Published online Aug 21, 2011. doi: 10.3748/wjg.v17.i31.3585
Revised: May 9, 2011
Accepted: May 16, 2011
Published online: August 21, 2011
Endoscopic submucosal dissection (ESD) is a highly refined technique compared to conventional endoscopic mucosal resection. It enables complete resection of early gastric cancer (EGC) which has no possibility of lymph node metastasis. Indication for ESD of EGC generally entails early gastric cancer confined to the mucosa with well differentiated histology, though there are clinically suitable expanded criteria. As ESD requires specific skill and expertise, endoscopists need to be familiarized with basic methods and the use of special devices. The essence of the technique is to dissect the submucosal layer with direct vision and maintain the cutting plane above the underlying proper muscle layer. Although there are some differences in the detailed technical aspect, the cardinal method of ESD is now well established and standardized. Furthermore, research and development of new ESD devices that render more efficient, safe ESD are still in progress to improve the overall result of ESD on early gastric cancer.
- Citation: Lee WS, Cho JW, Kim YD, Kim KJ, Jang BI. Technical issues and new devices of ESD of early gastric cancer. World J Gastroenterol 2011; 17(31): 3585-3590
- URL: https://www.wjgnet.com/1007-9327/full/v17/i31/3585.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i31.3585
Endoscopic submucosal dissection (ESD) is a novel endoscopic technique that enables en bloc resection of large superficial gastric cancer[1]. Since this technique was introduced, there has been remarkable improvement in technique and experience regarding the safety and efficiency of ESD[2-4]. Though reliable, long term results have not been obtained, there is a large body of evidence to suggest that ESD is the therapy of choice for early gastric cancer on occasions when the risk of lymph node metastasis can be excluded[5,6]. General indications for ESD for gastric cancer were proposed on the basis of Japanese studies[7,8]. The absolute indication was non-ulcerative, well-differentiated mucosal cancer less than 2 cm in diameter. Expanded criteria could encompass non-ulcerative well-differentiated cancer over 2 cm, ulcerative well-differentiated cancer under 3 cm and non-ulcerative, well-differentiated, submucosal invading (limited to 500 μm below the lamina propria) cancer under 3 cm in diameter[9].
Individual ESD technique could vary among endoscopists, but the cardinal aspect of this revolutionary technique is quite straightforward. The following section deals with a brief contemporary summary on the subject, which includes core technical issues and new devices pertaining to ESD procedure.
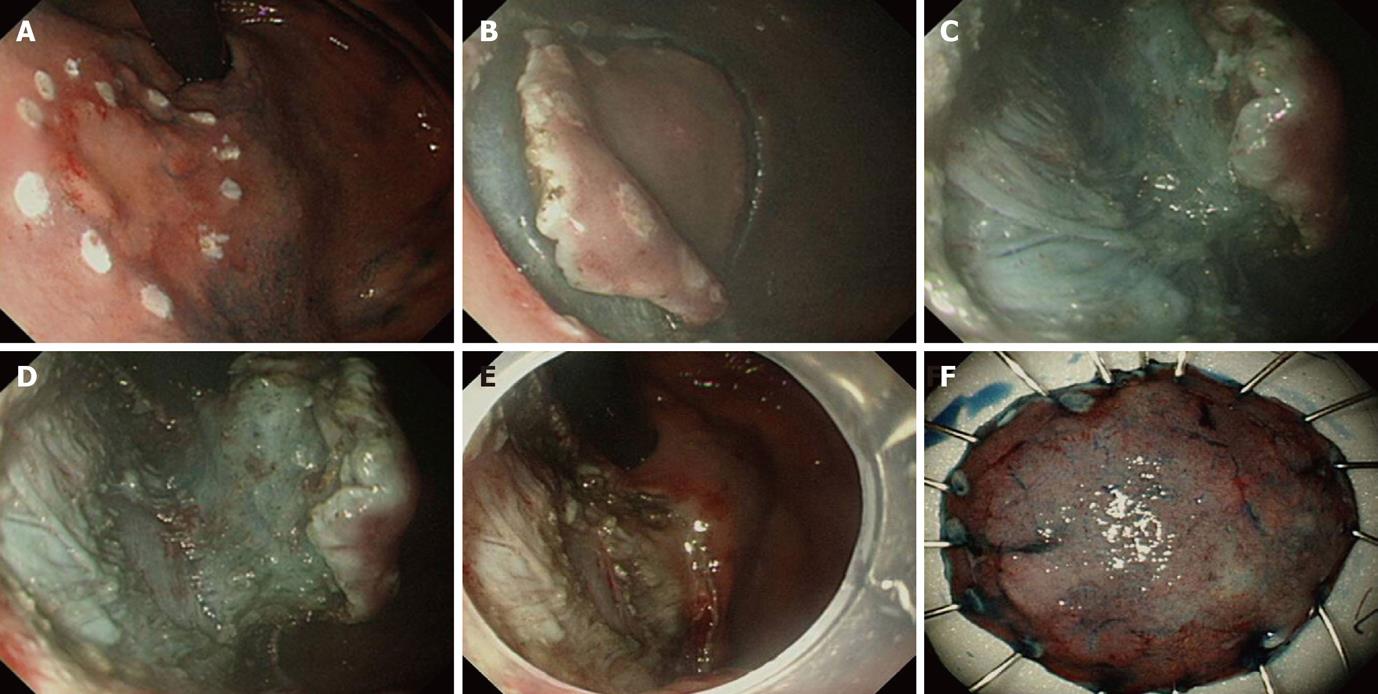
ESD is a unique, advantageous procedure over conventional endoscopic mucosal resection in that endoscopists can determine the extent of resection through establishing a outer imaginary line around the lesion[10]. This not only helps endoscopists to control the dissection process, but also ensures complete resection of the lesion confirmed histologically after ESD. Therefore, clarifying the boundaries of the lesion by careful observation before entering the procedure is highly important. Visual enhancing methods to improve detection of the lesion, such as chromoendoscopy, narrow band imaging or magnifying endoscopy, are sometimes helpful[11-14]. Using magnified pit pattern and microvascular pattern as a reference, magnification and endoscopy with or without a narrow band imaging system can give more information about the histological differentiation, depth of invasion and clarification of the extent of the EGC[15,16]. Preoperative diagnosis by image-enhanced endoscopy may have a significant supplementary role to conventional endoscopic ultrasonography in exact localization and clarification of indication, thereby improving the overall result of the procedure (Figure 1).
Marking around the lateral boundary of the target lesion is usually done by pointed devices at the coagulation setting of an electrosurgical unit. Devices such as the needle knife, flex knife, and hook knife are commonly used in careful contact with the mucosa, while coagulating force is fired only briefly to prevent deep thermal injury. Alternatively, argon plasma coagulation is conveniently used due to its non-contact thermal effect[17]. It is important to mark at least 5 mm apart from the outer circumferential margin of the lesion. After completion of marking, additional marking at the proximal or distal inner part of the lesion is usually required as a reference for determining the orientation of the resected specimen (Figure 1A).
Traditionally, hyper or isotonic saline mixed with indigocarmine and epinephrine has been used as basic injection solution. Characteristics of ideal injection materials would be to reduce submucosal blebs, have a hemostatic effect and be non-toxic to tissue. To fulfill these requirements, various injection solutions and mixtures, including hypertonic glucose, glycerol, sodium hyaluronic acid, and fibrinogen, have been tried. Some reported that high molecular hyaluronic acid solution was superior to others, albeit at high cost[18-20]. Maintaining the angle of injection needle 45 degrees tangentially for the mucosa is advised to avoid injecting into the muscle layer. It is recommended that injection starts from the anal part the lesion first and proceeds to the proximal part to avoid interruption of the visual field. For each endoscopist’s preference, partial injection and simultaneous submucosal dissection, beginning from a specific area, can be done initially instead of elevating whole circumference evenly.
Mucosal incision usually involves circumferential cutting around the lesion prior to submucosal dissection. It is important to incise deep enough into the muscularis mucosal layer that it expose the underlying submucosal layer, because shallow incision leads to unexpected bleeding and make subsequent submucosal dissection difficult. On doing this, immediate trimming by coagulation force with knives on the initial incised spot is really helpful in completing incision and preventing bleeding from the incised area. Available devices are the needle knife, insulation-tipped (IT) knife, flex knife, hook knife, and triangular-tipped knife[21-23]. With a needle knife, a small incision hole is made prior to insertion of the IT knife into the submucosal layer and beginning circumferential incision[1]. On the other hand, needle knife, flex knife, hook knife and triangular knife can be used alone to complete the incision. The sequence and direction of the mucosal incision is dependent on the location of the lesion and selection of the devices (Figure 1B).
Submucosal dissection is a continuing process related to mucosal incision (precut) and also the final stage of ESD. The entire procedure, from marking to dissection, should be carefully designed prior to ESD so that each step guarantees a smooth transition into the next. Direction of gravity, location of lesion, and presence of fibrosis and ulcer should all be taken into account thoroughly in order to apply different tactics and devices for individual lesions (Figure 1C and D).
The technique of submucosal dissection relies heavily on device selection. There are two different classes of knives currently used in clinical practice. One category is pointed tip devices such as needle, flex and hook knives, which are useful for horizontal dissection and have an easy maneuvering quality in all directions. The category contains linear blade devices such as the IT knife, which has an insulated ball tip to prevent perforation and provide fulcrum during dissection. While the IT knife has some disadvantages against fibrotic lesions and features diminished horizontal cutting ability, dissection is quicker and more efficient than other devices, especially in the stomach. Careful dissection with pointed tip knives may be the only reliable option for lesions with ulcer or dense fibrosis.
For successful submucosal dissection, measures to adjust to various situations, such as optimization of the operation field by repeated submucosal injection, utilization of transparent cap, adjusting air insufflations and frequent suction, are known to be essential. The cardinal aspect of dissection is maintaining an optimal dissection plane through the submucosal layer, while prudently avoiding injury to the underlying proper muscle layer. By this method, unexpected, blind dissection into the muscle layer and resultant perforation can be prevented.
Bleeding should be minimized for the clear operation field by recognition and meticulous coagulation of vessels before they are inadvertently injured by a cutting knife. Failure to do so often results in a poor field of view and a prolonged procedure time.
Dissected specimens can be dragged out of the stomach to prepare for histopathologic examination. Such specimens should be handled with care during stretching and fixation on the board. Processing the specimen should guarantee accurate analysis and correct diagnosis. Before submitting to pathology, endoscopists are obliged to determine spatial orientation and cutting direction for the preparation of pathologic specimens.
For successful ESD, the understanding and proper use of electrosurgical units is essential[24]. Earlier models of electrosurgical units were composed of a simple cutting and coagulation mode with only the output being adjustable. Recent models added multiple modes that could be used on different lesion characteristics. ICC 200 and VIO300D (ERBD, Germany) are equipped with sensors that pick up the changing signals from the cutting device and tissue interaction and automatically control output and maintain quality of cutting. Cutting mode was comprised of Endocut I&Q mode, dry cut, and swift coagulation, whereas coagulation mode incorporates forced, soft and sprays coagulation.
The time taken for ESD can be longer than 1 or 2 h if the lesion is large and in a difficult location. The patient often complains of abdominal distension and an urge for belching, owing to continuous air inflation of the stomach for maintaining visual field. It appears that there was less bloating and pain after procedures using CO2 for gut distension compared to air[25]. CO2 was found to be superior to air insufflations during balloon enteroscopy, endoscopic retrograde cholangio-pancreatography and invasive procedure such as colonic submucosal dissection[26-28]. Usage of CO2 clearly has a clear advantage when perforation occurs during ESD, because rapid absorption into splanchnic blood makes patients’ symptoms more tolerable and helps to stabilize vital signs.
Unlike the esophagus or large intestine, which is a long tubular structure, the stomach is a distensible bottle-shaped organ that requires diverse approaching techniques depending on the location. For a successful procedure, the anatomical structures and characteristics of each region should be first acknowledged so that individualized incision and dissection techniques can be applied.
As stated earlier, it is important to maintain a constant depth of incision or dissection while securing the desired operation field during the procedure by appropriately using turns of the endoscope (J-turns and U-turns), adjustments of the left/right levers, and changes of body position. In cases where fibrosis is severe, linear incision knives (such as needle, flex or hook knives) could be more advantageous, while incidence of perforation should be minimized by moving the knives elaborately by small amounts. Certain areas of stomach, such as the cardia or angularis, could be difficult to reach with a conventional endoscope. A multi-banding endoscope (GIF-2TQ260M, Olympus, Tokyo) can sometimes provide assistance in this situation, with its additional bending section enabling easier approximation to the lesion.
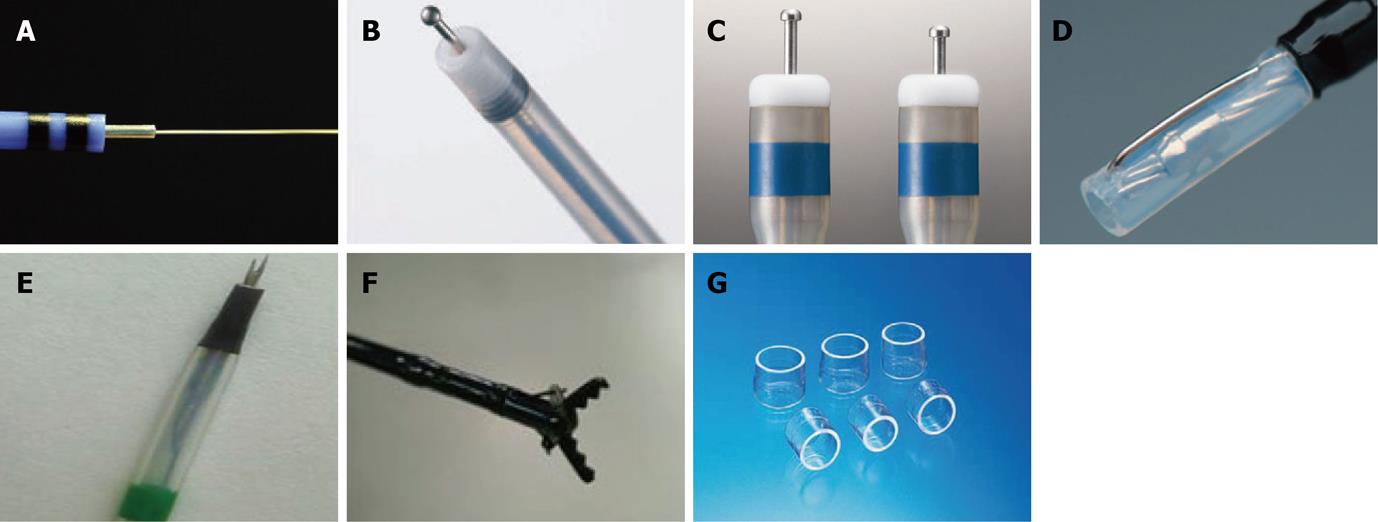
Knives are a basic instrument for ESD. Selection of the proper instrument influences the quality of the procedure and overall outcome. Every device has its own merits and disadvantages, with new devices usually giving specific modifications to cover up the weaknesses of earlier models.
Several knives, such as the needle, IT/IT-2 knife, hook, flex and flush knives are currently used. Constant effort has been paid to improve the dissection efficiency and safety of each knife. New devices have been devised to maximize ESD potential, while minimizing the ESD time, complication rate and patient discomfort.
The ERBE Hybrid Knife (ERBE, Tüebingen, Germany) combines an ultrafine high-pressure fluid jet with an electrocautery needle, making this device an attractive tool for performing ESD. This device allows submucosal fluid elevation with a preselected pressure and subsequent cutting or coagulation, and is used as a combination of a high-pressure water-jet and a radiofrequency surgical intervention. This allows needleless infusion and lifting of the lesion, as well as cutting and coagulation at the same time without the need to change instruments [29]. However, there is little experimental data and even less human experience with this device at the time of writing.
The ball tipped flush knife (Flush knife-BT) is the improved model of the flush knife, and was developed for the further improvement of the operability and ability of the hemostasis by the knife itself. It has a ball tip of 0.9 mm in diameter and 3 projecting parts of 1.5, 2 and 2.5 mm in length[30,31]. In one case-control study, Flush knife-BT appeared to improve hemostatic efficacy and dissection speed, compared with the standard flush knife.
The dual knife is a newly revised version of the pre-existing flex knife. Having a 0.3 mm needle tip shaped like a doorknob makes the needle less likely to slip, and simplifies marking and hemostasis. A two-step knife extrusion provides length adjustment, with no need for confirmation under endoscopic view, makes up for the weakness of the flex knife. These features enable a precise and effective cutting ability while reducing the burning effect and perforation.
Mucosectome is made of a non-conducted tip and endo-knife. Its blade is located at the side of the non-conducted tip and the tip is rotatable, so the blade can face the lumen and the non-conducted portion of the tip can face the wall of the hallow viscus[32].
The fork knife has two interchangeable knives; a fixed flexible snare and a forked knife, which forms a single working unit, and has an inlet for material injection or saline irrigation during the procedure. The knives can be changed during ESD by using two switches, the fork knob and core knob, located on the center of the instrument[33].
Grasping-type scissors forceps have a 0.8-mm-wide and 6-mm-long serrated cutting edge to facilitate grasping tissue. The outer side of the forceps is insulated so that electrosurgical current energy is concentrated at the blade to avoid burning the surrounding tissue. The forceps are also able to rotate to the desired orientation[34].
One of the most important devices in ESD is the distal attachment, which leads to safe and fast ESD. It can be fitted at the tip of the scope, making it possible to position the knives at the submucosal layer en face[1]. The distal attachment helps keep the field of view clear throughout the procedure and can be chosen from various sized and shaped models fitted with endoscopes. The most widely used hood is a disposable, transparent hood (D-201, Olympus, Japan). It is soft so that endoscopists can compress the submucosal layer without muscle injury and still get a good view. The small caliber tip transparent hood was reported to be useful in getting a higher complete resection rate and preventing perforation[35]. To facilitate the evacuation of blood and water that can be retained on the inner part of the hood and hinder field of the vision, hoods equipped with a irrigation port or side hole have been devised.
The technique of ESD for gastric cancer, though there can be slight differences between endoscopists, is relying on the basic concept of lifting the lesion and dissecting the submucosal layer under direct vision. Complications such as bleeding and perforation should be minimized for the invasive nature of the procedure. There are several knives and devices currently available for various purposes. However, the conclusion for which is superior is difficult to be judge because each set of devices has a unique advantage under specific circumstances. The development of new devices has focused on improving dissecting ability and shortening the procedural time while keeping safety in mind. It seems to be clear that current ESD techniques have certain limitations for the full application in all indications regarding early gastric cancer. Therefore, there should be a constant effort to refine and improve ESD devices to come up with ways to improve the ESD technique for early gastric cancer.
| 1. | Tanaka M, Ono H, Hasuike N, Takizawa K. Endoscopic submucosal dissection of early gastric cancer. Digestion. 2008;77 Suppl 1:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 484] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 3. | Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 529] [Article Influence: 31.1] [Reference Citation Analysis (1)] |
| 4. | Imagawa A, Okada H, Kawahara Y, Takenaka R, Kato J, Kawamoto H, Fujiki S, Takata R, Yoshino T, Shiratori Y. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38:987-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1350] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 6. | Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490-4498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 396] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 7. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 500] [Article Influence: 26.3] [Reference Citation Analysis (1)] |
| 8. | Ishikawa S, Togashi A, Inoue M, Honda S, Nozawa F, Toyama E, Miyanari N, Tabira Y, Baba H. Indications for EMR/ESD in cases of early gastric cancer: relationship between histological type, depth of wall invasion, and lymph node metastasis. Gastric Cancer. 2007;10:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Gotoda T, Iwasaki M, Kusano C, Seewald S, Oda I. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg. 2010;97:868-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 535] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 11. | Hyatt BJ, Paull PE, Wassef W. Gastric oncology: an update. Curr Opin Gastroenterol. 2009;25:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Sakai Y, Eto R, Kasanuki J, Kondo F, Kato K, Arai M, Suzuki T, Kobayashi M, Matsumura T, Bekku D. Chromoendoscopy with indigo carmine dye added to acetic acid in the diagnosis of gastric neoplasia: a prospective comparative study. Gastrointest Endosc. 2008;68:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009;41:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 345] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 14. | Uedo N, Ishihara R, Iishi H, Yamamoto S, Yamamoto S, Yamada T, Imanaka K, Takeuchi Y, Higashino K, Ishiguro S. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 270] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 15. | Otsuka Y, Niwa Y, Ohmiya N, Ando N, Ohashi A, Hirooka Y, Goto H. Usefulness of magnifying endoscopy in the diagnosis of early gastric cancer. Endoscopy. 2004;36:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 338] [Article Influence: 15.4] [Reference Citation Analysis (3)] |
| 17. | Yamamoto H. Endoscopic submucosal dissection of early cancers and large flat adenomas. Clin Gastroenterol Hepatol. 2005;3:S74-S76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Fujishiro M, Yahagi N, Kashimura K, Mizushima Y, Oka M, Enomoto S, Kakushima N, Kobayashi K, Hashimoto T, Iguchi M. Comparison of various submucosal injection solutions for maintaining mucosal elevation during endoscopic mucosal resection. Endoscopy. 2004;36:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 201] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Fujishiro M, Yahagi N, Nakamura M, Kakushima N, Kodashima S, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc. 2006;63:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Conio M, Rajan E, Sorbi D, Norton I, Herman L, Filiberti R, Gostout CJ. Comparative performance in the porcine esophagus of different solutions used for submucosal injection. Gastrointest Endosc. 2002;56:513-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Hoteya S, Iizuka T, Kikuchi D, Yahagi N. Endoscopic submucosal dissection for gastric submucosal tumor, endoscopic sub-tumoral dissection. Dig Endosc. 2009;21:266-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 461] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 23. | Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol. 2005;3:S71-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Morris ML, Tucker RD, Baron TH, Song LM. Electrosurgery in gastrointestinal endoscopy: principles to practice. Am J Gastroenterol. 2009;104:1563-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Janssens F, Deviere J, Eisendrath P, Dumonceau JM. Carbon dioxide for gut distension during digestive endoscopy: technique and practice survey. World J Gastroenterol. 2009;15:1475-1479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (5)] |
| 26. | Bretthauer M, Seip B, Aasen S, Kordal M, Hoff G, Aabakken L. Carbon dioxide insufflation for more comfortable endoscopic retrograde cholangiopancreatography: a randomized, controlled, double-blind trial. Endoscopy. 2007;39:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Domagk D, Bretthauer M, Lenz P, Aabakken L, Ullerich H, Maaser C, Domschke W, Kucharzik T. Carbon dioxide insufflation improves intubation depth in double-balloon enteroscopy: a randomized, controlled, double-blind trial. Endoscopy. 2007;39:1064-1067. [RCA] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Kozu T, Saito D. A pilot study to assess the safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection with the patient under conscious sedation. Gastrointest Endosc. 2007;65:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Neuhaus H, Wirths K, Schenk M, Enderle MD, Schumacher B. Randomized controlled study of EMR versus endoscopic submucosal dissection with a water-jet hybrid-knife of esophageal lesions in a porcine model. Gastrointest Endosc. 2009;70:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Toyonaga T, Man-I M, Fujita T, Nishino E, Ono W, Morita Y, Sanuki T, Masuda A, Yoshida M, Kutsumi H. The performance of a novel ball-tipped Flush knife for endoscopic submucosal dissection: a case-control study. Aliment Pharmacol Ther. 2010;32:908-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Toyonaga T, Man-I M, Morita Y, Kutsumi H, Inokuchi H, Azuma T. Effectiveness of the ball tipped flush knife in endoscopic submucosal dissection for the treatment of GI neoplasia. Gastrointest Endosc. 2009;69:AB263 [Doi: 10.1016/j.gie.2009.03.687]. |
| 32. | Kawahara Y, Imagawa A, Fujiki S, Shiratori Y. Novel procedure of endoscopic submucosal dissection (ESD) using a new device (mucosectome). Gastrointest Endosc. 2005;61:AB79. [Doi: 10.1016/S0016-5107(05)00599-7]. |
| 33. | Kim HG, Cho JY, Bok GH, Cho WY, Kim WJ, Hong SJ, Ko BM, Kim JO, Lee JS, Lee MS. A novel device for endoscopic submucosal dissection, the Fork knife. World J Gastroenterol. 2008;14:6726-6732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Akahoshi K, Honda K, Akahane H, Akiba H, Matsui N, Motomura Y, Kubokawa M, Endo S, Higuchi N, Oya M. Endoscopic submucosal dissection by using a grasping-type scissors forceps: a preliminary clinical study (with video). Gastrointest Endosc. 2008;67:1128-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 300] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
Peer reviewer: Antonello Trecca, MD, Usi Group Digestive Endoscopy and Gastroenterology, via Machiavelli, 22, 00185 Rome, Italy
S- Editor Tian L L- Editor Rutherford A E- Editor Xiong L