Published online Oct 21, 2011. doi: 10.3748/wjg.v17.i39.4434
Revised: April 5, 2011
Accepted: April 12, 2011
Published online: October 21, 2011
AIM: To investigate the effects of the somatostatin analogue, octreotide, on maltose and sucrase activities and expression of glucose transporter type 2 (GLUT2) in obese rat intestinal mucosa.
METHODS: We divided 49 Sprague-Dawley rats into a group of 31 high fat diet-induced obese rats and a group of 18 normal controls. The obese rats were separated into an octreotide treated group of 16 rats and an obese group of 15. The intervention group was injected with octreotide at 40 μg/kg body weight every 12 h for 8 d. Rat body weight was measured weekly to calculate Lee’s index. After euthanization, maltase and sucrase activities in the small intestine were measured by activity assays, and the fasting plasma glucose level was measured. The expression of GLUT2 in small intestinal mucosa was analyzed by immunohistochemistry, reverse transcriptase polymerase chain reaction and Western blotting assays.
RESULTS: Body weight, Lee’s index, fasting plasma glucose level, maltase activity in small intestinal mucosa, mucosa and apical GLUT2, GLUT2 mRNA and protein expression levels were all significantly higher in the obese group than in the normal control group (605.61 ± 141.00 vs 378.54 ± 111.75, 337.61 ± 10.82 vs 318.73 ± 20.10, 8.60 ± 1.38 vs 7.33 ± 0.70, 156.01 ± 58.81 vs 50.43 ± 30.49, 390 744.2 ± 62 469.21 vs 170 546.50 ± 50 646.14, 26 740.18 ± 3809.60 vs 354.98 ± 57.19, 0.26 ± 0.11 vs 0.07 ± 0.02, and 2.08 ± 0.59 vs 1.27 ± 0.38, respectively, all P < 0.01). Sucrase activity did not differ between the two groups. Octreotide intervention significantly decreased the body weight and fasting plasma glucose level of obese rats (508.27 ± 94.39 vs 605.61 ± 141.00, 7.58 ± 1.51 vs 8.60 ±1.38, respectively, all P < 0.05). The intestinal mucosa and apical GLUT2, expression of GLUT2 mRNA and protein were also significantly lower in the octreotide intervention group than in the obese group (269 975.2 ± 53 730.94 vs 390 744.2 ± 62 469.21, 3758.06 ±364.51 vs 26 740.18 ± 3809.60, 0.08 ± 0.02 vs 0.26 ±0.11, and 1.31 ± 0.27 vs 2.08 ± 0.59, respectively, all P < 0.01).
CONCLUSION: High fat diet-induced obesity is associated with elevated intestinal maltase activity, GLUT2 expression, and permanent apical GLUT2 in the small intestinal mucosa of rats. Octreotide can inhibit these effects.
- Citation: Wei N, Liu R, Ou Y, Li X, Qiang O, Guo W, Tang CW. Effects of octreotide on glucose transporter type 2 expression in obese rat small intestine. World J Gastroenterol 2011; 17(39): 4434-4439
- URL: https://www.wjgnet.com/1007-9327/full/v17/i39/4434.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i39.4434
Recently, obesity has become a worldwide health issue, as it increases the risk of a variety of human medical consequences[1] such as type 2 diabetes, cardiovascular and cerebrovascular disease. Nutritional obesity is a metabolic disorder involving chronic imbalance of energy. Feeding a high fat diet to rodents normally results in disorders of glucose metabolism, and leads to impaired glucose tolerance or diabetes[2].
Carbohydrates are the main source of energy in rats. With the exception of monosaccharides, which can be absorbed directly into the intestinal mucosa, carbohydrates and their intermediate metabolites must be hydrolyzed to disaccharides in the gastrointestinal tract. The disaccharides then broken down to glucose through maltose and sucrose on the surface of intestinal villi. There are two mechanisms of intestinal glucose absorption[3]. When the luminal glucose level is lower than the blood glucose level, absorption occurs via the sodium-dependent glucose transporter 1 (SGLT-1) through classical active transport. Once the glucose concentration in the intestine is beyond the transport saturation of SGLT-1, the diffusion is mediated primarily through the transient insertion of glucose transporter type 2 (GLUT2) into the apical membrane[3].
Somatostatin (SST), a multifunctional gut peptide synthesized and released by intestinal endocrine cells (D cells), regulates the physiological function of intestinal epithelial cells and immune cells, as well as gastric motor activity[4,5]. Somatostatin’s effects are mediated by the SST receptor (SSTR) which is located in the cell membrane. Somatostatin secreted from the intestine can thus suppress replication of gastrointestinal epithelial cells. The clinical utility of somatostatin is limited by its short half-life of about two minutes[6]. Octreotide is an artificial synthetic analogue of SST that is more convenient to use and has a longer half-life, duration of activity and fewer side effects.
The expression of SGLT-1 in intestinal mucosa is significantly elevated in high-fat-diet-induced obese rats, and octreotide can dramatically inhibit the expression of SGLT-1 (unpublished results). However, it is unknown whether obesity induced by a high fat diet is associated with maltase and sucrase activities and expression of GLUT2. It is also unknown whether octreotide can influence maltase and sucrase activities and GLUT2 expression. Thus, the authors conducted an experiment to investigate these questions.
All experiments were approved by the Institutional Animal Care and Use Committee of Sichuan University (Chengdu, China).
We used 66 healthy male 21-d-old Sprague-Dawley (SD) rats which had been weaned for 3 d. All animals were obtained from the Animal Center of Sichuan University. After adaptive feeding for 3 d, rats were placed into two groups, one group of 18 (the normal control group) fed with standard chow (290 kcal/100 g, in line with the People’s Republic of China National Standard GB 14924-2001), and another group of 48 fed with high-fat chow (430 kcal/100 g).
Food and water were supplied ad libitum, and the animals were housed in independently ventilated cages on a 12:12-h light: dark schedule and kept at 20 °C-25 °C. They were weighed and measured for body and tail length each week for 24 wk. After 24 wk, rats from the high-fat chow group with body weight at least 1.4 times the mean body weight of the normal control group were selected as obese rats. Obese rats were then placed into two groups, an obese group of 16 rats, and an octreotide-treated group of 15 rats. The octreotide-treated group was injected subcutaneously with octreotide (40 g/kg body weight) every 12 h for 8 d. Rat body weight was measured weekly to calculate Lee’s index [body weight (g)1/3× 1000/body length (cm)].
At the end of the experiment, after fasting for 12 h, rats were euthanized intraperitoneally with 2% sodium pentobarbital. The small intestine was removed from each rat. The mucosa from 15 cm of the small intestine was collected by scraping with a glass slide and kept at -80 °C for measurement of sucrase and maltase activities and Western blot analysis. Another 1-cm section of small intestine was kept at -140 °C until total RNA extraction. Plasma and serum were collected for blood glucose measurement. Blood glucose levels were measured by the enzymatic method. Each specimen was measured in duplicate.
Sucrase and maltase activities were measured using a sucrase and maltase activity assay kit (Jiancheng Bio-Engineering Institute, Nanjing, China) according to the manufacturer’s instructions. The sucrase and maltase activity unit was defined as the nanomoles of disaccharide hydrolyzed per milligram of protein in 1 min (U/mg protein, 37 °C, pH = 6.0), and activity was calculated as follows:
The small intestine mucosa was homogenized in phosphate buffered saline (PBS, pH = 7.4) with a homogenizer. After centrifugation at 1050 ×g, 4 °C for 5 min, the supernatant was used to measure sucrase and maltase activities. The protein concentrations in the homogenate were determined by a BCA Protein Assay Kit (Pierce Biotechnology, Inc., Rockford, IL, United States) according to the manufacturer’s instructions.
For the immunohistochemical detection of GLUT2, a paraffin-embedded tissue section was deparaffinized and antigen retrieval was performed at high pressure. For nonspecific blocking, 10% goat sera was added, each section was incubated for 20 min at 37 °C, then GLUT2 goat anti-rat polyclonal antibody was added (1:400; Santa Cruz Biotechnologies, Santa Cruz, CA, United States). After incubating overnight at 4 °C and rewarming to 37 °C, each section was stained with a ready-to-use streptavidin-catalase immunohistochemical reagent system for detection. The color reaction was developed with diaminobenzidine (DAB; Zhongshan Bioagent Company, Beijing, China). A semiquantitative immunohistochemical analysis of raw data with Image-Pro Plus 4.0 software (Media Cybernetics, Silver Spring, MD, United States) was used to score integrated optical density (IOD) for positive reaction area.
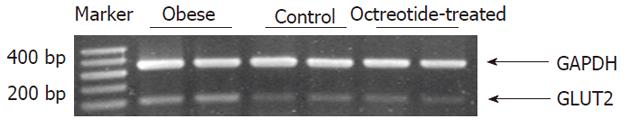
Total RNA was extracted from the frozen small intestine using Trizol reagent (Takara Bio-Engineering Co., Ltd., Kyoto, Japan). First-strand cDNA was synthesized from 2 mg of total RNA for each sample using reverse transcription kits (MBI, Fermentas Life Sciences Inc., Vilnius, Lithuania). The GLUT2 sense and antisense PCR primers were 5’-TGCTGGAAGAAGCGTAT CAG-3’ and 5’-GGCCAAGTAGGATGTGCCAG-3′, respectively; the GAPDH sense and antisense primers were 5’-CATGACCACAGTCCATGCCA-3’ and 5’-CACCCTGTTGCT CTAGCCATATTC-3’, respectively[7]. The PCR reaction was catalyzed using Taq DNA polymerase (MBI, Fermentas Life Sciences Inc., Vilnius, Lithuania). The thermal cycling parameters consisted of an initial denaturation step of 5 min at 94 °C, followed by 23 cycles of 1 min at 94 °C, 1 min at 63 °C, and 1 min at 72 °C. The final extension step lasted 7 min at 72 °C. PCR products were resolved by 2% agarose gel electrophoresis, and visualized by ethidium bromide staining. Densitometry was carried out using a Bio-Rad GelDoc image acquisition system and Quantity One (v4.3) quantitation software (Bio-Rad, Hercules, CA, United States).
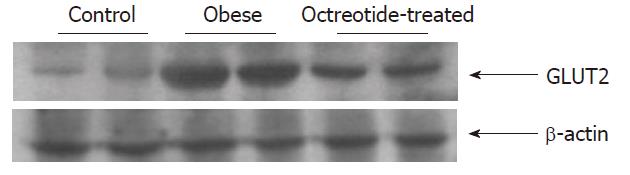
Small intestinal mucosa homogenate was prepared in lysis buffer containing protease inhibitor and phosphatase inhibitor (KeyGEN Biological Company, Nanjing, China). The protein concentrations were determined by a BCA Protein Assay Kit (Pierce Biotechnology, Inc., Rockford, IL, United States). The extracted protein (60 μg) was incubated in loading buffer and heated at 100 °C for 5 min. Samples were loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel, then transferred electronically to polyvinylidene difluoride membranes (Millipore, Bedford, MA, United States). The membranes were incubated with a 1:2000 dilution of rabbit polyclonal GLUT2 antibody (Millipore, Bedford, MA, United States) at 4 °C overnight. The membranes were then washed three times in blocking solution and incubated with a secondary antibody (Santa Cruz Biotechnologies, Santa Cruz, CA, United States, 1:50000). The signals were developed using Super-Signal West Pico chemiluminescent substrate (Pierce, Rockford, IL, United States). Band densities were quantified using Quantity One software 4.3.1 (Bio-Rad, Hercules, CA, United States). Each value was expressed as the ratio of the IOD of the GLUT2 band to that of β-actin.
Data are presented as means and standard deviations. Groups were compared using analysis of variance or the t test. All tests were two-tailed and P≤ 0.05 was considered statistically significant. All data met the assumptions of the tests used to analyze them. All data were analyzed by statistical software SPSS 13.0 (SPSS Inc., United States).
The rats with high-fat-diet-induced obesity showed typical features of obesity, such as higher body weight, Lee’s index and blood glucose levels than those in the normal control group (P < 0.01) (Table 1). The octreotide-treated group had significantly lower body weight and blood glucose levels than those in the obese comparison group (P < 0.05) (Table 1). Maltase activity in the small intestinal mucosa of the obese group was significantly higher than that in the normal control group (P < 0.01) (Table 1).
| Control (n = 18) | Obese (n = 16) | Octreotide treated (n = 15) | |
| Body weight (g) | 378.54. ± 111.75 | 605.61 ± 141.00b | 508.27 ± 94.39bc |
| Lee’s index | 318.73 ± 20.10 | 337.61 ± 10.82b | 334.67 ± 16.56a |
| Glucose (mmol/L) | 7.33 ± 0.70 | 8.60 ± 1.38b | 7.58 ± 1.51c |
| Sucrase activity (U/mg protein) | 53.84 ± 17.98 | 48.90 ± 13.95 | 35.85 ± 21.31 |
| Maltase activity(U/mg protein) | 50.43 ± 30.49 | 156.01 ± 58.81b | 112.85 ± 51.86a |
| Mucosa GLUT2 (IOD) | 170546.5 ± 50646.14 | 390744.2 ± 62469.21b | 269975.2 ± 53730.94bd |
| Apical GLUT2 (IOD) | 354.98 ± 57.19 | 26740.18 ± 3809.60b | 3758.06 ± 364.51ad |
| GLUT2 mRNA (IOD) | 0.07 ± 0.02 | 0.26 ± 0.11b | 0.08 ± 0.02bd |
| Western-blotting (IOD) | 1.27 ± 0.38 | 2.08 ± 0.59b | 1.31 ± 0.29d |
GLUT2 distribution and expression in the intestinal mucosa were measured by immunohistochemistry. GLUT2 resided mainly in the basolateral membrane (BLM) in the small intestinal mucosa (Figure 1). GLUT2 expression in the obese group was significantly higher than that in the normal control group (P < 0.01). In the octreotide-treated group, GLUT2 expression was significantly lower than that in the obese group (P < 0.01) (Figure 1 and Table 1).
Apical GLUT2 expression in the obese group was significantly higher than that in the normal control group (P < 0.01). After octreotide intervention, the level of apical GLUT2 was significantly lower than that in the obese group (P < 0.01) (Figure 1 and Table 1).
Reverse transcriptase polymerase chain reaction and Western blotting showed that GLUT2 mRNA and protein expression levels in the intestinal mucosa of the obese group were significantly higher than those in the normal control group (P < 0.01). GLUT2 mRNA and protein expression levels were significantly lower in the octreotide-treated group than in the obese rats (P < 0.01) (Figures 2 and 3, Table 1).
Starch is the major source of food glucose, and its digestion to maltose requires amylases in the gastrointestinal tract. Disaccharides, such as maltose, must be hydrolyzed to glucose by disaccharidase before being absorbed directly. As the most essential disaccharides, maltose and sucrose are broken down in the top of the intestinal brush border membrane (BBM)[8], which hydrolyzes sugar to glucose and fructose and plays a vital role in the utilization of carbohydrate.
Maltase and sucrase activities are regulated by diet, and are enhanced by increasing substrate concentration[9]. Our finding that maltose activity was markedly higher in the obese group suggests there is more robust intestinal digestion of starch in obese rats. Ferraris[10] reported that sucrase activity increased 68% when sucrose was added to the diets of mice. We fed rats a high fat diet with some sucrose to establish an obese model. In contrast with Ferraris’s results, we found no significant difference in sucrase activity between the obese and normal control groups. A possible explanation for this is that a high fat diet can stimulate secretion of trypsin, which reduces sucrase activity by degrading sucrose[11]. Thus, the obese rats’ high fat diet may account for the lack of variability in sucrase activity between the two groups.
According to Kellett’s review[3], there are two mechanisms of intestinal glucose absorption. When the luminal glucose level is lower than the blood glucose level, absorption occurs via SGLT-1 through classical active transport. Once the glucose concentration in the intestine is beyond the transport saturation of SGLT-1, the diffusion is mediated primarily by transient insertion of GLUT2 into the apical membrane (the apical membrane is opposed to the BLM). Lostao et al[12] reported that the diffusive component was three to five times greater than the active component at high luminal glucose concentrations. Therefore, GLUT2 is a key transport protein for glucose in the intestine.
We found that SGLT-1 and GLUT2 expression in the intestinal mucosa, as well as fasting plasma glucose levels, were all higher in obese rats than in non-obese rats. These findings are consistent with those of others[2,13]. A prolonged high-fat diet may downregulate muscle insulin receptors, inducing insulin resistance or hyperinsulinemia[3,13], and eventually leading to higher fasting plasma glucose levels. Sterol response element-binding protein (SREBP)-1c may exert beneficial effects in transporting glucose out of hepatocytes into blood, contributing to hyperglycemia[14]. SREBP-1c may mediate glucose-stimulated GLUT2 gene expression upregulation by binding to the -84/-76 region to activate the GLUT2 promoter[14]. In diabetic rats, GLUT2 expression increased hyperglycemia in the liver[15,16], intestine, and pancreatic β-cells[16]. Our results suggest that along with the increase in fasting plasma glucose levels, GLUT2 mRNA and protein expression are enhanced in the intestinal mucosa of obese rats, which results in greater glucose digestive ability and eventually obesity.
We observed that obese rats fed a high fat diet showed greater insertion of apical GLUT2 than did non-obese controls. It has been proposed that GLUT2 normally resides at the BLM but it can insert within min into the apical membrane of the intestine in response to high glucose concentrations; this facilitated component of absorption is called the apical GLUT2 pathway[3]. GLUT2 translocates to the apical membrane by at least two signaling pathways: one related to calcium absorption via the nonclassical neuroendocrine L-type voltage-dependent calcium channel (Cav1.3), and the other activated by sweet taste receptors stimulated by natural sugars and artificial sweeteners[17].
After the activation of protein kinase C (PKC) βII, a large number of GLUT2 proteins are inserted into the apical membrane to transport glucose[3]. As glucose is absorbed and its concentration in the intestine decreases, the signaling system is reversed, and GLUT2 leaves the apical membrane to restore the condition of low luminal glucose concentrations[18]. However, permanent apical GLUT2 insertion in a pathological state may result in increased sugar absorption and eventually lead to obesity, insulin resistance and diabetes[3,17]. All rats in our experiment were denied food for 12 h before being euthanized to avoid high glucose concentrations in the intestine. Thus, we speculate that the apical GLUT2 in obese rats was a kind of pathological permanent insertion[3]. Insulin binding to its enterocyte receptor (IR) inhibits the insertion of apical GLUT2, resulting in rapid trafficking of GLUT2 away from both the apical membrane and the BLM into the cell[19]. This function of insulin could be impaired by a prolonged high fat diet, leading to the permanent insertion of apical GLUT2.
Octreotide, a type of octopeptide, is an artificial synthetic analogue of SST. It has a longer half-life than natural SST. It binds to SSTR2 with high affinity, and has intermediate affinity for SSTR3 and SSTR5[4]. According to the work of Aydede et al[20], octreotide was safe and effective when administered subcutaneously (at 100 μg/kg 12 h apart) for 2 wk in rats with portal hypertensive colopathy. Thus, octreotide at 40 μg/kg 12 h apart for 8 d in our experiment seems reasonable. Some studies reported that children with hypothalamic obesity and hyperinsulinaemic obese adults received octreotide at a dosage of 5-15 μg/kg per day or long-acting release octreotide 40 mg/28 d for 6 mo, respectively, could decrease body weight by inhibiting β-cell insulin secretion[21,22]. In the present study, we also observed decreased body weight in obese rats after octreotide treatment. Lustig et al[21] found that almost half of children with hypothalamic obesity developed gallstone or sludge formation after treatment with octreotide for about 6 mo. However, ursodeoxycholic acid could reverse these problems[23].
Our experiments show that in comparison to the untreated obese rats, the octreotide-treated obese rats had much lower fasting plasma glucose levels. It may be that octreotide has a high affinity for SSTR2, which inhibits the release of glucagon by pancreatic α-cells[24] and results in a decrease in fasting plasma glucose levels. Moreover, decreased GLUT2 mRNA and protein levels were found in the octreotide-treated obese rats, which is consistent with the idea that glycemia is a systemic factor that can affect GLUT2 expression[25].
We found that the octreotide-treated group had significantly lower apical GLUT2 levels than the obese group. A possible explanation for this is that octreotide tightly binds to SSTR2, and SSTR2 binds with Gα02 to inhibit the L-type calcium channel and reduce Ca2+ transportation[4,26], which in turn suppresses the translocation of GLUT2 by depressing the activation of PKC βII[3].
In conclusion, our results indicate that high fat diet-induced obesity in rats is associated with increased fasting plasma glucose levels, intestinal maltase activity, GLUT2 expression, and permanent apical GLUT2 in small intestinal mucosa. After treatment with octreotide, an analogue of somatostatin, fasting plasma glucose levels, expression of GLUT2, and insertion of apical GLUT2 were inhibited, and eventually the obese rats lost weight. Therefore, the use of an analogue of somatostatin, such as octreotide, to suppress glucose absorption in intestinal mucosa may provide a novel approach in the pharmacological treatment of obesity.
Obesity induced by a high fat diet can result in disorders of glucose metabolism, and lead to impaired glucose tolerance or diabetes. Maltase and sucrase are the most important disaccharidases in sugar absorption. In addition, Glucose transporter type 2 (GLUT2) is a key transport protein for glucose in the small intestine. It is unknown whether obesity induced by a high fat diet is associated with glucose absorption. The relationship between the somatostatin analogue, octreotide, and glucose absorption in obesity has not yet been reported.
Although diet is an important way of treating nutritional obesity, many patients fail to lose weight with diet control. It is not know whether octreotide, a somatostatin analogue, which has an important role in the regulation of physiological functions in the small intestine, is able to inhibit glucose absorption. In the present study, the authors demonstrated that octreotide can inhibit glucose absorption and the potential mechanisms involved in obese rats.
Recently, several studies have shown the change in GLUT2 expression in diabetes or insulin resistance. Other researchers focused on the role of somatostatin analogues by suppressing β-cell insulin secretion in obesity in humans. This is the first study to provide evidence that the somatostatin analogue, octreotide, can inhibit glucose absorption in obese rats.
Since this study suggested that octreotide may be effective in inhibiting glucose absorption, octreotide might have potential applications as an alternative medicine in the treatment of obesity.
GLUT2 is normally thought to reside at the basolateral membrane in the small intestine, and it can be inserted within minutes into the apical membrane in vivo in response to high glucose concentrations. Somatostatin is a 14- or 28-amino-acid peptide, which mainly inhibits exocrine and endocrine secretion, motility and absorption via different somatostatin receptors in the gastrointestinal tract. Octreotide, a type of octopeptide, is an artificial synthetic analogue of somatostatin which is more convenient to use and has a longer half-life and duration of activity and fewer side effects.
The authors well demonstrated that high fat feeding produces permanent apical GLUT2 expression in addition to increased maltase activity, and octreotide improves all of them leading to decreased body weight and blood glucose levels compared to controls. The authors indicated the clinical significance of their findings. The authors need to discuss on the dosage of octreotide used in this and human studies. The potential side effects of octreotide such as gallstone should also be considered.
| 1. | Wang SN, Lee KT, Ker CG. Leptin in hepatocellular carcinoma. World J Gastroenterol. 2010;16:5801-5809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Hansen PA, Han DH, Marshall BA, Nolte LA, Chen MM, Mueckler M, Holloszy JO. A high fat diet impairs stimulation of glucose transport in muscle. Functional evaluation of potential mechanisms. J Biol Chem. 1998;273:26157-26163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr. 2008;28:35-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 342] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 4. | Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov. 2003;2:999-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 420] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 5. | Foxx-Orenstein A, Camilleri M, Stephens D, Burton D. Effect of a somatostatin analogue on gastric motor and sensory functions in healthy humans. Gut. 2003;52:1555-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Strosberg J, Kvols L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors. World J Gastroenterol. 2010;16:2963-2970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes. 2004;53:549-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 238] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Lentze MJ. Molecular and cellular aspects of hydrolysis and absorption. Am J Clin Nutr. 1995;61:946S-951S. [PubMed] |
| 9. | Wright EM. I. Glucose galactose malabsorption. Am J Physiol. 1998;275:G879-G882. [PubMed] |
| 10. | Ferraris RP. Dietary and developmental regulation of intestinal sugar transport. Biochem J. 2001;360:265-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Dunsford BR, Haensly WE. Effect of dietary cholesterol and carbohydrate on small intestinal structure and function in prematurely weaned rats. J Anim Sci. 1991;69:2894-2903. [PubMed] |
| 12. | Lostao MP, Berjón A, Barber A, Ponz F. On the multiplicity of glucose analogues transport systems in rat intestine. Rev Esp Fisiol. 1991;47:209-216. [PubMed] |
| 13. | Han DH, Hansen PA, Host HH, Holloszy JO. Insulin resistance of muscle glucose transport in rats fed a high-fat diet: a reevaluation. Diabetes. 1997;46:1761-1767. [PubMed] [DOI] [Full Text] |
| 14. | Im SS, Kang SY, Kim SY, Kim HI, Kim JW, Kim KS, Ahn YH. Glucose-stimulated upregulation of GLUT2 gene is mediated by sterol response element-binding protein-1c in the hepatocytes. Diabetes. 2005;54:1684-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Burcelin R, Eddouks M, Kande J, Assan R, Girard J. Evidence that GLUT-2 mRNA and protein concentrations are decreased by hyperinsulinaemia and increased by hyperglycaemia in liver of diabetic rats. Biochem J. 1992;288:675-679. [PubMed] |
| 16. | Thorens B. Glucose transporters in the regulation of intestinal, renal, and liver glucose fluxes. Am J Physiol. 1996;270:G541-G553. [PubMed] |
| 17. | Leturque A, Brot-Laroche E, Le Gall M. GLUT2 mutations, translocation, and receptor function in diet sugar managing. Am J Physiol Endocrinol Metab. 2009;296:E985-E992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Kellett GL, Brot-Laroche E. Apical GLUT2: a major pathway of intestinal sugar absorption. Diabetes. 2005;54:3056-3062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 259] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Tobin V, Le Gall M, Fioramonti X, Stolarczyk E, Blazquez AG, Klein C, Prigent M, Serradas P, Cuif MH, Magnan C. Insulin internalizes GLUT2 in the enterocytes of healthy but not insulin-resistant mice. Diabetes. 2008;57:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Aydede H, Sakarya A, Erhan Y, Kara E, Ilkgul O, Ozdemir N. Effects of octreotide and propranolol on colonic mucosa in rats with portal hypertensive colopathy. Hepatogastroenterology. 2003;50:1352-1355. [PubMed] |
| 21. | Lustig RH, Hinds PS, Ringwald-Smith K, Christensen RK, Kaste SC, Schreiber RE, Rai SN, Lensing SY, Wu S, Xiong X. Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2003;88:2586-2592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Velasquez-Mieyer PA, Cowan PA, Arheart KL, Buffington CK, Spencer KA, Connelly BE, Cowan GW, Lustig RH. Suppression of insulin secretion is associated with weight loss and altered macronutrient intake and preference in a subset of obese adults. Int J Obes Relat Metab Disord. 2003;27:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Williams C, Gowan R, Perey BJ. A Double-Blind Placebo-controlled Trial of Ursodeoxycholic Acid in the Prevention of Gallstones during Weight Loss after Vertical Banded Gastroplasty. Obes Surg. 1993;3:257-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology. 2000;141:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Cui XL, Jiang L, Ferraris RP. Regulation of rat intestinal GLUT2 mRNA abundance by luminal and systemic factors. Biochim Biophys Acta. 2003;1612:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Kleuss C, Hescheler J, Ewel C, Rosenthal W, Schultz G, Wittig B. Assignment of G-protein subtypes to specific receptors inducing inhibition of calcium currents. Nature. 1991;353:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 441] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
Peer reviewer: Akio Inui, MD, PhD, Professor, Department of Behavioral Medicine, Kagoshima University Graduate School of Medical and Dental Sciences, 8351 Sakuragaoka, Kagoshima 8908520, Japan
S- Editor Tian L L- Editor Webster JR E- Editor Xiong L