Published online Oct 21, 2011. doi: 10.3748/wjg.v17.i39.4396
Revised: April 21, 2011
Accepted: April 28, 2011
Published online: October 21, 2011
AIM: To assess nutrition, physical activity and healthful knowledge in obese children with biopsy-proven non-alcoholic steatohepatitis (NASH or NA) compared to children without liver disease.
METHODS: Children with biopsy-proven NASH comprised the NASH group. Age, sex and ethnicity matched control groups consisted of obese (OB) and lean (CO) children with no liver disease. Subjects were administered the School Physical Activity and Nutrition Survey and one blood draw was obtained.
RESULTS: Fifty-seven patients were enrolled with a mean age of 12.1 ± 2.1 years, and all were Hispanic. Even though the OB and NA had a similar increased body mass index (%), 35% of the NA group always read nutrition labels compared to none in the OB (P < 0.05), and more NA children felt their diet is “less healthy”. NA consumed the least amount of fruits with only 25% having ≥ 1 fruit/d vs 45% in OB and 64.7% in CO (P < 0.05 NA vs CO). Only 15% of NA subjects performed light exercise vs 35% and 59% of OB and CO groups, respectively (P = 0.02). The mean physical activity score was lowest in the NA group (P < 0.05). Amongst the subjects with NASH, we found that 100% of patients with grade 2 or 3 fibrosis had a sedentary score > 2 compared to only 63.6% of those with grade 1 or no fibrosis (P < 0.05).
CONCLUSION: Children with NASH had increased se-dentary behavior, decreased activity, and fruit intake. Larger studies may determine the benefit of changing these behaviors as treatment for NASH.
- Citation: Hattar LN, Wilson TA, Tabotabo LA, Smith EO, Abrams SH. Physical activity and nutrition attitudes in obese Hispanic children with non-alcoholic steatohepatitis. World J Gastroenterol 2011; 17(39): 4396-4403
- URL: https://www.wjgnet.com/1007-9327/full/v17/i39/4396.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i39.4396
The prevalence of obesity is increasing in epidemic proportions in both adults and children. This has tripled from 1980 for the age group 6 to 19 years, with national health and nutrition examination survey (NHANES) reporting 17.4%-18.8% of children being obese[1]. The evidence is clear; obese children become obese adults. In fact, obese 10 to 14 years old adolescents are more than 20 times more likely to become obese adults[2]. This is unfortunate, since obesity is associated with metabolic syndrome, which has been reported in up to 50% of severely obese children[3], and other obesity-associated diseases, such as fatty liver disease.
As the epidemic of childhood obesity progresses, it is predicted that the prevalence of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steato-hepatitis (NASH or NA) will also increase[4]. NAFLD is an umbrella term that includes simple steatosis and simple steatosis with the presence of lobular inflammation. These two forms of NAFLD are generally considered to be non-progressive. Once a patient has portal inflammation, ballooning degeneration of hepatocytes or fibrosis the patient has the type of NAFLD that has been shown to be progressive, or NASH. A diagnosis of NAFLD may be made when the patient has elevated liver enzymes in combination with radiography demonstrating fatty infiltration in the liver. NAFLD is now considered to be the most common cause of chronic liver disease in the pediatric population[5]. Studies using non-invasive techniques have been performed worldwide and have shown that NAFLD may be present in up to 77% of obese children[6]. Schwimmer et al[7] recently reported a population-based study where 13% of all children had biopsy-proven NAFLD and 3% had NASH.
The pathogenesis of the development of NAFLD is not completely understood. In 1999, Day and James[8] published the current theory “the two hit hypothesis”. The first “hit” is believed to be the development of steatosis, while the second “hit” leads to the progression to inflammation, necrosis and fibrosis. Oxidized by-products are harmful adducts that can cause liver injury, resulting in subsequent fibrosis[9]. Day[10] has subsequently pointed out that genetic factors influence the host response to endotoxin, oxidative stress, and severity of steatosis and that environmental factors, such as a diet high in saturated fat or low in antioxidant vitamins, influence the host’s susceptibility to fatty liver disease.
There are still no approved pharmacological therapies for NAFLD/NASH. Therefore, current management is based upon the presence of associated risk factors and aims to improve an individual’s quality of life, thus reducing NAFLD-associated morbidity and mortality. Today, lifestyle intervention (diet and exercise) is the treatment of choice for NAFLD/NASH. Significantly high levels of serum triglycerides, glucose, insulin, alanine aminotransferase (ALT), increased body mass index (BMI) and waist circumference (central adiposity) are all possible clinical features of pediatric NAFLD, which suggests that interventions on these variables can help to treat fatty liver, as well as to prevent progression to NASH[11]. On the other hand, resolution of histological abnormalities revealed by liver biopsy is, at this time, the main target of NASH treatment[12]. Studies on pediatric subjects have shown that moderate weight loss can improve BMI and serum levels of ALT, and reduce fatty liver infiltration and necro-inflammation, although no change has been demonstrated in degree of fibrosis[13].
One way to assess a child’s diet and physical activity is the School Physical Activity and Nutrition (SPAN) questionnaire[14], which was developed as a surveillance instrument to measure physical activity, nutrition attitudes, and dietary and physical activity behaviors in children and adolescents. This tool can assess many dietary and physical behaviors over the 24 h prior to questionnaire administration. The food behaviors measured by this instrument include recall of certain foods from the previous day. This type of short-term recall is better for children because their cognitive skill has not developed sufficiently to estimate averaging and frequency as found in a traditional food frequency questionnaire[15]. It has been validated and found to be reproducible[16,17].
We hypothesize that there are differences in the dietary behaviors, physical activity and healthful knowledge between obese children who develop NASH and obese and lean children with no evidence of liver disease. These differences would be of greater clinical relevance when examined in subjects who share a similar genetic background and who are affected most frequently by NASH, i.e., being of Hispanic origin. Therefore, identifying those potential environmental differences may have important implications which may guide us towards treatment and possibly even prevention of this condition in the future.
To test the hypothesis that there are differences in the dietary and physical activity habits between obese children with NASH and obese and lean children with no evidence of liver disease, we enrolled 57 children between the ages of 8 and 16 years into this pilot study. We categorized them into three different groups: the “NA” or NASH group which included 20 obese children with biopsy-proven NASH, the or obese control group (OB) which included 20 obese children with no evidence of liver disease, and the or lean control group (CO) which included 17 lean children with no evidence of liver disease.
Each subject in the NA group had a history of chronic serum ALT elevation defined by an ALT > 40 U/L on two separate occasions separated by at least 3 mo (90 d apart). Subjects also underwent a work-up for other causes of chronic hepatitis of unknown etiology including serologic evaluation for alpha-1-antitrypsin with phenotype, hepatitis B surface antigen, hepatitis C antibody, copper, ceruloplasmin, anti-nuclear antibody, anti-smooth muscle antibody (F-actin), anti-liver kidney microsomal antibody, and total immunoglobulin G. Subsequently, the NA subjects had a standard of care liver biopsy to identify the etiology and severity of chronic transaminitis. To be included in the study, the biopsy demonstrated a minimum of 5% of hepatocytes with macrovesicular fat, without other etiologies for the presence of fat being identified and a pattern of injury consistent with NASH, as determined by an anatomic pathologist. All biopsies were also scored according to the NAFLD Activity Score (NAS), which is the sum of grades for steatosis (0-3), ballooning degeneration (0-2) and lobular inflammation (0-3)[18]. Each biopsy also received a fibrosis score. All NA subjects had their liver biopsy confirming the diagnosis of NASH within 60 d of study enrollment.
In the OB group, each subject had a BMI percentile > 95% for age/sex as determined on growth chart by the Center for Disease Control (CDC), and no history of abnormal liver enzymes or chronic liver disease. If serum ALT levels were not drawn within the last 6 mo, subjects underwent screening ALT as a requirement for eligibility. If the subject had an ALT > 40 U/L on the day of the study visit, the subject was excluded (n = 2).
For inclusion in the CO group, each subject had a normal weight as defined by having a BMI percentile > 5% but less than 85% for age/sex as determined on a growth chart by the CDC. This was followed by Dual X-ray Absorptiometry (DXA) demonstrating less than 30% fat mass for females and less than 25% for males. Two male subjects were excluded for DXA % fat mass > 30% on the day of the study visit. CO subjects also had no history of abnormal liver enzymes or chronic liver disease.
We excluded any subject who had a disease considered by the study physician to be significant, including history of cancer or immunosuppressed state. We also excluded anyone with history of significant alcohol intake, use of medication known to cause NAFLD, or history of total parenteral nutrition. This protocol was reviewed and approved by the institutional review board of Baylor College of Medicine.
All 57 subjects underwent the same evaluation; one study visit which included obtaining one blood draw and the administration of the SPAN[14] questionnaire. This questionnaire was developed as a surveillance instrument to measure physical activity, nutrition attitudes, and food behaviors in children and adolescents. It has two versions, one for elementary students and the other for middle/high school students. We used the elementary school version, which is 10 pages long, with 54 questions. This version was found to have a reading level appropriate for a 9-year-old child and contains pictures to help the child understand the questions. Also, this questionnaire has been validated and has shown good to excellent reproducibility[16,17].
Each question is generally directed towards one of three parameters of interest: food intake, physical activity and healthful knowledge. Most of the questions that address the child’s food intake or physical activity are formatted to ask the child how much he/she has eaten of that food item on the day prior to the administration of the questionnaire (i.e., “Yesterday, did you eat fruit?”). This is intended to minimize any recall bias. However, the questions that assess their healthful knowledge are formatted in a more general way (i.e., “Do you ever read the nutrition labels on food packages?”).
For the purpose of better comparisons between the three groups, we created a scoring system for some of the questions of interest. For example, we created the physical activity score based on the sum of the answers of four different questions in the SPAN questionnaire that addressed physical activity. The higher the score, the more physical activity the child reported. We also added up the answers of two other questions and created the sedentary score (i.e., Sedentary score = the answer to question 35 “Yesterday, how many hours did you watch TV or video movies?” + the answer to question 40 “How many hours per day do you usually spend on the computer or playing video games, like Nintendo®, Sega®, or arcade games?”). Again the higher the score the more sedentary behavior the subject reported.
For other questions, we divided the children’s answers into “No” or “Yes”. “No” if they had no intake of that food item or “Yes” if they had at least one portion of that food item on the prior day but could be two, three or more portions. For example the answers to “Fruit intake ≥ once per day” are either “No”, which meant they had no fruit intake the day prior to the administration of the questionnaire or “Yes” which meant that they had at least one fruit the day before but could have had more.
One blood draw was performed at the time of the visit after an overnight fast of at least 8 h. Serological analysis was performed for insulin, glucose, lipid profile, and hepatic profile in Texas Children’s Hospital’s clinical laboratory. Heights and weights were measured for all children and BMI, BMI percentile for age/sex, and BMI Z-score were calculated. To assess insulin sensitivity, we determined the Quantitative Insulin Sensitivity Check Index (QUICKI) using the calculation: 1/[(log(fasting insulin in µU/mL) + log(fasting glucose in mg/dL)][19]. As a measure of insulin resistance, the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated using the equation: (fasting insulin in µU/mL X fasting glucose in mmol/L)/22.5[19]. A QUICKI value of < 0.339 represents impaired insulin sensitivity in children, while a HOMA-IR value of > 2 represents insulin resistance.
The data collected (serological levels and questionnaire answers) were analyzed using the Statistical Package for the Social Sciences (SPSS, Version 18.0). Descriptive statistics (means, standard deviations, and percentages) were calculated to describe sample characteristics. Analysis of Variance with Tukey’s multiple comparison analysis (Post Hoc Test) was used to compare the three experimental groups. Significance was defined as P < 0.05.
The study population (Table 1) included a total of 57 children; 20 in the NA group, 20 in the OB group and 17 in the CO group. All three groups were age, sex and ethnicity matched. The mean age was 12.1 ± 2.1 years. All subjects were Hispanic, and 74 % of the cohort was males (42 males and 15 females). The mean BMI % was 98.1% for the NA group (Z-score 2.2), 98.3% for the OB group (Z-score 2.3), and 48.9% for the CO group (Z-score -0.04). The mean fasting insulin levels were 19.4, 12.1 and 2.7 for the NA, OB and CO groups respectively (P < 0.05 NA vs OB). Increased insulin resistance and decreased insulin sensitivity were reflected in the HOMA-IR and the QUICKI (Table 1).
| CO | OB | NA | P valuec | |
| Male: Female (male %) | 12:5 (71) | 15:5 (75) | 15:5 (75) | 1 |
| Age, yr (mean ± SD) | 12.4 ± 2.1 | 11.9 ± 2 | 12.2 ± 2 | 0.9 |
| BMI (%) | 48.95 ± 21.85 (12.2-83.3) | 98.31 ± 1.42a (95.3-99.9) | 98.05 ± 1.73a (93.4-99.5) | 1 |
| Z-Score | -0.04 | 2.27a | 2.17a | 0.8 |
| BMI % males | 49.9 | 98.4a | 97.8a | 1 |
| BMI % females | 50.4 | 97.9a | 98.7a | 1 |
| Fasting insulin (μU/mL) | 2.7 ± 2.5 (1-11) | 12.1 ± 8.6a (1-31) | 19.4 ± 10.4ac (5-41) | 0.02 |
| Fasting lucose (mg/dL) | 78.7 ± 7.6 (62-91) | 84.1 ± 5.9 (75-95) | 85.9 ± 8.5a (68-111) | 0.7 |
| HOMA-IR | 0.5 ± 0.5 (0.1-2.0) | 2.5 ± 1.9a (0.2-7.0) | 4.1 ± 2.3ac (1-9.1) | 0.02 |
| QUICKI | 0.467 ± 0.069 (0.344-0.558) | 0.364 ± 0.073a (0.289-0.527) | 0.319 ± 0.027a (0.280-0.383) | 0.05 |
| Cholesterol (mg/dL) | 158.9 ± 25.2 (130-223) | 165.2 ± 30.4 (116-243) | 179.4 ± 37.8 (95-245) | 0.3 |
| TG (mg/dL) | 62.5 ± 28 (25-123) | 114.8 ± 38a (63-208) | 140.3 ± 81.4a (38-424) | 0.3 |
| HDL (mg/dL) | 56.7 ± 12.2 (37-87) | 43.4 ± 7.9a (32-62) | 43.9 ± 9.7a (22-68) | 1 |
While BMI % and BMI Z-score did not differ between NA and OB, differences in body image and dieting behavior between all 3 groups were elucidated (Table 2). We found that 55% of the NA group felt that they weighed too much compared to other students in their grade who were as tall as they were, while only 45% of the OB group and 5.9% of the CO group felt that way (P < 0.01, NA vs CO; P < 0.01, OB vs CO). Upon questioning the subjects if they have ever tried to lose weight, 85% of the NA group and 85% of the OB group answered yes compared to only 11.8% of the CO group. (P < 0.001, NA vs CO; P < 0.001, OB vs CO). In response to the question “Are you trying to lose weight now?” 95% of the NA group, 80% of the OB group and 5.9% in the CO group stated “Yes” (P < 0.001, NA vs CO; P < 0.001, OB vs CO).
| Question | CO | OB | NA |
| Compared to other students in your grade, who are as tall as you, do you think you weigh: | |||
| The right amount | 52.9 | 50 | 35 |
| Too much | 5.9 | 45a | 55a |
| Too little (or not enough) | 41.2 | 5a | 10a |
| Are you trying to lose weight? | |||
| No | 94.1 | 20a | 5a |
| Yes | 5.1 | 80a | 95a |
| Have you ever tried to lose weight? | |||
| No | 88.2 | 15a | 15a |
| Yes | 11.8 | 85a | 85a |
| Do you ever read the nutrition labels on food packages? | |||
| Almost never or never | 41.2 | 20 | 10a |
| Sometimes | 52.9 | 80 | 55 |
| Almost always or always | 5.9 | 0 | 35ac |
| The foods that I eat and drink now are healthy: | |||
| No | 0 | 0 | 10 |
| Yes, sometimes | 94.4 | 65 | 80 |
| Yes, all the time | 5.9 | 35a | 10 |
Looking into their healthful dietary knowledge, we found that 35% of the NA group answered that they always or almost always read the nutrition labels on food packages compared to only 5.9% of the CO group and none in the OB group (P < 0.05, NA vs OB; P < 0.05, NA vs CO). Interestingly, only 10% of the NA group and 5.9% of the CO group felt that they always eat and drink healthy foods, compared to 35% of the OB subjects (P < 0.05, OB vs CO).
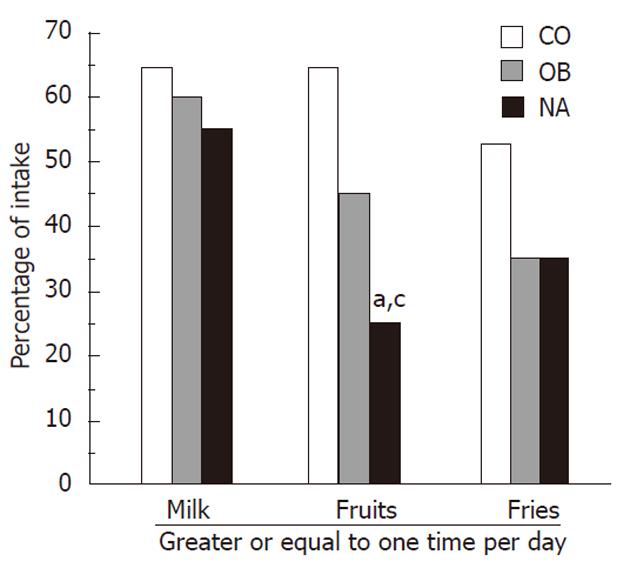
Children reported food intake of milk, fruits, and fries (Figure 1). The NA group consumed the least amount of fruits when compared to the other groups, with only 25% of NA subjects reporting that they consumed ≥1 fruit per day compared to 45% and 64.7% of the OB and CO groups respectively (P = 0.05, between the 3 groups). This difference was even more significant between the NA and CO groups (P < 0.05).
The NA group reported the least amount of dairy consumption, with only 55% having ≥ 1 cup of milk per day, compared to 60% of the OB group and 64.7% of the CO group; however this was not statistically significant. There were no statistically significant differences between the 3 groups regarding reported consumption of cereal, grains, vegetables, or sugar sweetened beverages. Also, there were no differences between the NA and OB groups in regards to having breakfast the day prior to the questionnaire or taking a vitamin pill.
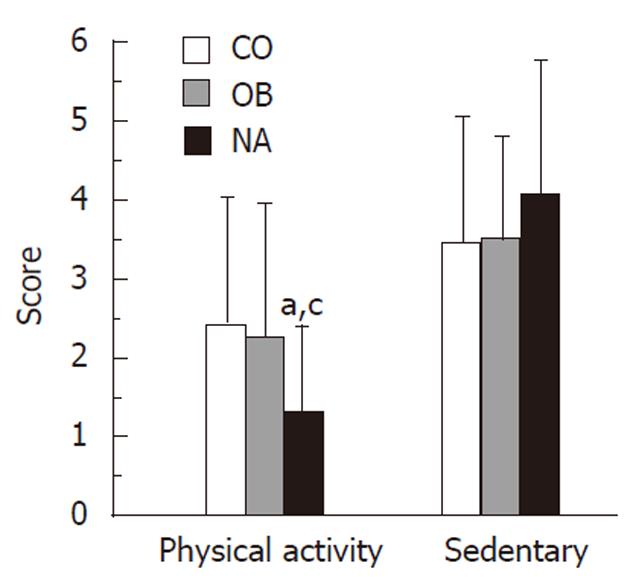
Differences in reported light to vigorous exercise and sedentary behaviors were evaluated in this cohort. We found that 45% of the NA group performed vigorous exercise (e.g., basketball, running or jogging, fast dancing, swimming laps, tennis, fast bicycling or similar aerobic exercises) for at least 20 min the day prior to the administration of the questionnaire, compared to 64.7% in the CO group and 25% in the OB (P = 0.05, between the 3 groups; P < 0.05, OB vs CO). However, only 15% of the NA subjects had performed light exercise (defined as fast walking, slow bicycling, skating, pushing a lawn mower, or mopping the floors) for at least 30 min the day before, compared to 35% and 59% of the OB and CO groups, respectively (P < 0.01, NA vs CO, P < 0.05 OB vs CO). The mean physical activity score was lowest in the NA group (NA = 1.3 vs OB = 2.3 vs CO = 2.4; P < 0.05), but the mean sedentary score was not significantly different between the groups (NA = 4.1 vs OB = 3.5 vs CO = 3.5; P = NS) (Figure 2).
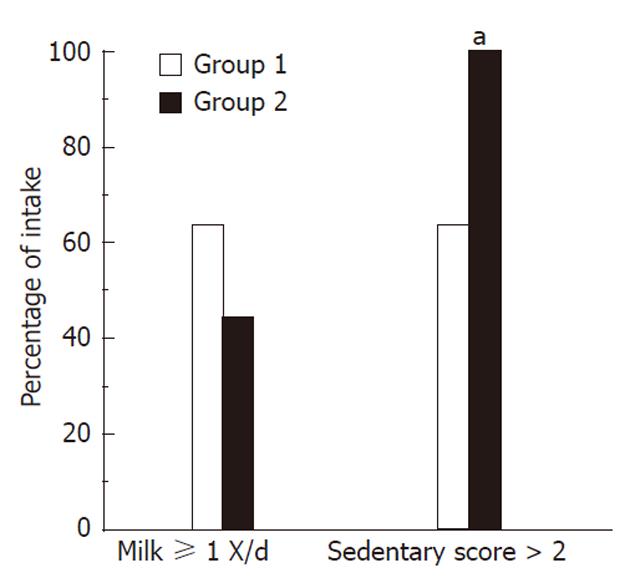
In an attempt to clarify possible differences in diet and activity amongst obese children with differing degrees of liver injury, a sub-group analysis of the NA group was performed (Figure 3). Amongst the subjects with NASH, we found that 100% of patients with grade 2 or 3 fibrosis had a sedentary score > 2 compared to only 63.6% of those with grade 1 or no fibrosis (P < 0.05). We also noted that 44.4% of patients with grade 2 or 3 fibrosis had ≥ 1 cup of milk per day, compared to 63.6% of patients with no fibrosis or with grade 1 fibrosis; however, this difference was not statistically significant. Also, 16.7% of patients with grade 3 steatosis reported having pasta on at least 2 occasions the day prior to the questionnaire while none of the subjects with grade 1 or 2 steatosis had that amount (P < 0.05). Comparing the patients’ NAFLD Activity Score (NAS), we found that there were no differences between subjects with NAS score ≥ 4 and those with NAS score < 4 in regards to their dietary habits, physical activity and healthful knowledge.
Upon comparing subjects with NASH to those with no evidence of liver disease (“non-NASH” = CO + OB groups), we found that 75% of the NA group reported no fruit intake at all compared to only 45.9% in the non-NASH group (P < 0.05). Additionally, 35% of the NA group “always” or “almost always” read nutrition labels, compared to only 2.7% of the non-NASH group (P < 0.01). In evaluating light exercise, we found that 85% of the NA group reported not performing any light exercise on the prior day, compared to 54.1% in the non-NASH group (P < 0.05). And finally, 95% of the NA group stated that they were currently trying to lose weight compared to only 45.9% of the non-NASH group (P < 0.001).
Our pilot study demonstrates clear differences in dietary behaviors, physical activity and healthful knowledge between lean healthy children, obese children with no evidence of liver disease and obese children with NASH. One potential caveat to our study is that we didn’t obtain a liver biopsy to confirm the absence of NAFLD in the CO and OB groups. However, given the invasive nature of liver biopsy and normal transaminases in these subjects, it would be unethical to perform this procedure on children with no other clinical indication. Hence, in order to minimize this possibility, we excluded any child with a history of liver disease of any sort, history of elevated ALT or ALT > 40 U/L on the day of the study visit.
We performed this study in a cohort of Hispanic children; this must be taken into account when considering generalizing this to the population as a whole. Ogden et al[19] recently reported that the highest rate of obesity in children of all age groups was among teenage African American girls followed by Hispanic boys and girls of all ages with Hispanic males having the highest percentage of obesity compared to white and African American males[20]. Additionally, the role of gender, race and ethnicity has been described in a population based study on NAFLD by Schwimmer et al[21], where they noted that obese adolescent boys were six times more likely to have fatty liver compared to obese adolescent girls. This study also supported the observation that NAFLD is more common in Hispanic adolescents compared to other ethnicities, with Hispanic ethnicity making a child five times more likely to have NAFLD than an African American child[7]. Given the prevalence and severity of obesity and NAFLD in Hispanic children, our study population provides a unique insight into this population.
In our cohort, all subjects were Hispanic, and 74% of them were males. This male:female ratio is similar to Schwimmer’s report of 82% of children with NASH being male[7]. There were no differences between the 3 groups in regards to age and sex; however, there were clear differences between the groups in regards to their fasting glucose and insulin levels - findings that are consistent with previous studies (e.g., Louthan et al[22] and Vos et al[23] studies). Evidence supports the concept that a trio of obesity, dyslipidemia, and insulin resistance play an important role in the pathogenesis and severity of NASH in children, and we found that children with NASH had an increase in HOMA-IR and a decrease in QUICKI when compared to the obese and lean control groups[19,22,23]. The NA and OB groups had a significantly higher level of triglyceride (TG) and lower level of high-density lipoprotein (HDL) when compared to the lean control subjects. Those values were comparable to the values from other studies such as Viva La Familia study by Butte et al[24] and Quirós-Tejeira et al[25], as the mean HDL in our NA subjects was 43.85 ± 9.7 (in Viva La Familia; HDL: 46.6 ± 0.5 mg/dL) and mean TG was 140.3 ± 81.4 (in Quirós-Tejeira et al[25]; TG for boys: 157.2 ± 86.9 mg/dL and for girls: 145.0 ± 71.7 mg/dL).
Recent studies have shown that the majority of Hispanic mothers of overweight children may not perceive their children as being overweight[26-28] . In another study by Mikhail et al[29] both children and parents underestimated the child’s body fat with parents’ estimates being slightly, but not significantly, better (unpublished data). Similarly, we found that only half of the NA children found themselves heavier than their classmates; however, the majority of them had tried and/or are currently trying to lose weight.
Interestingly, even though the NA and the OB groups had the same mean BMI % (98%), similar proportions reported feeling heavier than their classmates, and a similar percentage had tried or are currently trying to lose weight, the NA and OB groups had notable differences in their healthful knowledge. For example about one third of the NA children read food labels on a regular basis compared to none in the OB group, and more NA than OB children recognized that their diets are “less healthy”. This finding may be attributed to the fact that the NA children had multiple physician’s visits including seeing a specialist (i.e., a hepatologist), and had undergone multiple blood draws and a liver biopsy for obesity-related health issues, while the OB group were recruited generally during their well-child visit, through Baylor Pediatric Residents Primary Care clinics at Texas Children’s Hospital. Therefore, the NA subjects may have a heightened sense that their diet is not as healthy as it should be and may truly be reading food labels on a more regular basis.
A growing body of evidence supports that oxidative stress may play a vital role in the development of NASH[30,31]. Intake of antioxidants may help prevent the occurrence of this disease and antioxidant vitamin supplementation studies have been performed[13,32,33]. In our study, there were no differences between the three groups in regards to their daily intake of a vitamin pill to explain a difference in their antioxidant intake; however, we noted that the NA group had the lowest intake of fruits per day while the CO group had the highest intake. Fruits are rich in antioxidants[34-36], and this difference in fruit intake between the groups may give insight into why some obese Hispanic children develop NASH while others do not.
In an analysis of NHANES looking at whole grain consumption and weight status, it was found that women consuming at least one serving of whole grain had a significantly lower mean BMI and waist circumference than women with no whole grain consumption[37]. In another study done in the United Kingdom, it was found that a higher intake of whole grains (about three servings per day) was associated with lower BMI and central adiposity[38]. Nicklas et al[39] have found that consuming ready-to-eat cereal at breakfast was associated with improved weight and nutrient adequacy in African American children. Surprisingly, in our study there were no differences between the 3 groups in regards to having breakfast or cereal intake. This, however, may be due to the small sample size in our pilot study.
While differences between the 3 groups were noted in respect to vigorous activity, it was even more notable in regards to light exercise, with only 15% of the NA subjects performing light exercise for at least 30 min on the prior day, compared to one third and almost two thirds of the OB and CO groups, respectively. While we have not evaluated for patatin-like phospholipase domain-containing protein 3 (PNPLA 3) variants in our study subjects, it has been recognized that PNPLA 3 variants have been associated with NASH, especially in Hispanics. Johansson, et al[40] reported that PNPLA 3 mutations were associated with decreased light physical activity. This highlights the need for studies to evaluate PNPLA 3 mutations in children with NASH.
Amongst the subjects with NASH, we found that all patients with advanced fibrosis had a sedentary score > 2 compared to less than two thirds of those with grade 1 or no fibrosis. Realizing that this was a one-time study visit, we can not be certain as to where our subjects are in regards to the natural history of disease progression. However, in a study out of Italy by Nobili et al[41], children with NASH who improved their BMI, partially by increased exercise, decreased the degree of liver injury. This could explain the above finding in our study.
In conclusion, both genetic and environmental factors play major roles in the development of NASH and determine why only a minority of obese, insulin resistant individuals progress from simple steatosis to inflammation and fibrosis[10]. This pilot study aimed to use a validated tool, the SPAN questionnaire, to asses the role of specific environmental factors such as diet and physical activity in pediatric NAFLD. Our results suggest that pediatric hepatologists should consider counseling children with NASH regarding the potential benefits of decreasing sedentary behavior, increasing light activity level, and increasing fruit intake. We conclude that future studies identifying environmental factors involved in pediatric NASH may help target therapeutic measures and preventative measures.
As the epidemic of obesity in childhood is increasing, it is predicted that non-alcoholic fatty liver disease and non-alcoholic steato-hepatitis (NASH or NA) prevalence will increase as well. Currently, there is no approved therapy for this condition, so life style changes, e.g., diet and exercise have become the mainstays of therapy.
The authors hypothesize that there are differences in the dietary behaviors, physical activity and healthful knowledge between obese children who develop NASH and obese and lean children with no evidence of liver disease. Therefore, identifying those potential environmental differences may have important implications in regards to treatment and possibly even prevention of this condition in the future.
The authors demonstrated clear differences between obese children with NASH and obese and lean children with no evidence of liver disease. Children with NASH had increased sedentary behavior, decreased activity level, and decreased fruit intake.
This pilot study highlights the potential benefits of decreasing sedentary behavior, increasing light activity level, and increasing fruit intake in obese children as this may help prevent the development of NASH in this population.
In this manuscript the authors evaluated nutrition, physical activity and healthful knowledge in obese children with NASH compared to children without liver disease and concluded that children with NASH had increased sedentary behavior and decreased activity. This study is interesting.
Peer reviewer: Dr. Seyed Mohsen Dehghani, MD, Associate Professor, Department of Pediatrics, Nemazee Hospital, Shiraz University of Medical Sciences, Shiraz, Iran
S- Editor Tian L L- Editor O’Neill M E- Editor Xiong L
| 1. | Xanthakos S, Miles L, Bucuvalas J, Daniels S, Garcia V, Inge T. Histologic spectrum of nonalcoholic fatty liver disease in morbidly obese adolescents. Clin Gastroenterol Hepatol. 2006;4:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 2718] [Article Influence: 123.5] [Reference Citation Analysis (3)] |
| 3. | Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362-2374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2172] [Cited by in RCA: 2105] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 4. | Wieckowska A, Feldstein AE. Nonalcoholic fatty liver disease in the pediatric population: a review. Curr Opin Pediatr. 2005;17:636-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 5. | Lavine JE, Schwimmer JB. Nonalcoholic fatty liver disease in the pediatric population. Clin Liver Dis. 2004;8:549-558, viii-ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Chan DF, Li AM, Chu WC, Chan MH, Wong EM, Liu EK, Chan IH, Yin J, Lam CW, Fok TF. Hepatic steatosis in obese Chinese children. Int J Obes Relat Metab Disord. 2004;28:1257-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 197] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 1066] [Article Influence: 53.3] [Reference Citation Analysis (1)] |
| 8. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3159] [Article Influence: 112.8] [Reference Citation Analysis (36)] |
| 9. | Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 2010;55:560-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 238] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 10. | Day CP. Genes or environment to determine alcoholic liver disease and non-alcoholic fatty liver disease. Liver Int. 2006;26:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Loomba R, Sirlin CB, Schwimmer JB, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2009;50:1282-1293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Socha P, Horvath A, Vajro P, Dziechciarz P, Dhawan A, Szajewska H. Pharmacological interventions for nonalcoholic fatty liver disease in adults and in children: a systematic review. J Pediatr Gastroenterol Nutr. 2009;48:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Nobili V, Manco M, Devito R, Di Ciommo V, Comparcola D, Sartorelli MR, Piemonte F, Marcellini M, Angulo P. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology. 2008;48:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 298] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 14. | Available from: http://www.sph.uth.tmc.edu/catch/catch_em/4th%20SPAN%20Eng%20v8.pdf. |
| 15. | Baranowski T, Domel SB. A cognitive model of children's reporting of food intake. Am J Clin Nutr. 1994;59:212S-217S. [PubMed] |
| 16. | Thiagarajah K, Fly AD, Hoelscher DM, Bai Y, Lo K, Leone A, Shertzer JA. Validating the food behavior questions from the elementary school SPAN questionnaire. J Nutr Educ Behav. 2008;40:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Penkilo M, George GC, Hoelscher DM. Reproducibility of the School-Based Nutrition Monitoring Questionnaire among fourth-grade students in Texas. J Nutr Educ Behav. 2008;40:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8517] [Article Influence: 405.6] [Reference Citation Analysis (7)] |
| 19. | Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003;143:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 324] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 20. | Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003-2006. JAMA. 2008;299:2401-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1105] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 21. | Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561-e565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 266] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 22. | Louthan MV, Barve S, McClain CJ, Joshi-Barve S. Decreased serum adiponectin: an early event in pediatric nonalcoholic fatty liver disease. J Pediatr. 2005;147:835-838. [PubMed] |
| 23. | Vos MB, Barve S, Joshi-Barve S, Carew JD, Whitington PF, McClain CJ. Cytokeratin 18, a marker of cell death, is increased in children with suspected nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2008;47:481-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Butte NF, Cai G, Cole SA, Comuzzie AG. Viva la Familia Study: genetic and environmental contributions to childhood obesity and its comorbidities in the Hispanic population. Am J Clin Nutr. 2006;84:646-654; quiz 673-674. [PubMed] |
| 25. | Quirós-Tejeira RE, Rivera CA, Ziba TT, Mehta N, Smith CW, Butte NF. Risk for nonalcoholic fatty liver disease in Hispanic youth with BMI & gt; or =95th percentile. J Pediatr Gastroenterol Nutr. 2007;44:228-236. [PubMed] |
| 26. | Hackie M, Bowles CL. Maternal perception of their overweight children. Public Health Nurs. 2007;24:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Maynard LM, Galuska DA, Blanck HM, Serdula MK. Maternal perceptions of weight status of children. Pediatrics. 2003;111:1226-1231. [PubMed] |
| 28. | Contento IR, Basch C, Zybert P. Body image, weight, and food choices of Latina women and their young children. J Nutr Educ Behav. 2003;35:236-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Mikhail C, Raynaud S, Shepard V, Clark C, Young S, Burgess D. Body fat perception in overweight and normal weight children and their parents. Poster presentation at the 18th Annual Scientific Sessions of the Society for Behavioral Medicine, San Francisco. 1997;. |
| 30. | Sastre J, Pallardó FV, Llopis J, Furukawa T, Viña JR, Viña J. Glutathione depletion by hyperphagia-induced obesity. Life Sci. 1989;45:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Strauss RS, Barlow SE, Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr. 2000;136:727-733. [PubMed] |
| 32. | Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734-738. [PubMed] |
| 33. | Lavine JE, Schwimmer JB, Molleston JP, Scheimann AO, Murray KF, Abrams SH, Rosenthal P, Sanyal AJ, Robuck PR, Brunt EM. Treatment of nonalcoholic fatty liver disease in children: TONIC trial design. Contemp Clin Trials. 2010;31:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Stoner GD, Wang LS, Casto BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis. 2008;29:1665-1674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Balsano C, Alisi A. Antioxidant effects of natural bioactive compounds. Curr Pharm Des. 2009;15:3063-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18:567-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 491] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 37. | Good CK, Holschuh N, Albertson AM, Eldridge AL. Whole grain consumption and body mass index in adult women: an analysis of NHANES 1999-2000 and the USDA pyramid servings database. J Am Coll Nutr. 2008;27:80-87. [PubMed] |
| 38. | Harland JI, Garton LE. Whole-grain intake as a marker of healthy body weight and adiposity. Public Health Nutr. 2008;11:554-563. [PubMed] |
| 39. | Williams BM, O'Neil CE, Keast DR, Cho S, Nicklas TA. Are breakfast consumption patterns associated with weight status and nutrient adequacy in African-American children? Public Health Nutr. 2009;12:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Johansson LE, Lindblad U, Larsson CA, Rastam L, Ridderstrale M. Polymorphisms in the adiponutrin gene are associated with increased insulin secretion and obesity. Eur J Endocrinol. 2008;159:577-583. |
| 41. | Nobili V, Manco M, Ciampalini P, Alisi A, Devito R, Bugianesi E, Marcellini M, Marchesini G. Metformin use in children with nonalcoholic fatty liver disease: an open-label, 24-month, observational pilot study. Clin Ther. 2008;30:1168-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |