occlusion of all hepatic veins, a slit-like IVC, and a markedly enlarged caudate lobe.
| Ultrasound finding | Patients n (%) | |
| Hepatomegaly | 78 (83) | |
| Splenomegaly | 48 (51.1) | |
| No. of instances of occluded HV | 0 | 3 (3.2) |
| 1 | 2 (2.1) | |
| 2 | 12 (12.8) | |
| 3 | 77 (81.9) | |
| RHV occlusion | 85 (90.4) | |
| MHV occlusion | 91 (96.8) | |
| LHV occlusion | 87 (92.6) | |
| IVC occlusion | 22 (23.4) | |
| PV occlusion | 5 (5.3) | |
| Anatomical localization of thrombosis at presentation | Isolated HV | 70 (74.5) |
| Combined HV and IVC | 16 (17) | |
| Isolated IVC | 3 (3.2) | |
| Associated PV thrombosis | 5 (5.3) | |
Table 5 summarizes the relationship between BCS etiology and radiological data. A highly significant positive relationship was evident between the presence of Behçet’s disease and IVC occlusion, either isolated or combined with occlusion of the hepatic veins (P < 0.0001). No other significant relationship was evident between any etiological factor and radiological data.
| Etiology | Anatomical localization of thrombosis at presentation n (%) | ||||||||
| HV only | HV and PV | HV, PV and IVC | HV and IVC | IVC | χ2 | P value | Sig | ||
| PC deficiency | + | 3 (4.3) | 0 (0.0) | 0 (0.0) | 1 (6.3) | 0 (0.0) | 0.001 | 0.99 | NS |
| PS deficiency | + | 1 (1.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.32 | 0.58 | NS |
| AT III Deficiency | + | 3 (4.3) | 0 (0.0) | 0 (0.0) | 1 (6.3) | 0 (0.0) | 0.001 | 0.99 | NS |
| FVLM | Homo | 9 (17.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 9 (17.0) | 0.03 | 0.85 | NS |
| Hetero | 19 (35.8) | 2 (100.0) | 3 (37.5) | 0 (0.0) | 19 (35.8) | ||||
| PGM | Homo | 1 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.0) | 0.52 | 0.47 | NS |
| Hetero | 2 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.0) | ||||
| MTHFR | Homo | 7 (14.0) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 7 (14.0) | 0.29 | 0.59 | NS |
| Hetero | 19 (38.0) | 0 (0.0) | 3 (42.9) | 1 (10.0) | 19 (38.0) | ||||
| JAK2 (MPD) | + | 16 (31.4) | 2 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2.08 | 0.15 | NS |
| Primary APA | + | 9 (12.9) | 0 (0.0) | 1 (33.3) | 6 (37.5) | 0 (0.0) | 2.58 | 0.11 | NS |
| Secondary APA | + | 9 (12.9) | 0 (0.0) | 0 (0.0) | 1 (6.3) | 1 (33.3) | 0.02 | 0.88 | NS |
| Behçet’s disease | + | 2 (2.9) | 0 (0.0) | 2 (66.7) | 7 (43.8) | 1 (33.3) | 21.25 | < 0.0001 | HS |
| PNH | + | 2 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.62 | 0.43 | NS |
Our current epidemiological study of 94 Egyptian patients with confirmed diagnoses of primary BCS describes the socio-demographic features, etiology, and risk factors for BCS. Previous reports found that BCS affects all races, usually during the third or fourth decade of life, and is slightly more common in females[4]. More females than males (61.7% vs 38.3%) were present in our population and mean patient age at the time of first visit was 28.64 ± 8.35 years for males and 28.88 ± 9.08 years for females.
BCS patients from different geographic regions tend to show distinct disease etiologies. In particular, thromboses are more common in western BCS patients, whereas development of venous webs is more frequent in Eastern and Japanese BCS patients[11]. Recent studies have shown that primary BCS should be regarded as a multifactorial disease, and that co-occurrence of several prothrombotic disorders leads to thrombosis at this uncommon location. A previous study found that several prothrombotic conditions were evident in at least 35% of BCS patients[12]. Thus, in the workup of such patients, identification of a single etiology should not indicate that additional etiologies should not be sought[1]. A previous study by Uskudar et al[13] in Turkey identified at least one etiological factor in 72% of patients, two in 18.6%, and three in 1.3%. In our present study of Egyptian patients, we identified at least one etiological factor in 48.9% of patients, two in 30.9%, three in 8.5%, and four in 3.2%.
Table 6 shows the prevalence rates of the major causal factors of primary BCS as reported in the current study and in that of Valla, conducted in 2009[1] and based on the work of Primignani et al[12,14], Patel et al[15], Colaizzo et al[16], and Kiladjian et al[17]. In the current study, FVLM was the most common cause of disease (53.1% of patients); 37.5% of patients were FVLM heterozygotes and 15.6% FVLM homozygotes. Similarly, a study in India by Mohanty et al[11] found that FVLM was the most common etiology of BCS (26% of patients). Identification of FVLM as a risk factor for venous thrombosis was a major advance in understanding the pathogenesis of BCS[8]; the possible presence of FVLM should be routinely investigated when BCS is diagnosed[11].
| Etiology | Reported rate1 | Rate in current study | |
| PC deficiency | 20% | 4.30% | |
| PS deficiency | 7% | 1.10% | |
| AT III deficiency | 5% | 4.30% | |
| FVLM | Homozygous | NA | 15.60% |
| Heterozygous | 20% | 37.50% | |
| PGM | Homozygous | NA | 1.70% |
| Heterozygous | 7% | 3.30% | |
| MTHFR | Homozygous | NA | 13.30% |
| Heterozygous | NA | 38.30% | |
| JAK2-positive (MPD) | 50% | 29% | |
| Primary APA | 10% | 17% | |
| Secondary APA | 11.70% | ||
| Behçet’s disease | 5% | 12.80% | |
| PNH | 2% | 2.10% | |
| Hormonal therapy (58 females) | 50% | 15.50% | |
| Non-identified etiology | 5% | 8.50% | |
MTHFR gene mutation was the second most common etiology of BCS in the current study, evident in 51.6% of patients, of whom 38.3% were heterozygotes and 13.3% homozygotes. A previous study in China by Li et al[18] found that the prevalence of the MTHFR 677/T genotype in BCS patients was 45.12%, a figure similar to ours. Valla[19] reported that the G20210A prothrombin gene mutation (PGM), another recently discovered inherited marker, was less common in BCS patients than the other prothrombotic mutations mentioned above. In the present study, very few patients had a PGM (3.3% heterozygous, 1.7% homozygous).
MPD is the leading cause of BCS in western countries, foun d in 20%-53% of patients. Polycythemia vera was reported in 10%-40% of patients, whereas essential thrombocythemia and myelofibrosis were less common[6]. In the current study, we identified MPD in 29% of patients, of whom 28.5% had overt disease and 71.5% were of occult status. We confirmed the presence of such disorders by identification of the JAK2 V617F mutation. A recent study reported that clusters of dystrophic megakaryocytes evident in bone marrow biopsy samples were specific for MPD[20]. In our current study, a JAK2 mutation was present in 29% of patients; this figure was lower than that previously reported by Colaizzo et al[21] (34.4%), Spivak[22] (40%), and Patel et al[15] (58.5%). These discrepancies may be attributable to the use of different DNA sources (peripheral blood granulocytes in our present study and bone marrow films in the work of Patel et al[15]) or to differences in patient populations.
APA syndrome was one of the major causes of BCS in the current study, being present in 28.7% of patients; 17% with primary APA and 11.7% with APA secondary to systemic lupus erythematosus (SLE). A previous report found that 3%-17% of patients with venous thrombosis showed measureable levels of antiphospholipid antibodies[23]. In the current study, we were surprised to find Behçet’s disease in 12.8% of our patients. This disease is common in certain countries, and Uskudar et al[13] reported that the disease was present in 9% of Turkish patients with BCS. Thus, we suggest that patients with BCS should always be screened for Behçet’s disease. Such a diagnosis can be difficult, even in male patients from countries where the disease is endemic; use of the diagnostic criteria of the International Study Group of Behçet’s Disease may be helpful[24].
The prevalence of primary deficiencies in Protein C, Protein S, and Antithrombin III in BCS patients is difficult to determine, for several reasons. Firstly, the liver synthesizes these inhibitors of coagulation, and liver dysfunction related to BCS thus induces non-specific falls in the plasma levels of the inhibitors. Secondly, diagnosis of any primary deficiency is based on measurement of plasma protein level, because most mutations in the relevant genes are unique, rendering diagnosis using molecular biology techniques alone difficult. Finally, complete family screening is recommended to differentiate between inherited and false instances of deficiencies in Proteins C and S, but this is usually impractical. Thus, the question of whether a primary deficiency in the expression of one of the proteins mentioned above exists will remain unanswered in most instances[19]. However, a level below 20% of the normal value suggests the presence of an inherited deficiency[2]. In the present study, we identified deficiencies in Antithrombin III, Protein C, or Protein S in 4.3%, 1.1%, and 4.3% of our patients, respectively. Mohanty et al[11] studied BCS patients in India, and found deficiencies in Antithrombin III, Protein C, and Protein S in 3.8%, 13.2%, and 5.7% of patients, respectively. Uskudar et al[13] worked with BCS patients in Turkey, and reported deficiencies in Antithrombin III, Protein C, and Protein S in 3%, 9%, and 7% of patients, respectively.
A previous study found that hepatic vein (HV) thrombosis occurred in up to 12% of patients with PNH; this is the leading cause of mortality in BCS patients[7]. In the present study, PNH was documented in 2.1% of patients, similar to what was previously reported by Valla[2] (2%). Membranous obstruction of the IVC is the most common cause of BCS in South Africa and Asia, but is less common in western countries, where the condition is thought to be a consequence of IVC thrombosis[10]. One of the most striking results of the present study was that development of IVC venous webs was not responsible for any instance of BCS. In contrast, Uskudar et al[13] reported that venous webs were one of the most common etiological factors of BCS in Turkey.
Analysis of the relationship between gender and BCS etiology indicated that females were more likely to have secondary APA syndrome than males (17.2% vs 2.8%), but that males were more likely to have MTHFR gene mutations (71.5% vs 41%) and also had significantly higher rates of Behçet’s disease (30.6% vs 1.7%). We also found that potential risk factors for BCS included previous thrombosis elsewhere in the body (27.5%), recent pregnancy (17.2% of females), and hormonal therapy (15.5% of females). Each of our BCS patients on hormonal therapy or with pregnancy-related BCS displayed an additional etiological factor. Thus, many women with BCS who are pregnant or users of oral contraceptives appear to have additional thrombophilic conditions[25].
In the present study, we found isolated HV involvement in 74.5% of patients, isolated IVC involvement in 3.2%, and combined HV and IVC occlusion in 17%. In a previous study of 237 patients with BCS, Darwish Murad et al[26] reported obstruction of the HV, the IVC, and both veins, in 62%, 7%, and 31% of patients, respectively. The high rate of IVC thrombosis found in the cited study was associated with the high prevalence of Behçet’s disease. These findings were similar to those of the Turkish study by Uskudar et al[13], who reported HV involvement in 77% of patients and IVC involvement in 53%. Together, the results indicate that the etiology of BCS may be related to the site of obstruction.
Previous studies have indicated that the site of the lesion varies among patients of different countries. In particular, IVC involvement is more common in Nepal, South Africa, China, India, and Japan, whereas HV involvement is more common in the west[4]. In the current study, we assessed the extent of association between BCS etiology and the anatomical site of thrombosis as revealed using different imaging modalities. Our results indicate a significant association between the presence of Behçet’s disease and the anatomical site of thrombosis. In particular, Behçet’s disease was diagnosed in 12 patients, 9 (75%) with combined IVC and HV thrombosis, 2 (16.7%) with isolated HV thrombosis, and 1 (8.3%) with isolated IVC thrombosis. These results are similar to those reported by Bayraktar et al[27], who found that 85.7% of Behçet’s disease patients had combined IVC and HV thrombosis and 14.2% had isolated HV thrombosis.
An increasing number of studies support the concept that thrombosis is site-specific, depending on the underlying prothrombotic disorder[28]. For example, MPD is more common in BCS patients than in those with portal vein thrombosis; Factor V Leiden mutation is more strongly associated with BCS than is portal vein thrombosis; and the G20210A prothrombin gene mutation is more strongly associated with portal vein thrombosis than is BCS. Further site-specificity may be in play within the hepatic venous outflow tract per se. Indeed, the FVLM appears to be particularly common in patients with IVC obstruction[8] and the use of oral contraceptives, or pregnancy, is specifically associated with hepatic vein involvement[29].
In conclusion, our study of Egyptian BCS patients indicated that FVLM was the most common etiology, MTHFR the second most common, and that BCS commonly occurs during the third decade of life and is more prevalent in females. Comparison of our data with those of previous studies indicates that BCS patients from different geographic regions tend to differ in terms of etiology.
Budd-Chiari syndrome (BCS) results from hepatic venous outflow obstruction at any level, from the hepatic venules to the right atrium, and is estimated to affect every 1 out of 100 000 subjects in the general population worldwide. BCS affects all races, usually during the third or fourth decade of life, and is slightly more common in females.
BCS patients from different geographic regions tend to vary in terms of disease etiology. Previous reports have indicated that thromboses are more common in the West and venous webs more frequent in the East and Japan. In the present study of Egyptian patients, the authors demonstrate that Factor V Leiden mutation (FVLM) was the most common etiology, methylene tetrahydrofolate reductase gene mutation the second most common, and that the disease was of multiple etiologies in 42.6% of patients.
This is the first epidemiological study to examine the socio-demographic features, etiology and risk for development of BCS in Egyptian patients.
In the workup for determining the etiology of BCS, identification of a single cause should not preclude investigation of additional contributing factors. The presence of an underlying hypercoagulable state should be investigated in all patients.
FVLM is the most common cause of inherited thrombophilia. The mutated factor Va is resistant to degradation by protein C, a natural anticoagulant. The rise in the level of undegraded factor Va over time increases the risk of uncontrolled thrombin and thrombus formation. Methylene tetrahydrofolate reductase is an enzyme active in re-methylation of homocysteine. Deficiencies in the enzyme cause hyperhomocysteinemia; this condition compromises the functions of the anticoagulant and fibrinolytic systems.
The authors investigated the epidemiology of Budd-Chiari syndrome in Egyptian patients. The results indicate that BCS is most common during the third decade of life and is more often diagnosed in females than in males. In Egyptian patients, FVLM was the most common etiology, methylene tetrahydrofolate reductase gene mutation the second most common, and multiple etiologies were present in 42.6% of patients. Thus, in workup for determination of the etiology of BCS, identification of a single cause should not preclude investigation of additional relevant factors. The presence of an underlying hypercoagulable state should be investigated in all BCS patients. The present study provides valuable new information on BCS.
| 1. | Valla DC. Primary Budd-Chiari syndrome. J Hepatol. 2009;50:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 221] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 2. | Valla DC. The diagnosis and management of the Budd-Chiari syndrome: consensus and controversies. Hepatology. 2003;38:793-803. [PubMed] |
| 3. | Wang ZG, Jones RS. Budd-Chiari syndrome. Curr Probl Surg. 1996;33:83-211. [PubMed] |
| 4. | Valla DC. Hepatic vein thrombosis (Budd-Chiari syndrome). Semin Liver Dis. 2002;22:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Janssen HL, Garcia-Pagan JC, Elias E, Mentha G, Hadengue A, Valla DC. Budd-Chiari syndrome: a review by an expert panel. J Hepatol. 2003;38:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 334] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 6. | Denninger MH, Chaït Y, Casadevall N, Hillaire S, Guillin MC, Bezeaud A, Erlinger S, Briere J, Valla D. Cause of portal or hepatic venous thrombosis in adults: the role of multiple concurrent factors. Hepatology. 2000;31:587-591. [PubMed] |
| 7. | Socié G, Mary JY, de Gramont A, Rio B, Leporrier M, Rose C, Heudier P, Rochant H, Cahn JY, Gluckman E. Paroxysmal nocturnal haemoglobinuria: long-term follow-up and prognostic factors. French Society of Haematology. Lancet. 1996;348:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 334] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 8. | Deltenre P, Denninger MH, Hillaire S, Guillin MC, Casadevall N, Brière J, Erlinger S, Valla DC. Factor V Leiden related Budd-Chiari syndrome. Gut. 2001;48:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Dayal S, Pati HP, Pande GK, Sharma MP, Saraya AK. Multilineage hemopoietic stem cell defects in Budd Chiari syndrome. J Hepatol. 1997;26:293-297. [PubMed] |
| 10. | Okuda K. Inferior vena cava thrombosis at its hepatic portion (obliterative hepatocavopathy). Semin Liver Dis. 2002;22:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Mohanty D, Shetty S, Ghosh K, Pawar A, Abraham P. Hereditary thrombophilia as a cause of Budd-Chiari syndrome: a study from Western India. Hepatology. 2001;34:666-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Primignani M, Barosi G, Bergamaschi G, Gianelli U, Fabris F, Reati R, Dell'Era A, Bucciarelli P, Mannucci PM. Role of the JAK2 mutation in the diagnosis of chronic myeloproliferative disorders in splanchnic vein thrombosis. Hepatology. 2006;44:1528-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 170] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Uskudar O, Akdogan M, Sasmaz N, Yilmaz S, Tola M, Sahin B. Etiology and portal vein thrombosis in Budd-Chiari syndrome. World J Gastroenterol. 2008;14:2858-2862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Primignani M, Martinelli I, Bucciarelli P, Battaglioli T, Reati R, Fabris F, Dell'era A, Pappalardo E, Mannucci PM. Risk factors for thrombophilia in extrahepatic portal vein obstruction. Hepatology. 2005;41:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Patel RK, Lea NC, Heneghan MA, Westwood NB, Milojkovic D, Thanigaikumar M, Yallop D, Arya R, Pagliuca A, Gäken J. Prevalence of the activating JAK2 tyrosine kinase mutation V617F in the Budd-Chiari syndrome. Gastroenterology. 2006;130:2031-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 170] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Colaizzo D, Amitrano L, Tiscia GL, Scenna G, Grandone E, Guardascione MA, Brancaccio V, Margaglione M. The JAK2 V617F mutation frequently occurs in patients with portal and mesenteric venous thrombosis. J Thromb Haemost. 2007;5:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Kiladjian JJ, Cervantes F, Leebeek FW, Marzac C, Cassinat B, Chevret S, Cazals-Hatem D, Plessier A, Garcia-Pagan JC, Darwish Murad S. The impact of JAK2 and MPL mutations on diagnosis and prognosis of splanchnic vein thrombosis: a report on 241 cases. Blood. 2008;111:4922-4929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 223] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 18. | Li XM, Wei YF, Hao HL, Hao YB, He LS, Li JD, Mei B, Wang SY, Wang C, Wang JX. Hyperhomocysteinemia and the MTHFR C677T mutation in Budd-Chiari syndrome. Am J Hematol. 2002;71:11-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Valla DC. Budd-Chiari syndrome and veno-occlusive disease/sinusoidal obstruction syndrome. Gut. 2008;57:1469-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Chait Y, Condat B, Cazals-Hatem D, Rufat P, Atmani S, Chaoui D, Guilmin F, Kiladjian JJ, Plessier A, Denninger MH. Relevance of the criteria commonly used to diagnose myeloproliferative disorder in patients with splanchnic vein thrombosis. Br J Haematol. 2005;129:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Colaizzo D, Amitrano L, Tiscia GL, Iannaccone L, Gallone A, Grandone E, Guardascione MA, Margaglione M. Occurrence of the JAK2 V617F mutation in the Budd-Chiari syndrome. Blood Coagul Fibrinolysis. 2008;19:459-462. [PubMed] |
| 22. | Spivak JL. Phenotype and genotype in the myeloproliferative disorders. Eur J Haematol Suppl. 2007;9-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Manoussakis MN, Gharavi AE, Drosos AA, Kitridou RC, Moutsopoulos HM. Anticardiolipin antibodies in unselected autoimmune rheumatic disease patients. Clin Immunol Immunopathol. 1987;44:297-307. [PubMed] |
| 24. | International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s disease. Lancet. 1990;335:1078-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 283] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Janssen HL, Garcia-Pagan JC, Elias E, Mentha G, Hadengue A, Valla DC. Budd-Chiari syndrome: a review by an expert panel. J Hepatol. 2003;38:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 334] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 26. | Darwish Murad S, Valla DC, de Groen PC, Zeitoun G, Hopmans JA, Haagsma EB, van Hoek B, Hansen BE, Rosendaal FR, Janssen HL. Determinants of survival and the effect of portosystemic shunting in patients with Budd-Chiari syndrome. Hepatology. 2004;39:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 228] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Bayraktar Y, Balkanci F, Bayraktar M, Calguneri M. Budd-Chiari syndrome: a common complication of Behçet's disease. Am J Gastroenterol. 1997;92:858-862. [PubMed] |
| 28. | De Stefano V, Martinelli I, Mannucci PM, Paciaroni K, Rossi E, Chiusolo P, Casorelli I, Leone G. The risk of recurrent venous thromboembolism among heterozygous carriers of the G20210A prothrombin gene mutation. Br J Haematol. 2001;113:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Valla DC. Hepatic venous outflow tract obstruction etiopathogenesis: Asia versus the West. J Gastroenterol Hepatol. 2004;19:S204-S211. [RCA] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
Peer reviewers: Can Gonen, MD, Department of Gastroenterology, Kutahya State Hospital, 43100 Kutahya, Turkey; Dr. Herwig R Cerwenka, Department of Surgery, Medical University of Graz, Auenbruggerplatz 29, A-8036 Graz, Austria
S- Editor Sun H L- Editor Rutherford A E- Editor Zhang DN