Published online Sep 28, 2011. doi: 10.3748/wjg.v17.i36.4055
Revised: May 16, 2011
Accepted: May 23, 2011
Published online: September 28, 2011
Chronic hepatitis due to any cause leads to cirrhosis and end-stage liver disease. A growing body of literature has also shown that fatty liver due to overweight or obesity is a leading cause of cirrhosis. Due to the obesity epidemic, fatty liver is now a significant problem in clinical practice. Steatosis has an impact on the acceleration of liver damage in patients with chronic hepatitis due to other causes. An association between hepatitis C virus (HCV) infection, steatosis and the onset of insulin resistance has been reported. Insulin resistance is one of the leading factors for severe fibrosis in chronic HCV infections. Moreover, hyperinsulinemia has a deleterious effect on the management of chronic HCV. Response to therapy is increased by decreasing insulin resistance by weight loss or the use of thiazolidenediones or metformin. The underlying mechanisms of this complex interaction are not fully understood. A direct cytopathic effect of HCV has been suggested. The genomic structure of HCV (suggesting that some viral sequences are involved in the intracellular accumulation of triglycerides), lipid metabolism, the molecular links between the HCV core protein and lipid droplets (the core protein of HCV and its transcriptional regulatory function which induce a triglyceride accumulation in hepatocytes) and increased neolipogenesis and inhibited fatty acid degradation in mitochondria have been investigated.
- Citation: Basaranoglu M, Basaranoglu G. Pathophysiology of insulin resistance and steatosis in patients with chronic viral hepatitis. World J Gastroenterol 2011; 17(36): 4055-4062
- URL: https://www.wjgnet.com/1007-9327/full/v17/i36/4055.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i36.4055
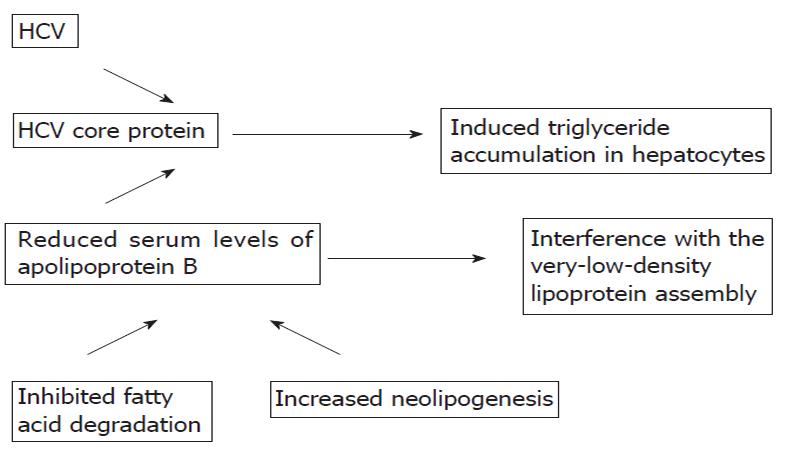
Chronic hepatitis due to any cause leads to cirrhosis and end-stage liver disease. A growing body of literature has also shown that fatty liver due to overweight or obesity is a leading cause of cirrhosis[1-3]. Due to the obesity epidemic, fatty liver is now a significant problem in clinical practice. An association between hepatitis C virus (HCV) infection, steatosis and the onset of insulin resistance has been reported[4-6]. Moreover, steatosis has an impact on the acceleration of liver damage in patients with chronic hepatitis due to other causes. The underlying mechanisms of this complex interaction are not fully understood. A direct cytopathic effect of HCV has been suggested. The genomic structure of HCV (suggesting that some viral sequences are involved in the intracellular accumulation of triglycerides), lipid metabolism, and the molecular links between the HCV core protein and lipid droplets (the core protein of HCV and its transcriptional regulatory function which induce a triglyceride accumulation in hepatocytes) and increased neolipogenesis and inhibited fatty acid degradation in mitochondria have been investigated (Figure 1).
Excessive accumulation of triglycerides in hepatocytes in the absence of significant alcohol consumption, defined as > 5% fat by weight, occurs in about 20%-30% of adults[1-3]. Excessive fat in the liver predisposes to the development of steatohepatitis which is a significant risk factor for developing cirrhosis and its complications, including hepatocellular carcinoma.
The frequency of type 2 diabetes is more common in patients with chronic HCV infection than in hepatitis B infection (21% vs 12%, respectively) which is evidence of a link between HCV infection and diabetes mellitus (DM)[4-6]. This relationship is independent of the existence of cirrhosis. A large cross-sectional United States study which included over 9000 individuals showed that the frequency of type 2 DM is 3-fold more common in hepatitis C patients. Both older age and higher body mass index (BMI) are more common among patients with both hepatitis C and type 2 diabetes.
Insulin resistance (IR) is a specific feature of chronic HCV, associated with genotypes 1 and 4 and high serum HCV RNA level[7]. Chronically HCV infected subjects present a 3-fold increased risk of IR and glucose metabolism impairment, with IR occurring in the very early stages of hepatic lesions (fibrosis stage 0 or 1), with a worsening tendency as hepatic fibrosis progresses[8-10]. There is also an association between IR severity and DM, with higher viral load and an improvement in IR after a sustained viral response.
A recently published study which investigated 600 patients [chronic hepatitis C (CHC) in 500 and chronic hepatitis B (CHB) in 100] reported that IR was present in 32.4% of the 462 nondiabetic CHC patients and was associated with the metabolic syndrome, genotypes 1 and 4, significant fibrosis, and severe steatosis[7]. IR was diagnosed in 15% of 145 CHC patients without metabolic syndrome or significant fibrosis, and was associated with genotypes 1 and 4, high serum HCV RNA level, and moderate-to-severe necroinflammation. IR was less frequent in CHB patients than in matched CHC patients (5% vs 35%, respectively, P < 0.001).
In our clinic, we investigated 76 patients. Of these 76, 12 had hepatitis B, 19 had hepatitis C, 34 had simple steatosis and 11 were control subjects. We found that IR was only significant and associated with severe fibrosis in patients with HCV[11].
Whether fat in the liver is an important determinant of IR is debatable. The study showed that insulin secretion assessed by intravenous glucose injection was not impaired in CHC patients compared to the controls[12]. When they studied the IR of 29 people with hepatitis C (14 with genotype 1 and 15 with genotype 3) and confirmed they had high IR, nearly all the IR was found to be in the muscle and hardly any in the liver. Of the 29 patients, 15 had very high levels of fat in the liver and had the same degree of IR as the 14 patients who did not have fatty livers.
IR is one of the leading factors for severe fibrosis in CHC infections independent of steatosis, as compensatory hyperinsulinemia is fibrogenic[12]. Moreover, a relationship between exogenous hyperinsulinemia and hepatocellular carcinoma has been reported. Hyperinsulinemia decreases therapy response and has a deleterious effect on the management of chronic HCV infection. Response to therapy is increased by decreasing insulin resistance by weight loss or the use of thiazolidenediones and metformin. Metformin improved virologic response when added to hepatitis C interferon-ribavirin therapy in those with IR[13].
A relationship between type 1 diabetes and hepatitis C, and type 2 diabetes and hepatitis B has not so far been reported[14].
The prevalence of steatosis is 20%-30% in the general population and is 50%-80% in patients with HCV infection. HCV itself has the ability to directly promote steatosis and IR[15-18]. If all steatogenic co-factors are excluded, the prevalence of steatosis remains at 50% resulting in a 2.5-fold increased prevalence as compared with the general population and other forms of chronic liver disease. The prevalence of steatosis is 18% in hepatitis B virus infection[19].
There are 2 types of liver steatosis: metabolic steatosis which is related to high BMI in patients with genotype 1, and viral steatosis which is related to hepatitis C genotype 3. Steatosis is more frequent in association with genotype 3a as compared to other genotypes such as genotype 1, 2 and 4 (74% vs 50%), which suggests that some sequences of the viral genome may be involved in intracellular lipid accumulation[16]. Additionally, steatosis correlates with viral load and can revert after effective treatment and reoccurs with re-infection in genotype 3 infection.
The localization of steatosis, particularly in genotype 3 infected patients, is predominantly in the periportal zone (acinar 1) and not in the centrilobular zone (acinar 3) and is more typical of metabolic associated steatohepatitis[20-23]. All genotypes are steatogenic, however, genotype 3 is three times more potent. Transgenic mouse models showed that the core protein can induce the appearance of lipid droplets. One possible molecular explanation for the greater steatogenic property of genotype 3, could be a phenylalanine residue at position 164 in core protein domain II, instead of tyrosine as in other genotypes, which results in a higher affinity to lipids.
Hyperinsulinism, IR related, directly activates stellate cells and, in association with hyperglycemia, increases connective tissue growth factor, a key cytokine in hepatic fibrogenesis[24,25]. Steatosis also relates to more advanced fibrosis and to accelerated fibrosis progression. Thus, treating HCV infected patients with evidence of hepatic steatosis, even if they only present mild inflammatory activity, has been suggested. How? Steatosis may sensitize the liver to inflammation and apoptosis, and subsequently enhance fibrosis. A recent study showed that hepatic steatosis is associated with higher programmed cell death by apoptosis with stellate cell activation[26].
A balance exists between energy demand and intake in the human body. Obesity and its consequences such as IR and the metabolic syndrome, is a growing threat to the health of people in developed nations. While insulin receptor defects cause severe IR, most patients with IR have impaired post-receptor intracellular insulin signaling[27].
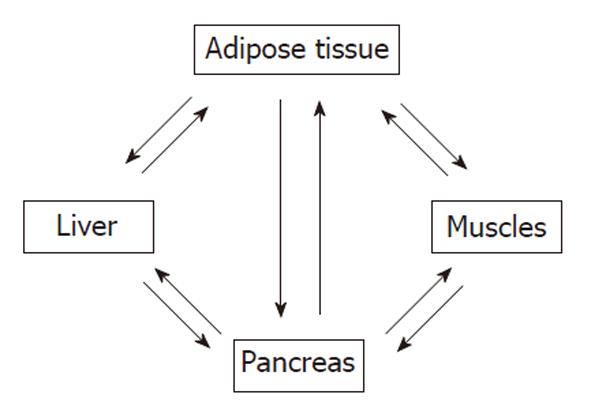
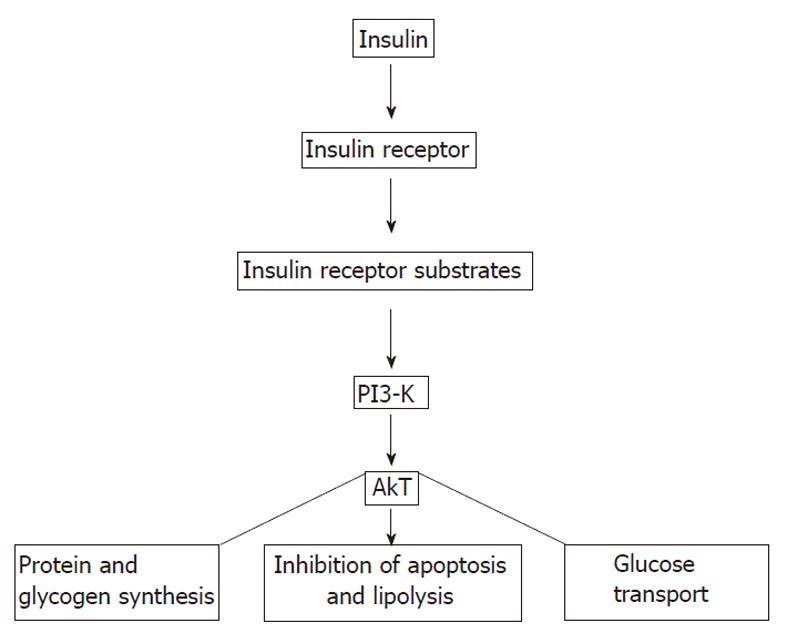
There is cross-talk among insulin sensitive tissues such as skeletal muscle, adipose tissue, and liver (Figure 2). Insulin binds α-subunits of its receptor which is a cell surface receptor on insulin sensitive cells such as skeletal muscle, adipocytes, and hepatocytes leading to autophosphorylation of the cytoplasmic domains (β-subunits) of the receptor[25-40]. The insulin receptor has intrinsic tyrosine kinase activity activated by insulin binding and the autophosphorylated receptor activates its substrates that include insulin receptor substrate (IRS)-1, IRS-2, Src homology collagen (Shc), and an adaptor protein with a pleckstrin homology (PH) and Src homology (SH) 2 domain by tyrosine phosphorylation (Figure 3). These phosphorylated docking proteins bind and activate several downstream components of the insulin signaling pathways. Activated IRS-1 associates with phosphatidyl inositol 3-kinase (PI3-K), which then activates Akt. Akt substrate of 160 kDa (AS160), a serine/threonine kinase, was identified in 3T3-L1 adipocytes. In both skeletal muscle and adipose tissue, these insulin-mediated phosphorylation-dephosphorylation signaling cascades induce the translocation of glucose transporters (GLUT), predominantly GLUT4-containing vesicles, from intracellular storage sites to the plasma membrane, increasing glucose uptake to prevent abnormal glucose and insulin elevations in the plasma (insulin-stimulated glucose transport). These events and insulin-dependent inhibition of hepatic glucose output maintain glucose homeostasis. Insulin also affects glucose homeostasis indirectly by its regulatory effect on lipid metabolism. Any interference in this insulin signaling pathway causes glucotoxicity, insulin resistance and, when islet β cells are capable of responding, compensatory hyperinsulinemia. Hepatitis C virus Genotype 1b diminishes IRS-1 levels and causes IR.
Hepatic expression of insulin receptor protein was decreased in chronic hyperinsulinemic states. IRS-1 was more closely linked to glucose homeostasis with the regulation of glucokinase expression, while IRS-2 was more closely linked to lipogenesis with the regulation of lipogenic enzymes sterol regulatory element-binding protein-1c (SREBP-1c) and fatty acid synthase. Moreover, insulin activates synthesis and inhibits catabolism of lipids, while shutting off the synthesis of glucose in the liver.
Adipose tissue is one of the major insulin sensitive organs in the human body and the process of differentiation of preadipocytes to adipocytes, induced by insulin, is called adipogenesis. Within the adipose tissue, insulin stimulates triglyceride synthesis (lipogenesis) and inhibits lipolysis by upregulating lipoprotein lipase activity which is the most sensitive pathway in insulin action, facilitating free fatty acid uptake and glucose transport, inhibiting hormone sensitive lipase, and increasing gene expression of lipogenic enzymes. Insulin also induces the degradation of apolipoprotein B100 (apo B100), a key component of very-low-density lipoprotein, in the liver[41].
Insulin resistance can be defined as the failure of insulin sensitive cells to respond to insulin normally. It is characterized by elevated plasma glucose and, before attrition of pancreatic β-cells develops, elevated insulin levels. Chronic hyperinsulinemia is a major contributor to glucose and lipid metabolism abnormalities. Insulin resistance also inappropriately activates peripheral lipolysis and stimulates free fatty acid mobilization from adipocytes in the fed state. Increased circulating free fatty acids contribute to fat accumulation in the liver and muscle, further causing these tissues to be insulin resistant by disturbing their downstream insulin signaling cascades.
Mechanisms of insulin resistance (role of tumor necrosis factor-αand plasma free fatty acids)
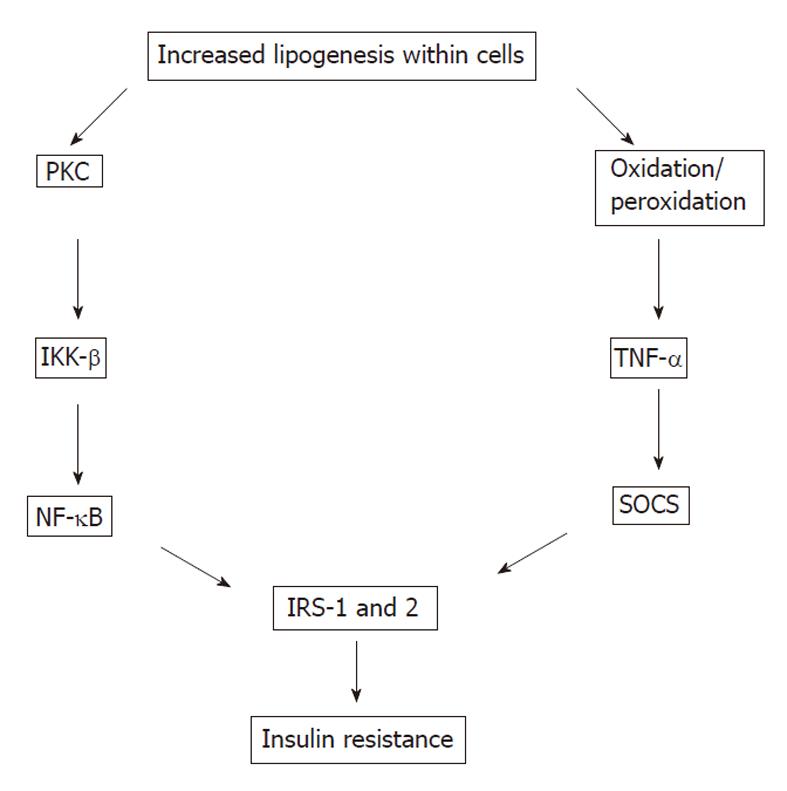
The most common mechanism of IR is disturbed post-receptor insulin signaling (Figure 4)[42-48]. Whereas most insulin signaling is propagated by tyrosine phosphorylation, serine (Ser) phosphorylation is often inhibitory. Ser phosphorylation of IRS-1 decreases both insulin stimulated tyrosine phosphorylation of IRS-1 (phosphorylated Ser residues of IRS-1 become poor substrates for insulin receptor) and PI3-K activation. This diminishes the downstream insulin signaling and insulin sensitivity of insulin target tissues. IRS-1 has several Ser residues including Ser 307, Ser 612 and Ser 632 which can be phosphorylated. Insulin and tumor necrosis factor-α (TNF-α) can phosphorylate the same Ser residues of IRS-1. IR occurs very early in HCV infection, in parallel with an elevation in TNF-α levels. HCV also directly promotes IR through the proteasomal degradation of IRS-1.
TNF-α and plasma free fatty acids have been shown to be major stimuli of Ser 307 phosphorylation of IRS-1. Inhibition of IRS-1 due to the phosphorylation of its Ser 307 residues also requires the activation of both c-Jun N-terminal kinase (JNK) and inhibitor κB kinase (IKK) β. Both TNF-α and free fatty acids induce JNK and IKK-β activation.
TNF-α stimulates phosphorylation of Ser residues of both IRS-1 and IRS-2 in hepatocytes[48-50]. It was recently reported that monocyte-derived macrophages increasingly accumulated within the adipose tissue of obese patients. In addition to the dysregulated production of adipocytokines by adipocytes, adipose tissue macrophages also produce proinflammatory cytokines such as TNF-α, interleukin-6, and C-reactive protein. Both adipose tissue and its macrophages contribute to the TNF-α burden. Indeed, its circulating concentrations are very low, commonly undetectable even in obese mice or humans.
Elevated free fatty acids in the circulation are also major contributors to IR in both humans and mice by stimulating Ser 307 phosphorylation of IRS-1. Adipose tissue triglycerides are the main source of circulating free fatty acids in obesity. One mechanism of elevated free fatty acid-induced IR in muscle is the impaired activation of protein kinase C lamda (PKCλ) and protein kinase C XI (PKCξ)[50-52]. PKCθ can also activate IKK-β which phosphorylates Ser 307 residues of IRS-1. Additionally, increased acyl CoAs or ceramide which is a derivative of acyl CoAs, promote IR by diminishing Akt1 activation. Increased ceramide activates a phosphatase (protein phosphatase 2A) that reverses tyrosine phosphorylation of Akt/protein kinase B (PKB). Inactivated PKB inhibits the insulin downstream signaling cascade leading to IR in muscles [Le]. Several oxidative stress mediators might also induce IR by affecting insulin downstream signaling. Phosphatases such as phosphatase and tensin homolog, small heterodimer partner 2, and protein tyrosine phosphatase 1B are now recognized to be major mediators involved in IR. Another possible mechanism for IR is defective glucose transport such as down-regulation of GLUT4.
JNK is one of the stress-related kinases and plays an important role in the development of IR[48,52]. The three members of the JNK group of serine/threonine kinases, namely JNK-1, -2, and -3 are activated by proinflammatory cytokines such as TNF-α as well as free fatty acids and endoplasmic reticulum stress due to metabolic overload, which is an intracellular abnormality found in obesity. Activated JNK induces Ser 307 phosphorylation of IRS-1, disturbs insulin downstream signaling, and subsequently causes insulin resistance. JNK activity has been found to be elevated in liver, muscle, and adipose tissue of experimental obese models. Additionally, the loss of JNK-1 activity such as in JNK-1 knockout mice has been shown to prevent the development of IR in leptin-deficient ob/ob mice or mice with high-fat induced dietary obesity.
PKCθ and IKK-β are two proinflammatory kinases involved in insulin downstream signaling. They are activated by lipid metabolites such as high plasma free fatty acid concentrations and there is a positive relationship between the activation of PKCθ and the concentration of intermediate fatty acid products. PKCθ activates both IKK-β and JNK, leading to increased Ser 307 phosphorylation of IRS-1 and IR. IKK-β is a mediator of IR and one of the other stress-related kinases[53,54]. Activation or overexpression of IKK-β diminishes insulin signaling and causes IR, whereas inhibition of IKK-β improves insulin sensitivity. IKK-β phosphorylates the inhibitor of nuclear factor (NF) -κB leading to the activation of NF-κB by the translocation of NF-κB to the nucleus. NF-κB is an inducible transcription factor and promotes specific gene expression in the nucleus. NF-κB has both apoptotic and anti-apoptotic effects. The finding that NF-κB deficient mice were protected from high-fat diet-induced IR suggests that NF-κB directly participates in processes that impair insulin signaling.
Suppressors of cytokine signaling (SOCS) and inducible nitric oxide synthase (iNOS) are two inflammatory mediators recently recognized to play a role in insulin signaling[54-61]. Induction of SOCS proteins [SOCS 1-7 and cytokine-inducible src homology 2 domain-containing protein (CIS)] by proinflammatory cytokines might contribute to the cytokine-mediated IR in obese subjects. SOCS-3 might also regulate central leptin action and play a role in the leptin resistance of obese human subjects. SOCS-1 knockout mice showed low glucose concentrations and increased insulin sensitivity. In animal studies, inactivation of SOCS-3 or SOCS-1 or both in the livers of db/db mice partially improved insulin sensitivity and decreased hyperinsulinemia, whereas overexpression of SOCS-1 and SOCS-3 in obese animals caused IR and also increased activation of SREBP-1c[62]. SREBP-1c is one of the key mediators of lipid synthesis from glucose and other precursors (de novo lipogenesis) in the liver. Indeed, SOCS proteins markedly induce de novo fatty acid synthesis in the liver by both the up-regulation of SREBP-1c and persistent IR with hyperinsulinemia which stimulates SREBP-1c-mediated gene expression.
The molecular mechanism that leads to IRS-1 degradation varies according to genotype in patients with heptitis C virus infection. Genotype 1 promotes the expression of SOCS-3 as genotype 3 promotes SOCS 7 expression, with a mechanism of IRS-1 degradation similar to that induced by SOCS 3; it also inhibits PPAR-γ, further worsening IR. One of the steatogenic mechanisms is the promotion of de novo fatty acids synthesis by ınduced expression of SREBP-1c by HCV infection.
Nitric oxide synthase-2 (NOS2) or iNOS production are also induced by proinflammatory cytokines[63,64]. High-fat diet in rats causes up-regulation of iNOS mRNA expression and increases iNOS protein activity. Increased production of NOS2 might reduce insulin action in both muscle and pancreas and decreased iNOS activity protects muscles from high-fat diet-induced IR.
HCV induces protein phosphatase 2A expression, through an endoplasmic reticulum stress response pathway, which dephosphorylates protein kinase B (PkB)/Akt (a main enzyme in the insulin signaling pathway), and thereby lowers its kinase activity[65].
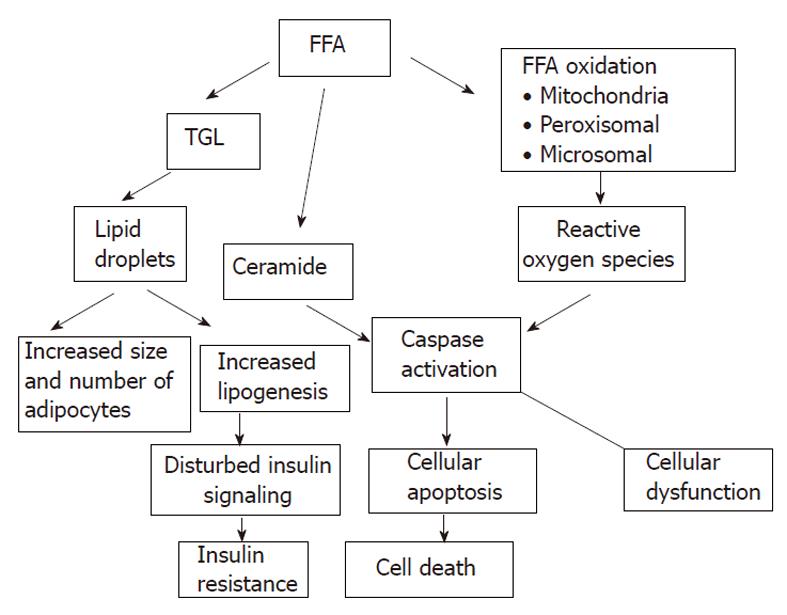
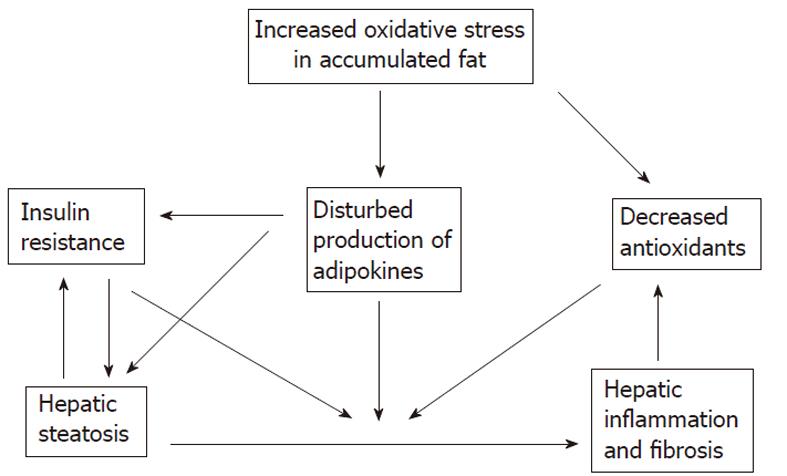
The accumulation of fat within the hepatocytes sensitizes the liver to injury from a variety of causes and the regenerative capacity of a fatty liver is impaired (Figures 5 and 6)[66-68]. An interacting network of cytokines and adipokines that regulate inflammation is disrupted. p53 is involved in the mechanisms of hepatocellular injury accompanied by steatosis[69].
Steatosis is one of the characteristic histopathologic features of HCV caused by chronic liver disease, and is also closely related to IR. Insulin resistance is one of the leading factors for severe fibrosis in chronic HCV infections. Moreover, hyperinsulinemia has a deleterious effect on the management of chronic HCV. The underlying mechanisms of this complex interaction are not fully understood. A direct cytopathic effect of HCV has been suggested. The genomic structure of HCV, lipid metabolism, the molecular links between the HCV core protein and lipid droplets and increased neolipogenesis and inhibited fatty acid degradation in mitochondria have been investigated.
This article is dedicated to my mentors Professor Stephen H Caldwell from the University of Virginia Health System and Professor Brent A Neuschwander-Tetri from the St. Louis University Hospital in gratitude for having guided me into Basic and Clinical Hepatology.
| 1. | Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 781] [Cited by in RCA: 751] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 2. | Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000;32:689-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 335] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 3. | Ratziu V, Bonyhay L, Di Martino V, Charlotte F, Cavallaro L, Sayegh-Tainturier MH, Giral P, Grimaldi A, Opolon P, Poynard T. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35:1485-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 317] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 4. | Romero-Gómez M. Insulin resistance and hepatitis C. World J Gastroenterol. 2006;12:7075-7080. [PubMed] |
| 5. | Arao M, Murase K, Kusakabe A, Yoshioka K, Fukuzawa Y, Ishikawa T, Tagaya T, Yamanouchi K, Ichimiya H, Sameshima Y. Prevalence of diabetes mellitus in Japanese patients infected chronically with hepatitis C virus. J Gastroenterol. 2003;38:355-360. [PubMed] |
| 6. | White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831-844. [PubMed] |
| 7. | Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP, Sobesky R, Martinot-Peignoux M, Maylin S, Nicolas-Chanoine MH. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416-423. [PubMed] |
| 8. | Lecube A, Hernández C, Genescà J, Esteban JI, Jardí R, Simó R. High prevalence of glucose abnormalities in patients with hepatitis C virus infection: a multivariate analysis considering the liver injury. Diabetes Care. 2004;27:1171-1175. [PubMed] |
| 9. | Sougleri M, Labropoulou-Karatza C, Paraskevopoulou P, Fragopanagou H, Alexandrides T. Chronic hepatitis C virus infection without cirrhosis induces insulin resistance in patients with alpha-thalassaemia major. Eur J Gastroenterol Hepatol. 2001;13:1195-1199. [PubMed] |
| 10. | Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, McCaughan GW, George J. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected]. Gastroenterology. 2003;125:1695-1704. [PubMed] |
| 11. | Akdogan M, Kuran SÖ, Kayhan B, editors . Is there a relationship between insulin resistance and fibrosis in patients with chronic viral hepatitis? Proceedings of the 17th Gastroenterology Conference; 2009 Sep 2-8; Antalya, TR. Ankara: Nobel 2009; 34. |
| 12. | Milner KL, van der Poorten D, Trenell M, Jenkins AB, Xu A, Smythe G, Dore GJ, Zekry A, Weltman M, Fragomeli V. Chronic hepatitis C is associated with peripheral rather than hepatic insulin resistance. Gastroenterology. 2010;138:932-941.e1-e3. [PubMed] |
| 13. | Romero-Gómez M, Diago M, Andrade RJ, Calleja JL, Salmerón J, Fernández-Rodríguez CM, Solà R, García-Samaniego J, Herrerías JM, De la Mata M. Treatment of insulin resistance with metformin in naïve genotype 1 chronic hepatitis C patients receiving peginterferon alfa-2a plus ribavirin. Hepatology. 2009;50:1702-1708. [PubMed] |
| 14. | Chen LK, Chou YC, Tsai ST, Hwang SJ, Lee SD. Hepatitis C virus infection-related Type 1 diabetes mellitus. Diabet Med. 2005;22:340-343. [PubMed] |
| 15. | Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, Adinolfi LE, Asselah T, Jonsson JR, Smedile A. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636-1642. [PubMed] |
| 16. | Machado MV, Cortez-Pinto H. Insulin resistance and steatosis in chronic hepatitis C. Ann Hepatol. 2009;8 Suppl 1:S67-S75. [PubMed] |
| 17. | Cua IH, Hui JM, Kench JG, George J. Genotype-specific interactions of insulin resistance, steatosis, and fibrosis in chronic hepatitis C. Hepatology. 2008;48:723-731. [PubMed] |
| 18. | Bedossa P, Moucari R, Chelbi E, Asselah T, Paradis V, Vidaud M, Cazals-Hatem D, Boyer N, Valla D, Marcellin P. Evidence for a role of nonalcoholic steatohepatitis in hepatitis C: a prospective study. Hepatology. 2007;46:380-387. [PubMed] |
| 19. | Thomopoulos KC, Arvaniti V, Tsamantas AC, Dimitropoulou D, Gogos CA, Siagris D, Theocharis GJ, Labropoulou-Karatza C. Prevalence of liver steatosis in patients with chronic hepatitis B: a study of associated factors and of relationship with fibrosis. Eur J Gastroenterol Hepatol. 2006;18:233-237. [PubMed] |
| 20. | Pazienza V, Clément S, Pugnale P, Conzelman S, Foti M, Mangia A, Negro F. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45:1164-1171. [PubMed] |
| 21. | Abid K, Pazienza V, de Gottardi A, Rubbia-Brandt L, Conne B, Pugnale P, Rossi C, Mangia A, Negro F. An in vitro model of hepatitis C virus genotype 3a-associated triglycerides accumulation. J Hepatol. 2005;42:744-751. [PubMed] |
| 22. | Moriya K, Yotsuyanagi H, Shintani Y, Fujie H, Ishibashi K, Matsuura Y, Miyamura T, Koike K. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J Gen Virol. 1997;78:1527-1531. [PubMed] |
| 23. | Chang ML, Chen JC, Yeh CT, Sheen IS, Tai DI, Chang MY, Chiu CT, Lin DY, Bissell DM. Topological and evolutional relationships between HCV core protein and hepatic lipid vesicles: studies in vitro and in conditionally transgenic mice. World J Gastroenterol. 2007;13:3472-3477. [PubMed] |
| 24. | Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, Conti M, Huet S, Ba N, Buffet C. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738-744. [PubMed] |
| 25. | Paradis V, Dargere D, Vidaud M, De Gouville AC, Huet S, Martinez V, Gauthier JM, Ba N, Sobesky R, Ratziu V. Expression of connective tissue growth factor in experimental rat and human liver fibrosis. Hepatology. 1999;30:968-976. [PubMed] |
| 26. | Walsh MJ, Vanags DM, Clouston AD, Richardson MM, Purdie DM, Jonsson JR, Powell EE. Steatosis and liver cell apoptosis in chronic hepatitis C: a mechanism for increased liver injury. Hepatology. 2004;39:1230-1238. [PubMed] |
| 27. | Basaranoglu M, Neuschwander-Tetri BA. Metabolic Aspects of Chronic Liver Disease. Pathophysiology of Nonalcoholic Steatohepatitis. Schattner A, Knobler H, editors. NY: Nova Science 2008; 1-70. |
| 28. | Eckel RH, Alberti KG, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;375:181-183. [PubMed] |
| 29. | Dahlman I, Arner P. Genetics of adipose tissue biology. Prog Mol Biol Transl Sci. 2010;94:39-74. [PubMed] |
| 30. | Le Marchand-Brustel Y, Gual P, Grémeaux T, Gonzalez T, Barrès R, Tanti JF. Fatty acid-induced insulin resistance: role of insulin receptor substrate 1 serine phosphorylation in the retroregulation of insulin signalling. Biochem Soc Trans. 2003;31:1152-1156. [PubMed] |
| 31. | Okamoto H, Obici S, Accili D, Rossetti L. Restoration of liver insulin signaling in Insr knockout mice fails to normalize hepatic insulin action. J Clin Invest. 2005;115:1314-1322. [PubMed] |
| 32. | Anai M, Funaki M, Ogihara T, Terasaki J, Inukai K, Katagiri H, Fukushima Y, Yazaki Y, Kikuchi M, Oka Y. Altered expression levels and impaired steps in the pathway to phosphatidylinositol 3-kinase activation via insulin receptor substrates 1 and 2 in Zucker fatty rats. Diabetes. 1998;47:13-23. [PubMed] |
| 33. | Taniguchi CM, Ueki K, Kahn R. Complementary roles of IRS-1 and IRS-2 in the hepatic regulation of metabolism. J Clin Invest. 2005;115:718-727. [PubMed] |
| 34. | Buettner R, Straub RH, Ottinger I, Woenckhaus M, Schölmerich J, Bollheimer LC. Efficient analysis of hepatic glucose output and insulin action using a liver slice culture system. Horm Metab Res. 2005;37:127-132. [PubMed] |
| 35. | Fisher SJ, Kahn CR. Insulin signaling is required for insulin's direct and indirect action on hepatic glucose production. J Clin Invest. 2003;111:463-468. [PubMed] |
| 36. | Jensen MD, Caruso M, Heiling V, Miles JM. Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes. 1989;38:1595-1601. [PubMed] |
| 37. | Smith U, Axelsen M, Carvalho E, Eliasson B, Jansson PA, Wesslau C. Insulin signaling and action in fat cells: associations with insulin resistance and type 2 diabetes. Ann N Y Acad Sci. 1999;892:119-126. [PubMed] |
| 38. | Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473-481. [PubMed] |
| 39. | Summers SA, Whiteman EL, Birnbaum MJ. Insulin signaling in the adipocyte. Int J Obes Relat Metab Disord. 2000;24 Suppl 4:S67-S70. [PubMed] |
| 40. | Formiguera X, Cantón A. Obesity: epidemiology and clinical aspects. Best Pract Res Clin Gastroenterol. 2004;18:1125-1146. [PubMed] |
| 41. | Karlsson HK, Zierath JR. Insulin signaling and glucose transport in insulin resistant human skeletal muscle. Cell Biochem Biophys. 2007;48:103-113. [PubMed] |
| 42. | Pittas AG, Joseph NA, Greenberg AS. Adipocytokines and insulin resistance. J Clin Endocrinol Metab. 2004;89:447-452. [PubMed] |
| 44. | Arner P. The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones. Trends Endocrinol Metab. 2003;14:137-145. [PubMed] |
| 45. | Nicholls HT, Kowalski G, Kennedy DJ, Risis S, Zaffino LA, Watson N, Kanellakis P, Watt MJ, Bobik A, Bonen A. Hematopoietic cell-restricted deletion of CD36 reduces high-fat diet-induced macrophage infiltration and improves insulin signaling in adipose tissue. Diabetes. 2011;60:1100-1110. [PubMed] |
| 46. | Fritsche L, Weigert C, Häring HU, Lehmann R. How insulin receptor substrate proteins regulate the metabolic capacity of the liver--implications for health and disease. Curr Med Chem. 2008;15:1316-1329. [PubMed] |
| 47. | Smith U. Impaired ('diabetic') insulin signaling and action occur in fat cells long before glucose intolerance--is insulin resistance initiated in the adipose tissue? Int J Obes Relat Metab Disord. 2002;26:897-904. [PubMed] |
| 48. | Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333-336. [PubMed] |
| 49. | Pittas AG, Joseph NA, Greenberg AS. Adipocytokines and insulin resistance. J Clin Endocrinol Metab. 2004;89:447-452. [PubMed] |
| 50. | Kanety H, Feinstein R, Papa MZ, Hemi R, Karasik A. Tumor necrosis factor alpha-induced phosphorylation of insulin receptor substrate-1 (IRS-1). Possible mechanism for suppression of insulin-stimulated tyrosine phosphorylation of IRS-1. J Biol Chem. 1995;270:23780-23784. [PubMed] |
| 51. | Kim YB, Shulman GI, Kahn BB. Fatty acid infusion selectively impairs insulin action on Akt1 and protein kinase C lambda /zeta but not on glycogen synthase kinase-3. J Biol Chem. 2002;277:32915-32922. [PubMed] |
| 52. | Lam TK, Yoshii H, Haber CA, Bogdanovic E, Lam L, Fantus IG, Giacca A. Free fatty acid-induced hepatic insulin resistance: a potential role for protein kinase C-delta. Am J Physiol Endocrinol Metab. 2002;283:E682-E691. [PubMed] |
| 53. | Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111-1119. [PubMed] |
| 54. | Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183-190. [PubMed] |
| 55. | Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med. 2001;7:1138-1143. [PubMed] |
| 56. | Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem. 2001;276:47944-47949. [PubMed] |
| 57. | Ueki K, Kondo T, Tseng YH, Kahn CR. Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc Natl Acad Sci USA. 2004;101:10422-10427. [PubMed] |
| 58. | Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394-42398. [PubMed] |
| 59. | Johnston JA, O'Shea JJ. Matching SOCS with function. Nat Immunol. 2003;4:507-509. [PubMed] |
| 60. | Farrell GC. Signalling links in the liver: knitting SOCS with fat and inflammation. J Hepatol. 2005;43:193-196. [PubMed] |
| 61. | Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10:739-743. [PubMed] |
| 62. | Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125-1131. [PubMed] |
| 63. | Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796-1808. [PubMed] |
| 64. | Rockey DC, Shah V. Nitric oxide biology and the liver: report of an AASLD research workshop. Hepatology. 2004;39:250-257. [PubMed] |
| 65. | Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171-176. [PubMed] |
| 66. | Wan G, Ohnomi S, Kato N. Increased hepatic activity of inducible nitric oxide synthase in rats fed on a high-fat diet. Biosci Biotechnol Biochem. 2000;64:555-561. [PubMed] |
| 67. | Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci USA. 1997;94:2557-2562. [PubMed] |
| 68. | Yang S, Lin H, Diehl AM. Fatty liver vulnerability to endotoxin-induced damage despite NF-kappaB induction and inhibited caspase 3 activation. Am J Physiol Gastrointest Liver Physiol. 2001;281:G382-G392. [PubMed] |
| 69. | Yahagi N, Shimano H, Matsuzaka T, Sekiya M, Najima Y, Okazaki S, Okazaki H, Tamura Y, Iizuka Y, Inoue N. p53 involvement in the pathogenesis of fatty liver disease. J Biol Chem. 2004;279:20571-20575. [PubMed] |
Peer reviewer: Nagarajan Perumal, Dr., Compliance Veterinarian, Center for Life Science, IACUC OFFICE, National University of Singapore, 117456, Singapore
S- Editor Tian L L- Editor Webster JR E- Editor Zhang DN