Published online Oct 28, 2011. doi: 10.3748/wjg.v17.i40.4456
Revised: March 2, 2011
Accepted: March 9, 2011
Published online: October 28, 2011
Multiphoton microscopy, relying on the simultaneous absorption of two or more photons by a fluorophore, has come to occupy a prominent place in modern biomedical research with its ability to allow real-time observation of a single cell and molecules in intact tissues. Multiphoton microscopy exhibits nonlinear optical contrast properties, which can make it possible to provide an exceptionally large depth penetration with less phototoxicity. This system becomes more and more an inspiring tool for a non-invasive imaging system to realize “optical biopsy” and to examine the functions of living cells. In this review, we briefly present the physical principles and properties of multiphoton microscopy as well as the current applications in biological fields. In addition, we address what we see as the future potential of multiphoton microscopy for gastroenterologic research.
- Citation: Cho HJ, Chun HJ, Kim ES, Cho BR. Multiphoton microscopy: An introduction to gastroenterologists. World J Gastroenterol 2011; 17(40): 4456-4460
- URL: https://www.wjgnet.com/1007-9327/full/v17/i40/4456.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i40.4456
A remarkable evolution of biological science has induced the need to visualize cells in an intact whole organism. To date, most applications using microscopy are limited to fixed cells or excised tissues. However, characterization of morphological features and establishment of cell function of native tissues is important for the diagnosis of disease in the early stage and has improved understanding of the associated pathophysiological processes. Therefore, the need for real-time observation of cells and their subcellular components in intact tissues is of great interest and imaging techniques have been developed to pursue this goal.
One of these techniques is fluorescence imaging. Although the combination of microscopy with fluorescent labeling has improved sensitivity, this approach provides only a two-dimensional view of samples. The use of confocal microscopy allows for the observation of subcellular material with three-dimensional resolution. However, confocal microscopy is limited by the effective imaging depth of typically less than 100 μm and phototoxicity, which is caused by using a short wavelength laser[1].
Recent advances in nonlinear optical processes of multiphoton microscopy compensate single photon-linear microscopy technologies such as confocal microscopy by the capacity for deeper tissue penetration with clear images and the reduction of direct ultraviolet damage[2]. Thus, multiphoton microscopy has been applied to various parts of the imaging task and has now become the technique of choice for subcellular observations of thick tissues and in living animals[3].
In addition, endoscopists often want to know the relationship between the gross endoscopic findings and the microscopic diagnosis during routine endoscopy. Although a mucosal biopsy is the standard method for histopathological diagnosis of an abnormal mucosal lesion, this approach is limited by sampling error, bleeding risk and the time lag for results. Therefore, endoscopists would like to have the ability to directly observe and promptly identify pathology of cellular and/or subcellular structures without biopsy. Multiphoton microscopy has the full potential to achieve this goal because it can provide thin optical sections from thick specimens.
In this article, the principles of multiphoton microscopy and its applications in bioscience are reviewed, as well as the prospects for clinical use.
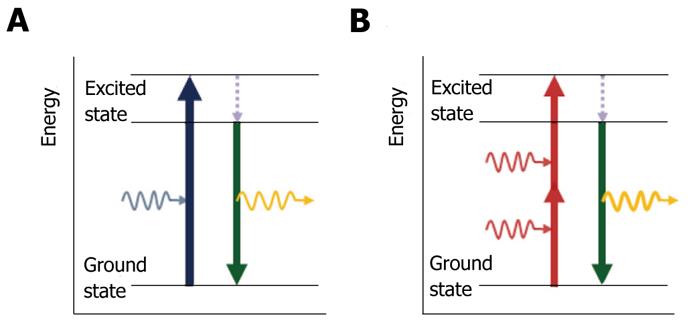
Early in the development of quantum mechanics, the theoretical concept was first proposed by Göppert-Mayer in 1931. Multiphoton excitation is based on the probability that fluorophore molecules are excited by multiple low energy photons that can arrive “simultaneously” at the fluorophore and interact with it. The fluorophore molecule absorbs the sum of the energy from each photon, and an electron in the fluorophore is transferred to the excited state, which can induce an electronic transition similar to a single high-energy photon[4]. Soon after, the molecule in the excited state falls back to the ground state with emission of fluorescence, which has most, but not all, of the initial energy, owing to non-radiative relaxation (Figure 1).
Because the energy of a photon is inversely proportional to its wavelength (λ), the emitted fluorescence is a longer wavelength than the exciting light. However, in the case of multiphoton excitation, the fluorophore molecule almost simultaneously absorbs the energy from multiple photons, each of which contributes a part of the total energy required to induce the fluorescent emission. Thus, the emitted fluorescent photon has a shorter wavelength than each of the photons involved in excitation. For this reason, multiphoton microscopy can induce fluorescence equal to the energy of single photon excitation microscopy by low energy photons.
However, multiphoton excitation requires enormously high light intensities that, if continous, would almost instantly vaporize the specimen. Therefore, to generate enough fluorescence practical for multi-photon microscopy, a pulsed laser source is needed. In other words, using a laser that produces extremely brief pulses (femtosecond laser, about 10-5) at a high repetition rate, thus generating high instantaneous energy but low average energy[5].
Multiphoton microscopy exhibits nonlinear optical contrast properties that are predicated upon second and third-order nonlinear interactions between light and particles[6]. The nonlinear optical effects are proportional to the square or cube of the fundamental light intensity; this gives multiphoton microscopy the intrinsic characteristics of 3-dimensional images. This is because the photon density is high at the focal point, and it falls off steeply from the focal point[7]. This eliminates out-of-focus contributions and allows multiphoton microscopy to obtain high resolution images from the scattered photons of the fluorophore emission used to produce the image. In addition, photobleaching is restricted to a narrow region around the plane of focus.
To date, the most widely used imaging modalities associated with multiphoton microscopy are multiphoton excitation with fluorescence, second harmonic generation, multiphoton fluorescence lifetime imaging microscopy, and spectral lifetime imaging microscopy[1]. For example, with two-photon microscopy, a fluorophore molecule is excited by the nearly simultaneous absorption of two photons, each twice the wavelength required for a single photon excitation[8]. A molecule of fluorescein can be excited by two photons of near-infrared light (λ ≈ 780 nm), each of which has approximately half the energy of a single blue photon (λ ≈ 480 nm), and then emit a photon of green light, in the same manner as for standard (one-photon) excitation with blue light[7]. According to nonlinear excitation, fluorescent emission from fluorophore molecules is proportional to the square of the excitation intensity. This intensity-squared dependence of two-photon microscopy provides “optical sectioning” capability, without using an adjustable pinhole aperture in front of the detector to reject out-of-focus fluorescence like confocal microscopy[9].
Multiphoton microscopy has several advantages over confocal microscopy. Most of all, the use of long excitation wavelengths has major advantages. Since light scattering declines rapidly with an increasing wavelength, deeper penetration can be achieved by using a longer wavelength of light than with single-photon confocal microscopy. In addition to an increase in the penetration depth, a longer wavelength of light, such as infrared light (700-1000 nm) used for multiphoton microscopy, has much less energy than confocal microscopy, and therefore causes negligible photodamage and phototoxicity to cells and tissues. Cells and molecules deep inside living tissues can be observed for long periods of time. Moreover, because excitation and emission take place only at the focal plane, multiphoton microscopy reduces the photobleaching outside of the focal plane, unlike confocal microscopy. Therefore, it results in high fluorescence collection efficiency and thus greater signal intensity at any given tissue depth.
Table 1 details comparative differences in excitation wavelength, tissue imaging depth, resolution and photo damage/bleaching between two-photon microscopy and confocal microscopy.
| Confocal microscopy | Two-photon microscopy | |
| Excitation wavelength | Short (ultraviolet light) | Long (infra-red light) |
| Tissue imaging depth | About 50-100 μm | About 400-1000 μm |
| Spatial resolution | nm (3D-resolution with pinhole aperture ) | nm (3D-resolution with inherent optical sectioning) |
| Photodamage and photobleaching | High | Low |
Multiphoton microscopy is a powerful tool for visualizing cellular and subcellular events within living tissue with its inherent “optical sectioning” capability, deeper penetration and minimal phototoxicity and photobleaching. Multiphoton microscopy can capture whole organisms or embryos on a large scale. Though transparent organisms such as the zebra fish and drosophila are ideal candidates for such studies, the development of the hamster embryo model has allowed for observations over long periods of time, for several days[10].
In addition to morphological studies, multiphoton microscopy can be used for dynamic and functional cellular imaging with the development of various fluorescent probes. For example, two-photon microscopy of the calcium sensitive fluorophore allows for the collection of subcellular spatial and temporal information on (Ca2+) ion entry through voltage-gated channels or release from intracellular stores within a single myocyte at depths of up to 200 μm below the epicardial surface. Therefore, two-photon microscopy is well suited to determine the functional state of donor cells following intracardiac transplantation[11].
Neuroscientists use multiphoton microscopy for the observation of neuronal plastic changes within brain slices, measuring ionized-calcium dynamics deep in brain tissues[12]. The dendritic spines, which are a major functional component of the nervous system associated with learning and memory activated by chemical and electrical transmission mechanisms, are very tiny structures. Since neurons are very sensitive to phototoxicity and brain tissue is highly scattered, it has been difficult to visualize these dynamic processes in live tissues[13,14]. However, multiphoton microscopy overcomes these obstacles by using long wavelength light and providing high resolution deep imaging without causing injury to the living material[15]. It allows visualization of fine structures of the brain in the head and neck area, including unique signaling and dynamic motility of the dendritic spines 300-400 μm into the brain tissue[16,17].
Multiphoton microscopy enables imaging of dynamic and heterogeneous immune processes at the cellular and molecular levels deep within intact organs of living animals. Due to the depth of penetration and minimal photodamage, multiphoton microscopy permits six-dimensional (x, y, z, time, intensity, wavelength) imaging of intact lymphoid organs and can be used to observe naïve lymphocytes for hours without loss of viability or motility[18]. Dynamic movements and cellular interactions of viable T- and B-cells can be revealed, as well as the antigen presenting cells in the in vivo setting[7,19].
Multiphoton microscopy is also a preferred imaging technique for cancer research, for example in studies on angiogenesis and metastasis in vivo[20,21]. Tumor microinvasion and metastasis involves complex interactions between cells and extracellular matrix proteins, most notably collagen[22]. Due to the ability of imaging more deeply in tissues with less toxicity, multiphoton microscopy facilitates imaging of tumor-stroma interactions and thus facilitates improved understanding of the processes of cell migration, metastasis, and tumor progression with direct observation in vivo[23].
Gastrointestinal endoscopists have to rely on visual inspection for the diagnosis of disease. Therefore, multiphoton excitation imaging may be helpful in the diagnosis and offer additional diagnostic benefit. Indeed, a pilot study of multiphoton microscopy to diagnose gastric cancer has been reported recently[24]. The results of the study showed that multiphoton microscopy can be used to diagnose gastric cancer by optical biopsy. Multiphoton microscopy has proved to be a promising tool for real-time histological diagnosis. Recent developments in imaging technology now make this possible.
Multiphoton microscopy also has the ability to penetrate deeper inside the tissue and excite endogenous autofluorescence molecules such as intracellular nicotinamide adenine dinucleotide phosphate (reduced form), flavin, melanin and lipofuscin, instead of using fluorescent dyes which must be used for in vivo confocal laser microscopy[25]. It provides the ability to detect cellular and subcellular details of the gastrointestinal mucosa without fixation or staining. Multiphoton imaging of intact human gastrointestinal mucosa ex vivo provides improved cellular detail compared to confocal imaging, without the need for fluorescent dyes[2].
Suitable indicators for two-photon microscopy are required in order to get a clearer image. Recently, our collaborators have developed many new two-photon tracers. One tracer, a hydrogen probe, AH2, which emits fluorescence at pH < 4 can be used to obtain images of live esophageal tissue from the mucosal surface to 100 μm in depth. Emitted fluorescence of the hydrogen probe in reflux esophagitis tissue was stronger than that in control tissue. Multiphoton-emitted fluorescence of low esophageal tissue of the reflux model was similar to that of stomach[26]. Visible images of pH changes in reflux esophageal tissue can be obtained by use of the multiphoton hydrogen probe.
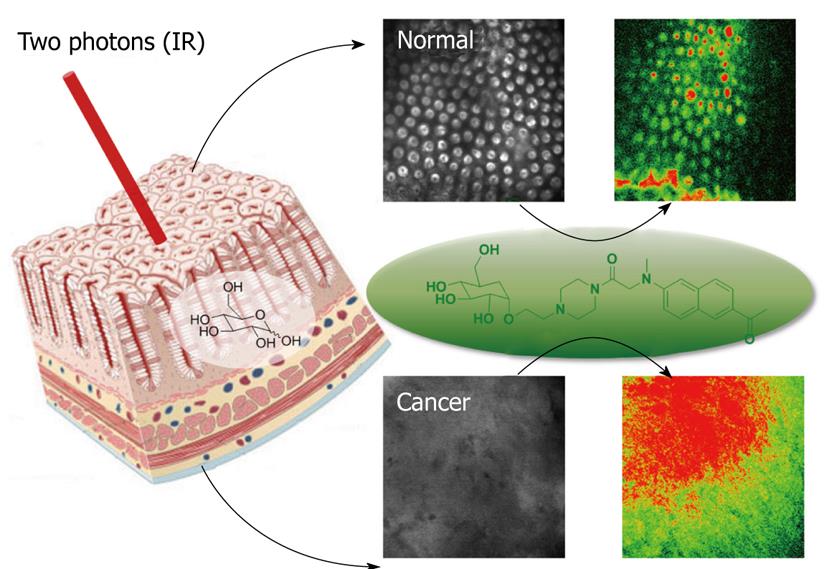
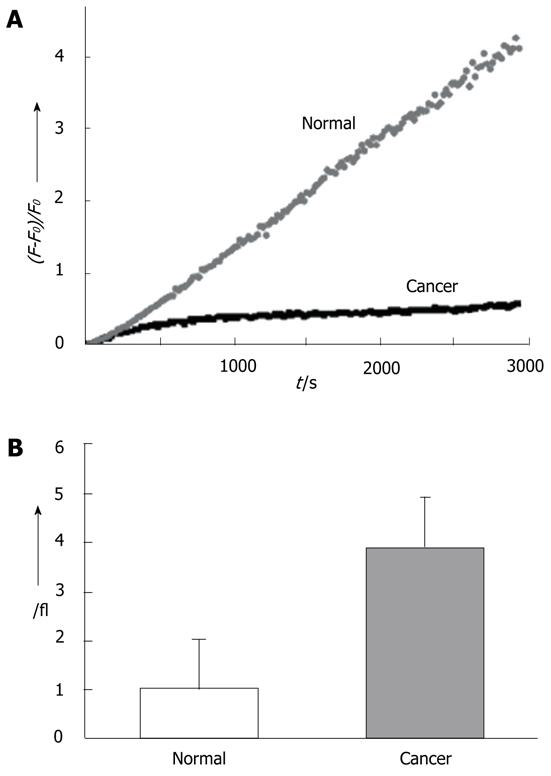
Another new probe, AG2, that can be easily taken up by cancer cells and tissues through glucose-specific translocation has been developed. AG2 shows negligible cytotoxicity and high photostability. It can monitor glucose uptake in colon cancer tissues and visualize at depth of 75-150 μm by two-photon microscopy (Figure 2). This compound may be useful in diagnosing the early stages of cancer and make it possible to develop customized cancer therapy according to the uptake rates of AG2 in normal and cancerous tissues (Figure 3). In addition, this laboratory has used multiphoton laser scanning microscopy to study gastric and colon cancer with other probes. Multiphoton images of normal and cancer cell lines, as well as normal mucosa and dysplastic tissues, (adenoma, adenocarcinoma) labeled with the multiphoton microscopy probes AZn1 and ACu1, have been studied. The findings showed that the Cu1 content was higher, Zn1 content was lower, and the ratio of Cu1 to Zn1 was much higher in adenomas and adenocarcinoma than in the normal mucosa. These results suggest the possibility that multiphoton endomicroscopy might be developed further to use as a technique for performing virtual biopsies during the course of routine endoscopy.
Multiphoton microscopy has rapidly evolved and become a standard device for cell-based biological research in the fields of genomics, proteomics and tissue engineering. A major advantage of multiphoton microscopy is the ability to observe deep within intact organs and cells. Its applications are being extended beyond basic research to the clinical setting, such as detection of skin cancers, mucosal dysplasia of the intestinal tract, Alzheimer’s disease, and metabolic disorders just by visualizing patient’s tissue at the cellular level of resolution[27].
Although multiphoton microscopy has already been used by many biologists for research and some clinicians, as mentioned briefly above, its advantages are partly limited by the bulkiness of the system including lasers, objective lenses, and scanning devices. Therefore, several groups currently are trying to develop smaller fluorescence microscopes, either by using a gradient index lens as a thin, rodlike probe to extend the working distance of a conventional objective[15] or by using fiber optics to construct multiphoton endoscopes[28]. Imaging of goblet cells as a marker for intestinal metaplasia of the stomach by two-photon endomicroscopy has been reported[29]. Its techniques can three-dimensionally observe goblet cells in mouse large intestine, and it provides the possibility that two photon endomicroscopy is advantageous in diagnoses.
The development of miniature laser scanning multiphoton endoscopes will provide advantages over currently available endomicroscopy technologies and be of great utility to gastroenterologists. Moreover, miniature multiphoton endoscopy may be used for minimally invasive endoscopic procedures and has enormous potential for histological evaluation of organs outside the gastrointestinal tract, namely, the liver, pancreas, and ovaries by transluminal endoscopic approaches[30].
With the development of novel laser sources, new fluorophores and more specific probes, multiphoton microscopy and its applications will open up a wide range of possibilities. In addition, it can be combined with other imaging modalities such as ultrasound or magnetic resonance imaging, which provide complementary information.
The development of multiphoton microscopy marks a significant step in the advancement of imaging modalities and will likely aid in our understanding of the basis of disease as well as the management of the clinical manifestations of disease.
| 1. | Provenzano PP, Eliceiri KW, Keely PJ. Multiphoton microscopy and fluorescence lifetime imaging microscopy (FLIM) to monitor metastasis and the tumor microenvironment. Clin Exp Metastasis. 2009;26:357-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Rogart JN, Nagata J, Loeser CS, Roorda RD, Aslanian H, Robert ME, Zipfel WR, Nathanson MH. Multiphoton imaging can be used for microscopic examination of intact human gastrointestinal mucosa ex vivo. Clin Gastroenterol Hepatol. 2008;6:95-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol. 2003;21:1369-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2935] [Cited by in RCA: 2316] [Article Influence: 100.7] [Reference Citation Analysis (0)] |
| 4. | Williams RM, Zipfel WR, Webb WW. Multiphoton microscopy in biological research. Curr Opin Chem Biol. 2001;5:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 245] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Wang BG, König K, Halbhuber KJ. Two-photon microscopy of deep intravital tissues and its merits in clinical research. J Microsc. 2010;238:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | 6 Carriles R, Schafer DN, Sheetz KE, Field JJ, Cisek R, Barzda V, Sylvester AW, Squier JA. Invited review article: Imaging techniques for harmonic and multiphoton absorption fluorescence microscopy. Rev Sci Instrum. 2009;80:081101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Cahalan MD, Parker I, Wei SH, Miller MJ. Two-photon tissue imaging: seeing the immune system in a fresh light. Nat Rev Immunol. 2002;2:872-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 353] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 8. | Stutzmann GE, Parker I. Dynamic multiphoton imaging: a live view from cells to systems. Physiology (Bethesda). 2005;20:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Webb RH. Theoretical basis of confocal microscopy. Methods Enzymol. 1999;307:3-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Squirrell JM, Wokosin DL, White JG, Bavister BD. Long-term two-photon fluorescence imaging of mammalian embryos without compromising viability. Nat Biotechnol. 1999;17:763-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 347] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 11. | Rubart M. Two-photon microscopy of cells and tissue. Circ Res. 2004;95:1154-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Helmchen F, Waters J. Ca2+ imaging in the mammalian brain in vivo. Eur J Pharmacol. 2002;447:119-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Jung JC, Mehta AD, Aksay E, Stepnoski R, Schnitzer MJ. In vivo mammalian brain imaging using one- and two-photon fluorescence microendoscopy. J Neurophysiol. 2004;92:3121-3133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 14. | Oheim M, Beaurepaire E, Chaigneau E, Mertz J, Charpak S. Two-photon microscopy in brain tissue: parameters influencing the imaging depth. J Neurosci Methods. 2001;111:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 268] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 15. | Levene MJ, Dombeck DA, Kasischke KA, Molloy RP, Webb WW. In vivo multiphoton microscopy of deep brain tissue. J Neurophysiol. 2004;91:1908-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 284] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 16. | Holthoff K, Tsay D, Yuste R. Calcium dynamics of spines depend on their dendritic location. Neuron. 2002;33:425-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Majewska A, Tashiro A, Yuste R. Regulation of spine calcium dynamics by rapid spine motility. J Neurosci. 2000;20:8262-8268. [PubMed] |
| 18. | Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 899] [Article Influence: 37.5] [Reference Citation Analysis (8)] |
| 19. | Meyer-Hermann ME, Maini PK. Interpreting two-photon imaging data of lymphocyte motility. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71:061912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Brown EB, Campbell RB, Tsuzuki Y, Xu L, Carmeliet P, Fukumura D, Jain RK. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nat Med. 2001;7:864-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 417] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 21. | McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9:713-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 671] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 22. | Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1315] [Cited by in RCA: 1370] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 23. | Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, Jain RK. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 2003;9:796-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 584] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 24. | Yan J, Chen G, Chen J, Liu N, Zhuo S, Yu H, Ying M. A pilot study of using multiphoton microscopy to diagnose gastric cancer. Surg Endosc. 2011;25:1425-1430. [PubMed] |
| 25. | Hoffman A, Goetz M, Vieth M, Galle PR, Neurath MF, Kiesslich R. Confocal laser endomicroscopy: technical status and current indications. Endoscopy. 2006;38:1275-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Tian YS, Lee HY, Lim CS, Park J, Kim HM, Shin YN, Kim ES, Jeon HJ, Park SB, Cho BR. A two-photon tracer for glucose uptake. Angew Chem Int Ed Engl. 2009;48:8027-8031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci USA. 2003;100:7075-7080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1329] [Cited by in RCA: 1115] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 28. | Helmchen F. Miniaturization of fluorescence microscopes using fibre optics. Exp Physiol. 2002;87:737-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Bao H, Boussioutas A, Reynolds J, Russell S, Gu M. Imaging of goblet cells as a marker for intestinal metaplasia of the stomach by one-photon and two-photon fluorescence endomicroscopy. J Biomed Opt. 2009;14:064031. [PubMed] |
| 30. | Kalloo AN, Singh VK, Jagannath SB, Niiyama H, Hill SL, Vaughn CA, Magee CA, Kantsevoy SV. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 912] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
Peer reviewer: Kevin J Spring, Dr., PhD, Conjoint Gastro-enterology Laboratory, The Queensland Institute of Medical Research, the Bancroft Centre, Rm H07, PO Royal Brisbane Hospital, Herston, QLD 4029, Australia
S- Editor Tian L L- Editor O’Neill M E- Editor Li JY