Published online Jan 28, 2011. doi: 10.3748/wjg.v17.i4.506
Revised: September 27, 2010
Accepted: October 3, 2010
Published online: January 28, 2011
AIM: To evaluate cladribine [2-chlorodeoxyadenosine (2-CdA)] therapy in refractory celiac disease (RCD) II.
METHODS: An open-label cohort-study of RCD II patients treated with 2-CdA was performed between 2000 and 2010. Survival rate, enteropathy associated T-cell lymphoma (EATL) occurrence, clinical course, and histological and immunological response rates were evaluated.
RESULTS: Overall, 32 patients were included with a median follow-up of 31 mo. Eighteen patients responded well to 2-CdA. Patients responsive to 2-CdA had a statistically significant increased survival compared to those who were unresponsive. The overall 3- and 5-year survival was 83% in the responder and 63% and 22% in the non-responder group, respectively. The overall 2-year clinical, histological and immunological response rates were 81%, 47% and 41%, respectively. Progression into EATL was reported in 16%, all of these patients died.
CONCLUSION: Treatment of RCD II with 2-CdA holds promise, showing excellent clinical and histological response rates, and probably less frequent transition into EATL.
- Citation: Tack GJ, Verbeek WH, Al-Toma A, Kuik DJ, Schreurs MW, Visser O, Mulder CJ. Evaluation of Cladribine treatment in refractory celiac disease type II. World J Gastroenterol 2011; 17(4): 506-513
- URL: https://www.wjgnet.com/1007-9327/full/v17/i4/506.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i4.506
Coeliac disease (CD) is characterized by a permanent state of intolerance to dietary gluten leading to an inflammatory T-cell response in the small intestine with mucosal injury in genetically susceptible individuals[1,2]. Although reduction of this intestinal inflammatory activity is usually seen upon strict adherence to a gluten free diet (GFD), a small percentage (2%-5%) of the adult-onset CD patients, especially those diagnosed above the age of 50 years, display a lack of response to such a diet. They are regarded as suffering from refractory celiac disease (RCD) when clinical and histological symptoms persist or recur after a former good response to a strict GFD, despite strict adherence to the diet for more than 12 mo, unless earlier intervention is necessary[3,4]. Immunologically, this syndrome can be subdivided into RCD I and RCD II, with immunophenotypically normal and aberrant intraepithelial T lymphocytes (IELs) in the small intestinal mucosa, respectively. Clonal expansion of these aberrant IELs, lacking the T-cell surface markers T cell receptor (TCR), CD3, CD4 and CD8[5], but expressing cytoplasmic CD3, is supposed to be responsible for the occurrence of enteropathy associated T-cell lymphoma (EATL)[4-6]. This type of lymphoma occurs in 60%-80% of RCD II patients within 5 years and has a poor prognosis with a 2-year survival of only 15%[7,8]. The latter is mainly due to incomplete response to currently available therapies, high rates of life-threatening complications such as perforation of the gut, and poor nutritional conditions[7,8]. Therefore, it is of utmost importance to evaluate new treatment strategies for RCD II in order to improve clinical course and prevent or delay progression to overt EATL.
Since the late 90’s, researchers have become increasingly interested in therapeutic alternatives for treating RCD II, however as far as has been published there is no standardised approach yet[9,10]. RCD II is, at least in part, resistant to most evaluated therapies so far[10-12]. Until 2005 in our tertiary referral centre for RCD II, patients were initially treated with conventional immunosuppressive drugs, mainly azathioprine and prednisone, and if clinically and histologically unresponsive cladribine [2-chlorodeoxyadenosine (2-CdA)] was prescribed. Since then, a modified treatment strategy has been initiated with cladribine being drug of first choice.
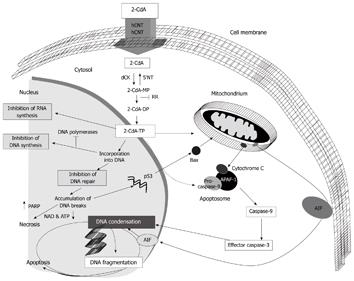
2-CdA is a synthetic purine nucleoside homologue being equally toxic to proliferating as to non-dividing lymphoid cells. Because of this unique feature it is supposed to be especially active against low-grade malignancies, including hairy cell leukaemia. Cladribine is metabolised into its pharmacologically active cladribine triphosphate, which induces apoptosis, necrosis and inhibition of DNA/RNA synthesis (Figure 1). Clinically, 2-CdA is of proven value in the treatment of a number of haematological malignancies and selected auto-immune disorders, including multiple sclerosis[13-15]. Our pilot studies showed that 2-CdA therapy[16] is feasible and well tolerated in these patients. Survival at that time seemed promising, but follow-up was short.
This analysis evaluates cladribine therapy in a large prospectively studied open-label cohort of RCD II patients, during a mean follow-up time of 3 years.
This cohort study includes reports of extended follow-up of 14 out of the 17 RCD II patients included in the open-label prospective phase I study performed by Al-Toma et al[16] with 18 new patients added. Three patients out of the previous study were not included in this study as they were followed over time outside our hospital. Between January 2000 and April 2010, 2-CdA was prescribed to 32 RCD II patients at the VU University Medical Centre in The Netherlands.
Patients diagnosed as having RCD II and treated with one or two courses of 2-CdA at the VU University Medical Centre were included. Cladribine was intravenously given in a dose of 0.1 mg/kg per day for 5 consecutive days. The diagnosis of RCD II was based on persisting or recurring clinical symptoms and small intestinal villous atrophy after a former good response to a strict GFD, despite strict adherence to the diet for more than 12 mo. Furthermore, the clinically validated cut-off value of more than 20% aberrant intraepithelial lymphocytes (IELs) detected by flow cytometric (FACS) analysis was used to distinguish RCD type I and type II[5]. Although a clonal TCR gamma rearrangement determined by PCR is still a widely accepted method to define RCD II, Verbeek et al[5] showed that the percentage of aberrant IELs detected by FACS analysis is a more accurate way to define RCD II. The presence of EATL was excluded by using selected investigations[17-20] and its diagnosis was confirmed according to the World Health Organization Classification of Tumors of Haematopoietic and Lymphoid tissues[21]. Furthermore, pre-treatment with immunomodulatory drugs within 6 mo or any experimental drug within 30 d of the study entry was not allowed.
Before and during follow-up after cladribine treatment a clinical assessment was carried out noting in particular signs and symptoms of malabsorption, body mass index (BMI), albumin, and haemoglobin (Hb). A nutritional screening was performed by a dietician who specialised in CD in almost all patients prior to treatment and nutritional support was given when indicated. Clinical remission was defined as improvement of the diarrhoea, abdominal discomfort and/or signs of malabsorption, combined with at least 2 out of the following parameters of intestinal integrity within the normal range or an improvement of ≥ 1 point: (1) Hb; (2) BMI; and (3) albumin. Multiple duodenal biopsies were taken by upper gastrointestinal endoscopy in order to detect histopathological abnormalities and to perform immunophenotyping of IELs by FACS analysis at different time points (planned at 3, 6 and 12 mo, and then every year during follow-up). Isolation of small intestinal T-lymphocytes and staining for immunophenotyping were performed as previously described[5]. Complete histological remission has been defined as a normalisation of the architecture of the duodenum, classified as a Marsh 0 or I lesion according to the Modified Marsh classification[22]. A decline of 20% or more in the percentage of aberrant IELs was considered a significant immunological remission. In addition, survival rate and EATL occurrence were evaluated during follow-up.
Furthermore, following on from Verbeek et al[10] who hypothesised that pre-treatment with common immunosuppressive drugs, including azathioprine and prednisone might influence the response to 2-CdA treatment, this study compared RCD II patients pre-treated with immunosuppressive agents before 2-CdA was prescribed (group I) to those treated with upfront 2-CdA (group II).
Approval for this open label study protocol was obtained from the local ethics committee in 2000 and all patients gave their informed consent.
Quantitative data were expressed as medians and means. Kaplan-Meier survival curves were constructed using SPSS software (SPSS Inc., Chicago, Illinois, USA). In addition, the log rank test was used to assess the statistical significance. A P value of less than 0.05 was considered statistically significant.
In total, 32 RCD II patients who were treated with 2-CdA were included with a median follow-up time of 31 mo (range 4-120 mo). Patient characteristics (Table 1) show a median age of > 50 years, with a male predominance. Prior to the start of 2-CdA therapy, 10 patients failed to respond clinically and histologically to conventional immunosuppressive drugs (defined as group I), including high dose prednisone in 2 and azathioprine or 6-thioguanine added to prednisone in 8 patients. The remaining 22 patients (defined as group II) were initially treated with 2-CdA following diagnosis of RCD.
| Gender (M:F) | 18:14 |
| Age of CD diagnosis: median in years (range) | 58.5 (38-74) |
| Age of RCDII diagnosis: median in years (range) | 64 (42-78) |
| Age at the start of 2-CdA treatment: median in years (range) | 64 (45-78) |
| Treatment prior to 2-CdA | |
| None | 22 |
| Immunosuppressive drugs | 10 |
| Follow-up time: median in months (range) | 31 (4-120) |
| HLA-DQ status | |
| DQ2 heterozygous | 17 |
| DQ2 homozygous | 12 |
| DQ2 and DQ8 | 2 |
| Unknown | 1 |
| TCR-γ gene rearrangement | |
| Monoclonal | 18 |
| Polyclonal | 9 |
| Unknown | 5 |
| Marsh classification before 2-CdA | |
| Marsh IIIA | 13 |
| Marsh IIIB | 11 |
| Marsh IIIC | 8 |
| Intestinal aberrant IELs before 2-CdA: median in % (range) | 61 (21-96) |
| Body mass index before 2-CdA: median in kg/m2 (range) | 21 (16-27) |
| Albumin level before 2-CdA: median (range, reference value 35-52 g/L) | 36 (23-47) |
| Haemoglobin level before 2-CdA: median in mmol/L (range) | 7.8 (6.0-9.8) |
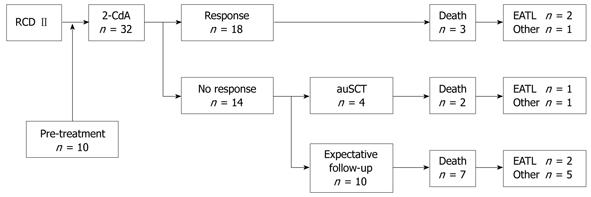
In agreement with our previous study, 2-CdA was feasible and well tolerated without serious adverse events[10]. Overall, 18 (56%) of the RCD II patients were responsive to one or two courses of 2-CdA based on the clinical and complete histological and/or immunological response (Figure 2). Seven showed a clinical and histological response, 4 a clinical and immunological response, and 7 a clinical, histological and immunological remission. Out of the remaining 14 patients unresponsive to 2-CdA, 2 were admitted for a second course of 2-CdA in the last 6 mo and in anticipation of evaluation of response. Six non-responsive patients were evaluated for high dose chemotherapy followed by autologous hematopoietic stem cell transplantation (auSCT). In 2 of them stem cells could not be harvested, therefore 4 patients were actually transplanted[23]. Another 6 patients had an expectative follow-up, 2 of them had an exacerbation after an initial response to 2-CdA for almost 2 and 3.5 years.
Table 2 shows the clinical, histological and immunological 1- and 2-year response rates to 2-CdA treatment. In total, clinical remission was observed in 26 (81%), complete histological remission in 15 (47%) and immunological remission in 13 (41%) of the RCD II patients. The median levels of BMI, Hb and albumin increased from 20.9 kg/m2, 7.8 mmol/L and 36 g/L at baseline to 23 kg/m2, 7.9 mmol/L and 39 g/L at the end of follow-up, respectively. In addition to the 15 patients in whom complete histological remission, defined as Marsh 0 or I, was observed, 2 patients had a partial histological remission from Marsh 3B and 3C lesions at baseline to Marsh 2 and 3A at the end of follow-up respectively. The median percentage of intestinal aberrant IELs before 2-CdA treatment was 61% and declined to 56% after 2-CdA treatment. The time to a 50% response rate was 3 years. Approximately 33% of the patients lacked a clonal TCR-gamma gene rearrangement, although all patients had an aberrant IELs of more than 20%. A statistical significance between the percentage of aberrant IELs and the clonality status was not found.
| Treatment(n) | Response (%) | Time to a 50% response rate (mo) | |||
| 12 mo | 24 mo | ||||
| Histological response | Group I | 10 | 13 | 25 | 36 |
| Group II | 22 | 20 | 58 | 24 | |
| Overall | 32 | 10 | 47 | 36 (3-96) | |
| Immunological1 response | Group I | 10 | 11 | 11 | > 60 |
| Group II | 22 | 52 | 58 | 12 | |
| Overall | 32 | 39 | 41 | 41 (2-96) | |
| Clinical2 response | Group I | 10 | 67 | 67 | 6 |
| Group II | 22 | 95 | 95 | 3 | |
| Overall | 32 | 81 | 81 | 3 (2-72) | |
| Overall response | Group I | 10 | 22 | 38 | 36 |
| Group II | 22 | 28 | 43 | 30 | |
| Overall | 32 | 25 | 41 | 36 | |
Analysis of the overall response in patients pre-treated with immunosuppressive drugs prior to 2-CdA (group I) and those with up-front 2-CdA treatment showed no statistical significance (log rank, P = 0.856). Immunological (log rank, P = 0.030) response, however, was significantly higher in group II. In addition, a trend towards a higher clinical response in group II (log rank, P = 0.058) was found. Yet, for the histological response rate a statistical significance was not observed (Table 2). Cox-regression analysis showed that the following parameters have no predictive value for response to 2-CdA: age at 2-CdA infusion (P = 0.06), sex (P = 0.60), TCR-gamma-clonality (P = 0.604), and percentage of aberrant IELs (P = 0.646), degree of small intestinal villous atrophy (P = 0.610), BMI (P = 0.095), albumin (P = 0.936) and Hb (P = 0.953) before treatment.
The overall median survival was almost 4.5 years. In total, 37% (12/32) of all included patients died, due to an EATL in 42% (5/12). All patients diagnosed with EATL (16%) died subsequently, having a median survival of only 4.4 mo (range 0-12 mo) after diagnosis.
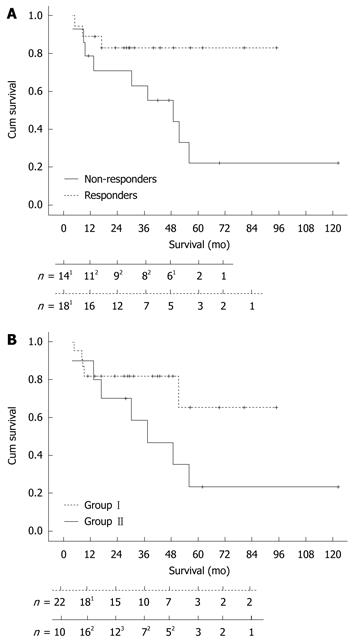
The survival curve (Figure 3A) shows a statistically significant difference (P = 0.037) between responders and non-responders to 2-CdA treatment. The 3- and 5-years survival rate was 83% in the responding group and 63% and 22% in the non-responding group, respectively. One-sixth (3/18) of the responders died, 1 due to refractory disease status and 2 were diagnosed with EATL within 3 and 9 mo after the last infusion of 2-CdA. Approximately 65% (9/14) of the non-responders died, 3 as a consequence of developing an EATL within 8, 12 and 44 mo of 2-CdA treatment (Figure2). The exceptionally delayed progression into EATL in the latter patient might be explained by treatment with high dose of chemotherapy followed by auSCT 8 mo after 2-CdA treatment. The remaining 6 unresponsive patients died as a result of refractory disease status; 1 treated with chemotherapy and auSCT died after 1 year of follow-up and 5 with expectative follow-up died despite lack of response to 2-CdA after a median follow-up of 18 mo (range: 4.5-60 mo). The patient in the latter group who died 5 years after 2-CdA treatment had an exacerbation after an initial remission to 2-CdA for 3.5 years and then refused further treatment.
In addition, there was no statistical significance (P = 0.23) between the overall survival in the pre-treatment group (I) and the upfront 2-CdA treatment group (II) as depicted in Figure 3B.
Approximately half of RCD II patients have an unfavourable clinical course as a consequence of non or partial response to current available therapies and subsequently the progression into EATL[7,8]. For that reason clinicians have become increasingly interested in treating this condition alternatively and more effectively. However, there is no standardised approach reported so far. In this study a large cohort of RCD II patients treated with 2-CdA has been analyzed.
In agreement with Al-Toma et al[16], this report describing almost twice as many patients with extended follow-up, showed that 2-CdA is indeed feasible and well tolerated without serious adverse events after short and long-term follow-up. Our data show that RCD II patients responsive to 2-CdA treatment have a statistically significant increased survival compared to those who are unresponsive. Although the median follow-up time was only 31 mo, after 1.5 year of follow-up none of the responders died compared to 4 non-responders, indicating a clear difference in survival rate. The overall 5-year survival rate of RCD II patients in this analysis is 46% so far, which is in line with other single-centre studies[11,12], and is expected to improve after extended follow-up. Previous reports included RCD II patients treated with diverse steroid-like drugs[11,12], whereas in this study 10 RCD II patients unresponsive to such immunosuppressive drugs were included as well. However, unexpectedly the overall survival did not significantly differ between patients pre-treated with conventional immunosuppressive drugs (group I) compared to those with up-front 2-CdA treatment (group II). Nevertheless, a clearly increased median survival rate in group II was observed. The lack of statistical significance might be due to the small cohort included in group I. In addition, some of the RCD II patients treated with conventional immunosuppressive drugs died before 2-CdA treatment could be initiated as previously reported by our group[24] and were therefore not included in the current analysis. Apart from favouring clinical course and increasing survival, preventing EATL represents the ultimate goal of treating RCD II. Although treatment with 2-CdA could not prevent progression into EATL, compared to previous reports showing EATL in 32%-52% of the RCD II patients within 5 years[7,11], our results showed a much lower rate of lymphomagenesis (16%), yet approximately 50% had a 5-year follow-up.
Furthermore, our open-label and observational data showed that 2-CdA is an effective treatment in obtaining a clinical and immunohistological response in more than half of the RCD II patients. Previous studies reported a good clinical response after treatment, but histological response rates up to only 30% were found depending on the type of treatment[11,12]. Consistent with these findings, a clinical response rate of 81% was found. The potential advantage of 2-CdA, however, seems to be a good histological response. In fact, histologic healing (Marsh 0 or I) was observed in almost half of the cases and a partial response of at least two Marsh scores in another 3 cases. Although not statistically significant, the 2-year histological response rate in up-front 2-CdA group (II) was twice that of the pre-treatment group (I), possibly indicating a beneficial effect of up-front 2-CdA treatment.
Furthermore, Malamut et al[11] recently described a 74% steroid-dependency in a large cohort of RCD II patients treated with corticosteroids. Since there is no need for corticosteroids if 2-CdA therapy is prescribed, steroid-dependency and its complications will not occur.
In this cohort, the proportion of RCD II patients lacking a clonal TCR-gamma gene rearrangement was relatively high (33%) compared with that reported in other studies[4,11,12], although all patients had more than 20% aberrant IELs determined by FACS analysis. This discrepancy might be the result of some technical aspects of the DNA analysis, for instance a reduced DNA quality due to formalin fixation of the biopsies and a limited sensitivity of PCR in the case of a low percentage of aberrant IELs. Therefore, the tested polyclonal status in this cohort is most likely overestimated. However, we have previously shown[5] that flow cytometry of aberrant IELs is superior to clonality analysis for risk stratification in RCD II.
Approximately 40% of the RCD II patients showed an immunological response after 2-CdA treatment, defined as a decrease of more than 20% of the aberrant intestinal IELs determined by FACS analysis. However, the majority still showed more than 30% aberrant IELs during follow-up. Expansion of these aberrant T-lymphocytes, which reside in the intraepithelial as well as lamina propria layer of the small intestine[25], is generally accepted as the culprit factor in the progression into EATL. Conversely, in our series the persisting high percentage of aberrant mucosal T-lymphocytes did not correlate with the relatively low EATL occurrence found during the time of follow-up so far. Unfortunately, reports from other centres on the immunological response determined by FACS analysis after treatment are lacking. Whereas the percentage of aberrant IELs is established to be important in the diagnostic work-up for distinguishing RCD type I and II[5], apparently it is questionable whether this percentage is a predictive marker for progression into EATL during monitoring of the therapeutic response. Further research is mandatory to further elucidate EATL risk stratification. Immunophenotyping of the aberrant T-cells by FACS analysis of small intestinal biopsies and probably also genotyping seem to be appropriate methods to search for predictive markers. In addition, future studies on quantifying the mass of aberrant IELs using immuno-PET techniques, instead of the currently determined percentage of aberrant IELs, given the relatively equal depletion of normal and aberrant IELs upon 2-CdA treatment, have to be conducted.
In the current analysis approximately half of the RCD II patients were unresponsive to 2-CdA, for yet unknown reasons. A significant association with the degree of mucosal villous atrophy, the percentage of aberrant IELs, and levels of BMI, Hb and albumin before 2-CdA treatment, clonal TCR-gamma gene rearrangement and HLA-DQ status was not revealed. Although dose-finding studies with 2-CdA infusion in refractory celiac disease are not conducted, clinical dose-finding studies in lymphoproliferative diseases showed good response rates with an identical treatment schedule[13,26]. In addition, compared to intravenous infusions and subcutaneous injections which provide identical plasma 2-CdA levels, oral administration has a much lower bioavailability (approximately 40%) due to degradation through acid in the stomach and intestinal bacteria[14]. A higher dose and/or a prolonged treatment schedule might result in a higher response rate, yet the maximum tolerated dose established in lymphoproliferative diseases without serious adverse events was 0.1 mg/kg per day for 7 d[13]. A recent clinical trial of oral cladribine for relapsing multiple sclerosis showed that short-course and high dose (3.5 mg/kg) therapy is effective, yet lymphocytopenia is frequently reported[15]. Furthermore, the high sensitivity of hairy cell leukaemia (HCL) to treatment with 2-CdA showing low resistance levels is hypothesised to be the result of p53-dependent pathways required for killing resting cells and its inhibitory effect on the cholesterol metabolism which is highly active in HCL cells[14]. Physiological conditions such as increased repair of DNA, increased anti-apoptotic effects and decreased activation of deoxycytidine kinase, an enzyme required for the cytotoxicity of 2-CdA, might be contributing factors to resistance as well[27]. Whether the same results regarding administration route, treatment schedule and resistance pattern of 2-CdA also correlate with such good response rates in RCD II, remains to be further elucidated. The registration of oral 2-CdA for multiple sclerosis in Europe, might be a further step forward towards the application of this drug in RCD as well as some other gastroenterological diseases refractory to currently available therapies, including Crohn’s disease, ulcerative colitis and autoimmune hepatitis.
In conclusion, 2-CdA appears to be a promising treatment in RCD II. This analysis showed excellent clinical and histological response rates after 2-CdA treatment. Furthermore, 2-CdA therapy does not necessitate the additional use of corticosteroids and subsequently prevents steroid-dependency and its complications. Although EATL could not be fully prevented, its incidence was restricted to 16%. Multicentre, randomised clinical trials with 2-CdA and/or other new treatment options are mandatory to standardise the treatment strategy for RCD II, in order to further decrease morbidity and mortality in this patient group.
A small percentage (2%-5%) of patients with adult-onset celiac disease, especially those diagnosed above the age of 50, show a lack of response to a gluten-free diet. They are diagnosed as suffering from refractory celiac disease (RCD) when clinical and histological symptoms persist or recur after a former good response to a strict gluten-free diet and despite strict adherence to the diet for more than 12 mo, unless earlier intervention is necessary. RCD can be subdivided into type I with phenotypically normal and type II with aberrant intraepithelial T lymphocytes in the small intestinal mucosa. Patients with RCD I have a less dismal prognosis compared with those diagnosed as having RCD II: the 5-year survival rates are 96% and 58%, respectively. As the majority of RCD type II patients are unresponsive to common immunosuppressive therapy, it is of utmost importance to evaluate new treatment strategies. Apart from clinical and histological remission, the ultimate goal of treatment is to prevent progression into a lethal enteropathy associated T-cell lymphoma (EATL) which occurs in more than half the patients within 4-6 years.
This is the first report evaluating a standardised treatment strategy for RCD type II with cladribine [2-chlorodeoxyadenosine (2-CdA)] therapy in a large cohort (n = 32) with a long-term follow-up (median 31 mo).
This report shows that cladribine was feasible and well tolerated without serious adverse events. More than half of the RCD II patients were responsive to cladribine therapy and had a significantly increased 3 and 5 year survival compared to those who were unresponsive. In line with previous reports, very high clinical response rates were observed. The potential advantage of cladribine, however, seems to be a good histological response. In fact, histologic healing (Marsh 0 or I) was observed in almost half of the cases and a partial response of at least two Marsh scores in another 3 cases, compared to reported histological response rates up to only 30%. Although EATL could not be fully prevented, its incidence was restricted to 17%. Furthermore, 2-CdA therapy does not necessitate the additional use of corticosteroids and subsequently prevents steroid-dependency and its complications.
The results suggest that cladribine therapy may represent a promising option for RCD II, however, multicentre randomised clinical trials with 2-CdA and/or other new treatment options are mandatory to standardise the treatment strategy for this group, in order to further decrease morbidity and mortality in this patient group.
Aberrant IELs: Intraepithelial T lymphocytes are considered aberrant when expressing cytoplasmic CD3, but lacking surface expression of the T-cell markers CD3, CD4, CD8 and the T-cell receptor. 2-CdA: Cladribine, 2-chlorodeoxyadenosine, a synthetic purine nucleoside homologue. EATL: Enteropathy associated T-cell lymphoma. RCD: Refractory celiac disease. Immunologically, this disorder can be subdivided into two types: type I without and type II with aberrant IELs in the small intestinal mucosa. TCR: T-cell receptor.
In this manuscript, the authors reported an open-label study on the treatment of type II refractory coeliac disease (RCD II) with cladribine (2-chlorodeoxyadenosine) in a cohort of 32 patients. After a mean follow up time of 3 years, cladribine, which was given to 22 patients as the first line treatment and 10 patients who failed to respond to conventional immunosuppressive drugs, was shown to have induced apparent clinical, histological and/or immunological response in over half of the patients. In addition, all the responders had a significantly increased 3 and 5 year survival compared to those who were unresponsive although progression of RCD-II to enteropathy associated T-cell lymphoma had not been prevented and overall 5 year survival (46%) was not different from that reported by other centres using alternative therapies. This open-label trial was uncontrolled. However, a favourable outcome was observed in most of patients treated with cladribine. The results suggest that cladribine therapy may represent a promising option for RCD II patients.
| 1. | Sollid LM. Molecular basis of celiac disease. Annu Rev Immunol. 2000;18:53-81. |
| 3. | Daum S, Cellier C, Mulder CJ. Refractory coeliac disease. Best Pract Res Clin Gastroenterol. 2005;19:413-424. |
| 4. | Cellier C, Delabesse E, Helmer C, Patey N, Matuchansky C, Jabri B, Macintyre E, Cerf-Bensussan N, Brousse N. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet. 2000;356:203-208. |
| 5. | Verbeek WH, Goerres MS, von Blomberg BM, Oudejans JJ, Scholten PE, Hadithi M, Al-Toma A, Schreurs MW, Mulder CJ. Flow cytometric determination of aberrant intra-epithelial lymphocytes predicts T-cell lymphoma development more accurately than T-cell clonality analysis in Refractory Celiac Disease. Clin Immunol. 2008;126:48-56. |
| 6. | Daum S, Hummel M, Weiss D, Peters M, Wiedenmann B, Schäper F, Stein H, Riecken EO, Foss H. Refractory sprue syndrome with clonal intraepithelial lymphocytes evolving into overt enteropathy-type intestinal T-cell lymphoma. Digestion. 2000;62:60-65. |
| 7. | Al-Toma A, Verbeek WH, Hadithi M, von Blomberg BM, Mulder CJ. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut. 2007;56:1373-1378. |
| 8. | Bishton MJ, Haynes AP. Combination chemotherapy followed by autologous stem cell transplant for enteropathy-associated T cell lymphoma. Br J Haematol. 2007;136:111-113. |
| 9. | Daum S, Ullrich R, Heise W, Dederke B, Foss HD, Stein H, Thiel E, Zeitz M, Riecken EO. Intestinal non-Hodgkin’s lymphoma: a multicenter prospective clinical study from the German Study Group on Intestinal non-Hodgkin’s Lymphoma. J Clin Oncol. 2003;21:2740-2746. |
| 10. | Verbeek WH, Schreurs MW, Visser OJ, von Blomberg BM, Al-Toma A, Mulder CJ. Novel approaches in the management of refractory celiac disease. Expert Rev Clin Immunol. 2008;4:205-219. |
| 11. | Malamut G, Afchain P, Verkarre V, Lecomte T, Amiot A, Damotte D, Bouhnik Y, Colombel JF, Delchier JC, Allez M. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology. 2009;136:81-90. |
| 12. | Rubio-Tapia A, Kelly DG, Lahr BD, Dogan A, Wu TT, Murray JA. Clinical staging and survival in refractory celiac disease: a single center experience. Gastroenterology. 2009;136:99-107; quiz 352-353. |
| 13. | Robak T, Korycka A, Robak E. Older and new formulations of cladribine. Pharmacology and clinical efficacy in hematological malignancies. Recent Pat Anticancer Drug Discov. 2006;1:23-38. |
| 14. | Juliusson G, Liliemark J. Purine analogues: rationale for development, mechanisms of action, and pharmacokinetics in hairy cell leukemia. Hematol Oncol Clin North Am. 2006;20:1087-1097. |
| 15. | Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, Soelberg Sørensen P, Vermersch P, Chang P, Hamlett A, Musch B. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362:416-426. |
| 16. | Al-Toma A, Goerres MS, Meijer JW, von Blomberg BM, Wahab PJ, Kerckhaert JA, Mulder CJ. Cladribine therapy in refractory celiac disease with aberrant T cells. Clin Gastroenterol Hepatol. 2006;4:1322-1327; quiz 1300. |
| 17. | Mallant M, Hadithi M, Al-Toma AB, Kater M, Jacobs M, Manoliu R, Mulder C, van Waesberghe JH. Abdominal computed tomography in refractory coeliac disease and enteropathy associated T-cell lymphoma. World J Gastroenterol. 2007;13:1696-1700. |
| 18. | Hadithi M, Al-toma A, Oudejans J, van Bodegraven AA, Mulder CJ, Jacobs M. The value of double-balloon enteroscopy in patients with refractory celiac disease. Am J Gastroenterol. 2007;102:987-996. |
| 19. | Hadithi M, Mallant M, Oudejans J, van Waesberghe JH, Mulder CJ, Comans EF. 18F-FDG PET versus CT for the detection of enteropathy-associated T-cell lymphoma in refractory celiac disease. J Nucl Med. 2006;47:1622-1627. |
| 20. | Rondonotti E, Villa F, Mulder CJ, Jacobs MA, de Franchis R. Small bowel capsule endoscopy in 2007: indications, risks and limitations. World J Gastroenterol. 2007;13:6140-6149. |
| 21. | Isaacson PG, Chott A, Ott G, Stein H. Enteropathy-associated T-cell lymphoma. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer 2008; 289-291. |
| 22. | Rostami K, Kerckhaert J, Tiemessen R, von Blomberg BM, Meijer JW, Mulder CJ. Sensitivity of antiendomysium and antigliadin antibodies in untreated celiac disease: disappointing in clinical practice. Am J Gastroenterol. 1999;94:888-894. |
| 23. | Tack GJ, Wondergem MJ, Al-Toma A, Verbeek WH, Schmittel A, Machado MV, Perri F, Ossenkoppele GJ, Huijgens PC, Schreurs MW. Auto-SCT in refractory celiac disease type II patients unresponsive to cladribine therapy. Bone Marrow Transplant. 2010;Epub ahead of print. |
| 24. | Goerres MS, Meijer JW, Wahab PJ, Kerckhaert JA, Groenen PJ, Van Krieken JH, Mulder CJ. Azathioprine and prednisone combination therapy in refractory coeliac disease. Aliment Pharmacol Ther. 2003;18:487-494. |
| 25. | Verbeek WH, von Blomberg BM, Coupe VM, Daum S, Mulder CJ, Schreurs MW. Aberrant T-lymphocytes in refractory coeliac disease are not strictly confined to a small intestinal intraepithelial localization. Cytometry B Clin Cytom. 2009;76:367-374. |
| 26. | Liliemark J, Albertioni F, Juliusson G, Eksborg S. A limited sampling strategy for estimation of the cladribine plasma area under the concentration versus time curve after intermittent i.v. infusion, s.c. injection, and oral administration. Cancer Chemother Pharmacol. 1996;38:536-540. |
| 27. | Peters GJ, Jansen G. Resistance to antimetabolites. Principles of antineoplastic drug development and pharmacology. New York: Marcel Dekker, Inc 1996; 543-585. |
Peer reviewer: Hong-Xiang Liu, PhD, Department of Pathology, Division of Molecular Histopathology, University of Cambridge, Box 231, Level 3, Lab Block, Addenbrooke’s Hospital, Hills Road, Cambridge CB2 2QQ, United Kingdom
S- Editor Wang YR L- Editor O’Neill M E- Editor Ma WH