Published online Aug 14, 2011. doi: 10.3748/wjg.v17.i30.3560
Revised: January 4, 2011
Accepted: January 11, 2011
Published online: August 14, 2011
Pancreatic fistula is a quite rare complication in patients who undergo living donor liver transplantation (LDLT). However, in the cases that show pancreatic fistula, the limited volume of the graft and the resultant inadequate liver function may complicate the management of the fistula. As a result, the pancreatic fistula may result in the death of the patient. We present 2 cases in which endoscopic treatment was effective against pancreatic fistulas that developed after LDLT. In case 1, a 61-year-old woman underwent LDLT for primary biliary cirrhosis. Because of a portal venous thrombus caused by a splenorenal shunt, the patient underwent portal vein reconstruction, and a splenorenal shunt was ligated on postoperative day (POD) 7. The main pancreatic duct was injured during the manipulation to achieve hemostasis, thereby necessitating open drainage. However, discharge of pancreatic fluid continued even after POD 300. Endoscopic naso-pancreatic drainage (ENPD) was performed, and this procedure resulted in a remarkable decrease in drain output. The refractory pancreatic fistula healed on day 40 after ENPD. In case 2, a 58-year-old man underwent LDLT for cirrhosis caused by the hepatitis C virus. When the portal vein was exposed during thrombectomy, the pancreatic head was injured, which led to the formation of a pancreatic fistula. Conservative therapy was ineffective; therefore, ENPD was performed. The pancreatic fistula healed on day 38 after ENPD. The findings in these 2 cases show that endoscopic drainage of the main pancreatic duct is a less invasive and effective treatment for pancreatic fistulas that develop after LDLT.
- Citation: Nagatsu A, Taniguchi M, Shimamura T, Suzuki T, Yamashita K, Kawakami H, Abo D, Kamiyama T, Furukawa H, Todo S. Endoscopic naso-pancreatic drainage for the treatment of pancreatic fistula occurring after LDLT. World J Gastroenterol 2011; 17(30): 3560-3564
- URL: https://www.wjgnet.com/1007-9327/full/v17/i30/3560.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i30.3560
The incidence of complications associated with living donor liver transplantation (LDLT) is known to be greater than that associated with deceased donor liver transplantation (DDLT)[1-4]. In the patients who undergo LDLT, the incidence of complications, including mild complications, during the perioperative period can be as high as 82.8%. In particular, the rate of development of biliary complications after LDLT is twice that after DDLT[1]. Moreover, patients who undergo LDLT often have inadequate liver function because of the limited volume of the graft. Therefore, the management of complications is very difficult, and the mortality rate in critical cases is high.
Compared to other abdominal surgery, pancreatic fistula is a quite rare complication after LDLT[1-5], but it is theoretically possible because LDLT involves surgery in the area surrounding the portal vein, the pancreas, and the spleen. Pancreatic fistula causes hemorrhage, abscess, etc., and may result in the death of the patient[6-8]. Only 1 previous study has reported 2 cases of leakage of pancreatic fluid after liver transplantation. In those cases, leakage of a mixture of pancreatic fluid and bile was observed at the anastomosis site of the bile duct after DDLT[9]. However, there is no clear consensus on the management of pancreatic fistula after liver transplantation. Generally, conservative therapy is the first line of treatment, and surgery is performed only when the patient does not respond to conservative therapy[6-8]. Recently, however, endoscopic treatment has attracted more attention because it is less invasive than surgical treatment. We present 2 cases in which endoscopic treatment was effective against refractory pancreatic fistulas that developed after LDLT.
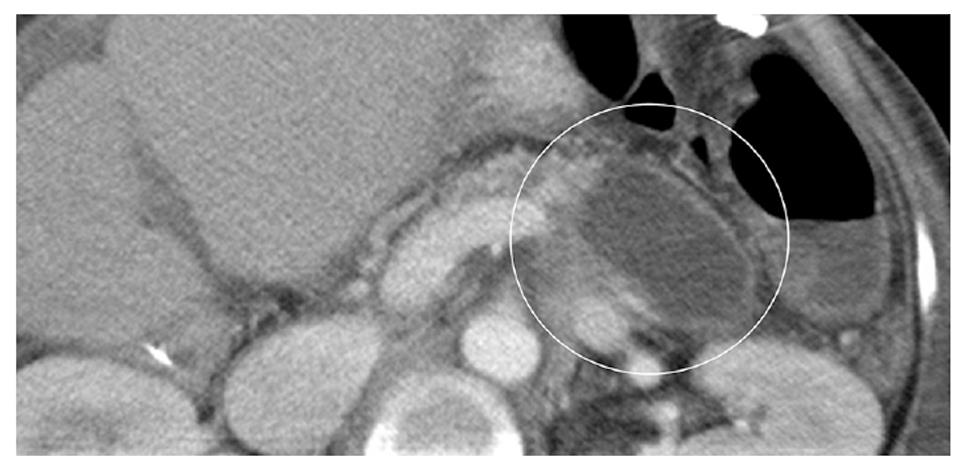
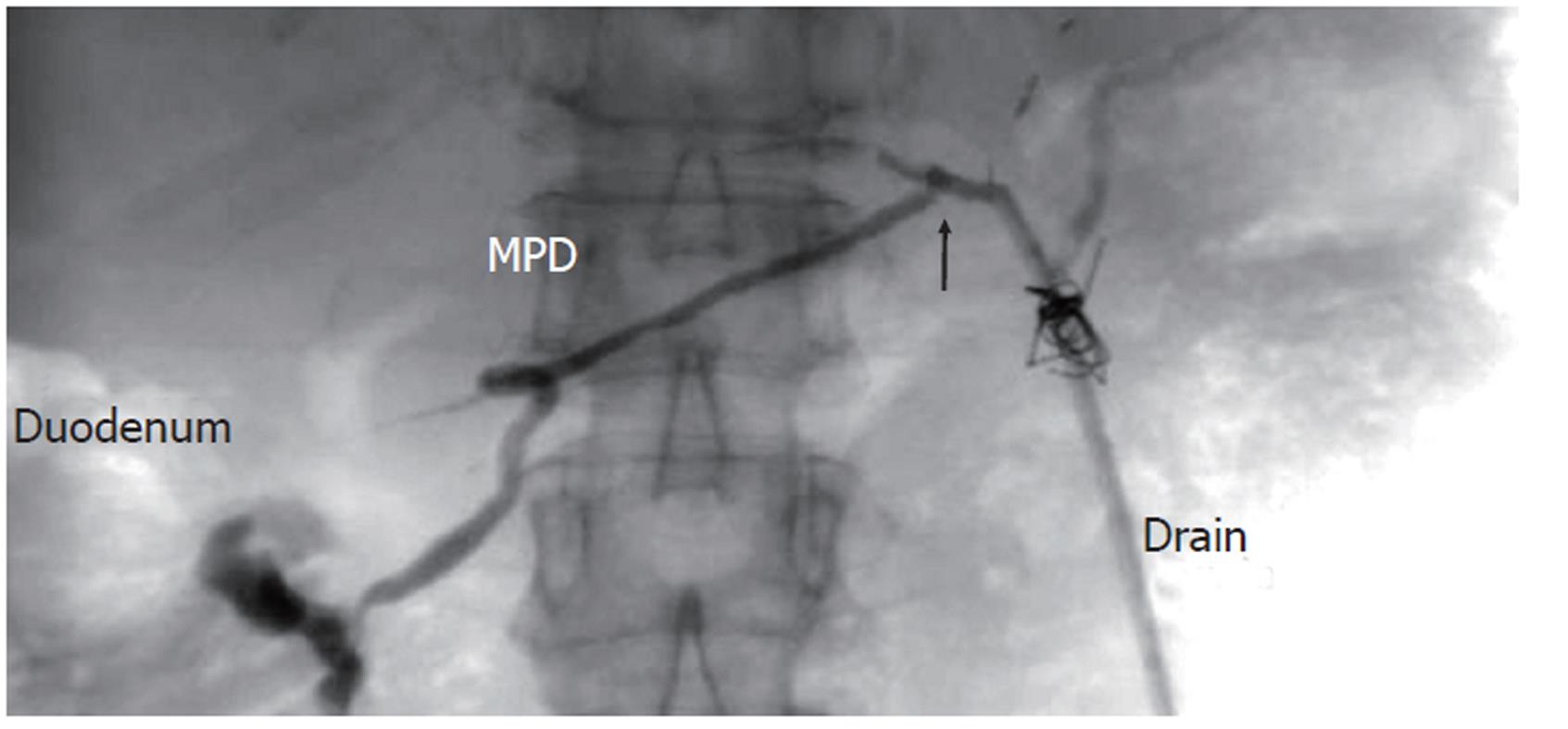
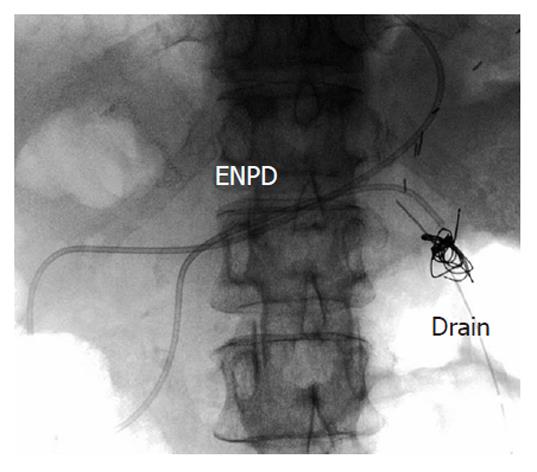
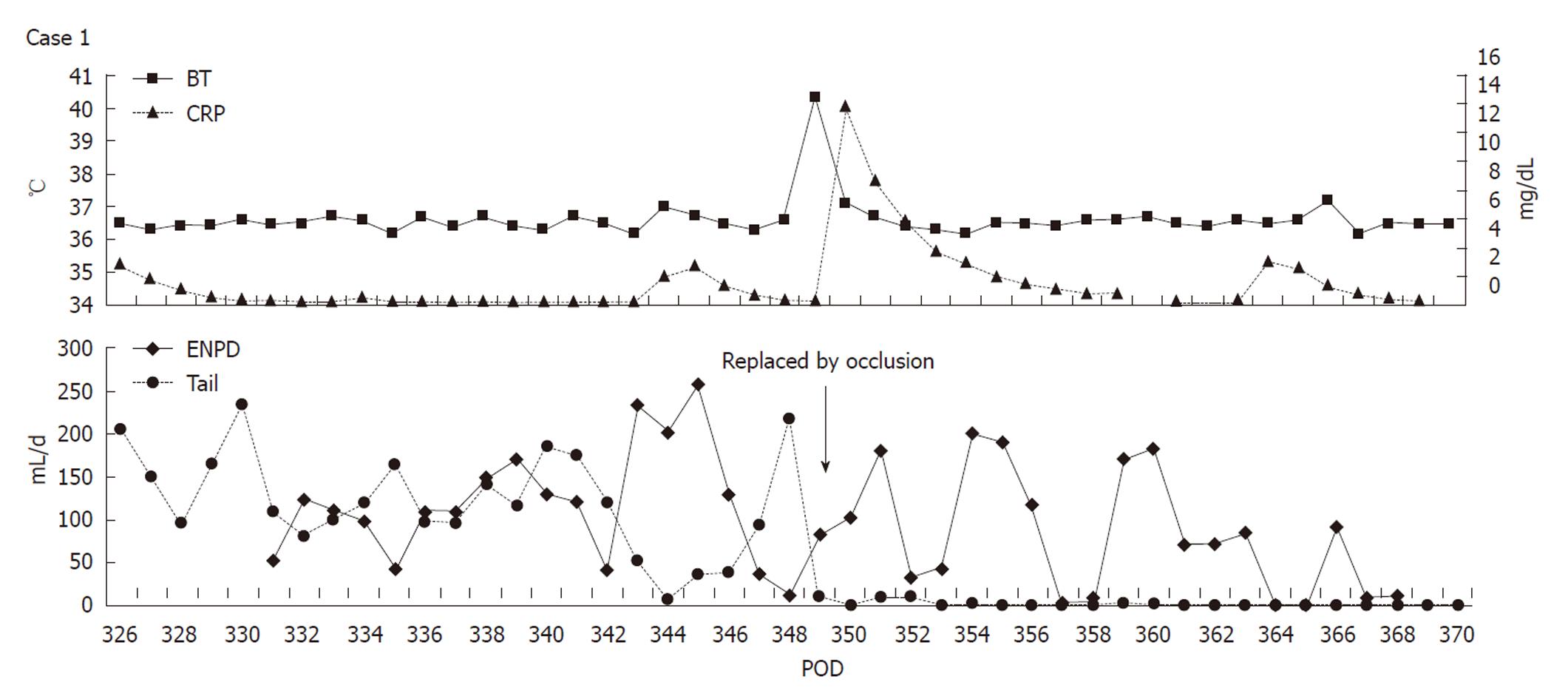
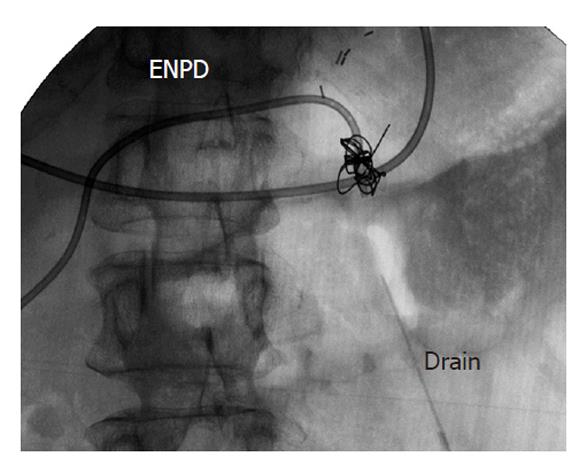
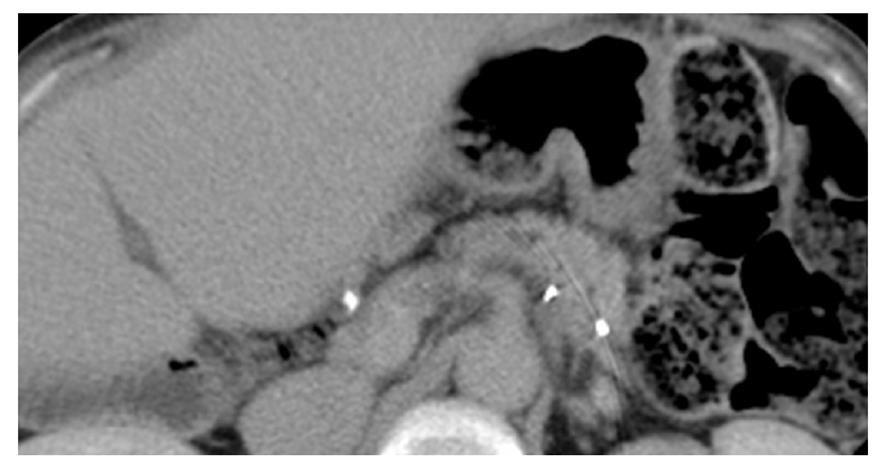
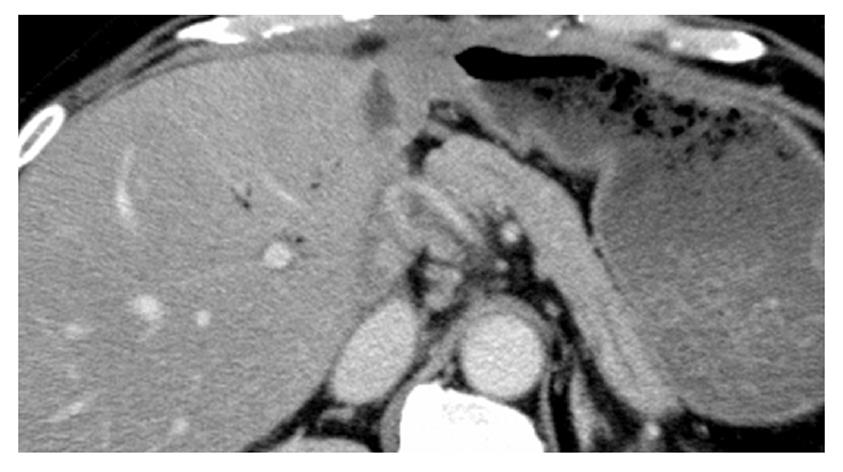
A 61-year-old woman underwent LDLT for primary biliary cirrhosis; the graft was obtained from the left liver lobe of her son. Abdominal computed tomography (CT) performed on postoperative day (POD) 7 revealed a portal vein thrombus; therefore, urgent exploratory laparotomy was performed. The thrombus was believed to have been induced by reduced portal blood flow, which was caused by splenorenal steal by an artificial shunt. After removing the thrombus, portal vein reconstruction was performed by using the right external iliac vein, and this procedure was followed by splenectomy. To increase the portal blood flow, a splenorenal shunt was ligated. The main pancreatic duct on the dorsal side of the pancreas was injured at the time of hemostatic manipulation; however, this injury was not identified immediately. CT performed on POD 13 revealed a hematoma at the lower edge of the pancreas. CT on POD 21 revealed that the hematoma under the pancreas had decreased in size (Figure 1). The amylase level of the drainage fluid was 22 690 IU/L; therefore, the hematoma at the inferior edge of the pancreas was considered to have ruptured because of a pancreatic leak. Another CT examination revealed fluid collection in the mesentery on the ventral side of the upper pole of the left kidney; therefore, open drainage was performed. To this end, a drain was placed at the tail of the pancreas and administration of octreotide was started. However, the leakage of pancreatic fluid from the drain did not stop. After several days, the patient’s general status stabilized, but surgical treatment for pancreatic fistula was still unsafe because of inadequate liver function. Therefore, the patient was discharged on POD 128 with the drain in place and was followed up. The patient was in a stable state at discharge. However, on POD 318, she was readmitted to our department because of fever. Examinations revealed that the drain at the tail of the pancreas had deviated and that the patient had developed liver necrosis, supposedly because of contact with the drain. On POD 320, we repositioned the drain by using a fluoroscope. A contrast test performed at that time revealed that the main pancreatic duct was completely disrupted (Figure 2). The patient was diagnosed with refractory pancreatic fistula, and an endoscopic naso-pancreatic drainage (ENPD) tube was inserted to the proximal side of the leakage on POD 331 (Figure 3); this procedure resulted in a remarkable decrease in drain output (Figures 4 and 5). The ENPD tube was removed on POD 368, and the drains at the tail of the pancreas were removed on POD 371. The patient was discharged on POD 375 without abnormal fluid collection around the pancreas (Figure 6). The patient is well without the recurrence of pancreatic fistula up to this time.
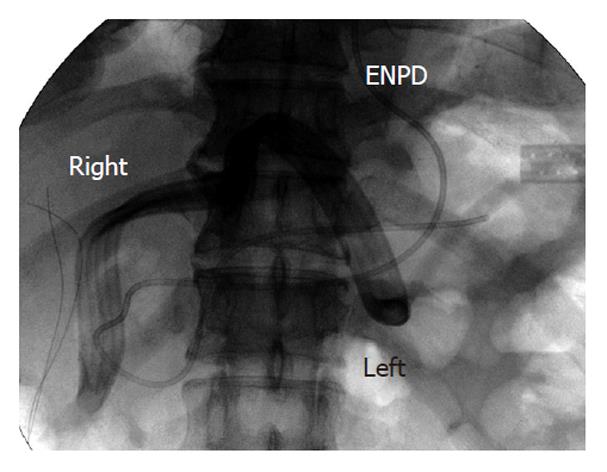
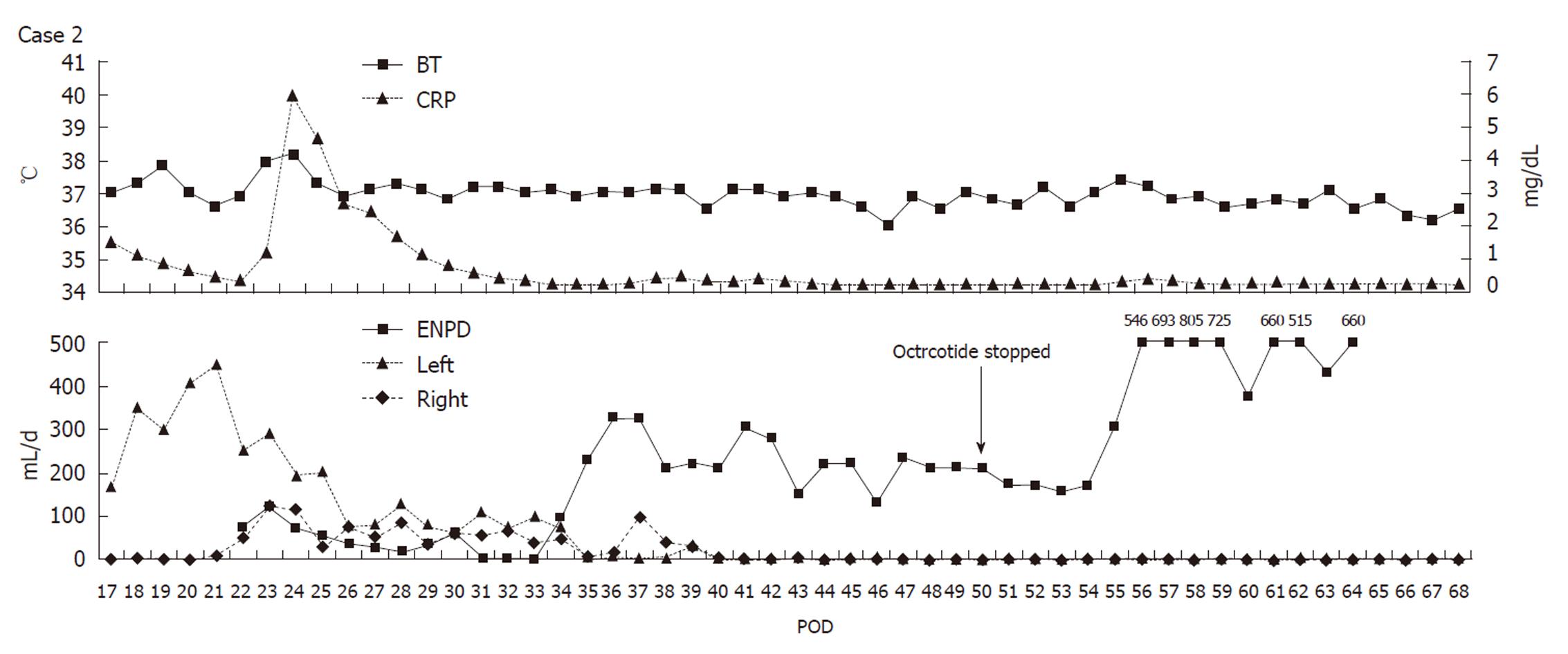
A 58-year-old man underwent LDLT for cirrhosis C; the graft was obtained from the right liver lobe of his daughter. Because the portal vein was occluded by a thrombus, the portal and splenic veins were stripped off from the surrounding tissue and were exposed in order to remove the thrombus. However, the upper edge of the pancreatic head was injured during this process. The amylase level measured at the upper edge of the pancreatic drain was high on POD 1; therefore, the patient received octreotide on POD 2. On POD 5, the patient showed high fever and acute peritonitis. Therefore, an emergency exploratory laparotomy was performed. Because fluid collection was observed around the pancreas, drains were placed at both the right and left edges of the pancreas. On POD 21, the total output from the drain was 460 mL/d, and the amylase level of the drainage fluid was 166 700 IU/L. The patient was diagnosed with high-output pancreatic fistula, and ENPD was performed on POD 22 (Figure 7). The drain output decreased very rapidly (Figure 8); therefore, the patient was allowed to consume solid foods on POD 49, and octreotide administration was stopped on POD 50. The ENPD tube was removed on POD 65, and the drains placed at the right and left edges of the pancreas were removed on POD 68 and POD 70, respectively. The patient was discharged on POD 100 without abnormal fluid collection around the pancreas (Figure 9). The patient is well and receiving regular out-patient treatment and is showing no recurrence of pancreatic fistula.
Pancreatic fistula is primarily treated by conservative therapy, which includes rapid total infusion or enteral nutrition along with administration of octreotide. The recovery rate after conservative therapy ranges from 44% to 85%[6-8]; thus, a number of cases are not resolved by conservative treatment. Surgical treatment has been performed in such cases. However, surgical treatment is highly invasive and may lead to various complications. Further, surgical treatment is associated with high mortality rates, with the mortality rate being as high as 23%-67% in the cases showing early peritonitis after the operation[9]. Endoscopic drainage of the main pancreatic duct via the ampulla of Vater, which was first reported in 1991[10], has drawn considerable attention. Boerma et al[11] (2006) reported an excellent recovery rate (87%) after endoscopic treatment of 15 cases of pancreatic fistula. In addition, other studies have reported recovery rates of about 58%-100% in the cases of pancreatic fistulas that do not respond to conservative therapy and involve endoscopic treatment[12-20]. To date, only 1 death caused by acute pancreatitis has been reported. However, since this death may also have been caused by inadequate drainage, a direct relationship between the death and endoscopic treatment could not be confirmed[13]. Unlike LDLT, endoscopic treatment for pancreatic fistula allows greater accessibility to the ampulla of Vater. Further, endoscopic treatment is less invasive than surgical treatment; therefore, it can easily replace conservative therapy if sufficient drainage is achieved. Thus, patients who undergo endoscopic treatment for pancreatic fistula can be expected to make an early recovery. Irrespective of their merits and demerits, both ENPD and endoscopic pancreatic stenting (EPS) have been referred to in the reports. ENPD causes a sense of discomfort in the pharynx; however, this technique enables easy diagnosis of occlusion and dropout because it allows monitoring of the pancreatic fluid. In contrast, in EPS the diagnosis of occlusion and dropout is difficult; however, this technique causes no sense of discomfort in the pharynx. We selected ENPD to enable safe monitoring of 2 channels of drainage: the endoscopic retrograde pancreatic drain as well as the intraperitoneal drain. In case 1, the drain tube had to be replaced because of the fever caused by occlusion; therefore, the choice of ENPD was considered to be reasonable. The patient in case 1 could have recovered earlier if the endoscopic treatment for pancreatic fistula had been initiated earlier. In each case, the patient recovered within approximately 40 d after ENPD. Further, the treatment had no influence on the patients’ general status. Endoscopic treatment is considered to be safe for treating pancreatic fistulas that develop after LDLT. New endoscopic techniques, such as ultrasonography (US)-guided drainage, have also been used to treat refractory cases that do not respond to drainage via the ampulla of Vater; however, only few reports have described these techniques. These new techniques may also be less invasive than surgical treatment[21, 22].
In conclusion, we described 2 cases of pancreatic fistula after LDLT that were not responsive to conservative therapy. In each case, the patient recovered within approximately 40 d after ENPD. Thus, endoscopic treatment for pancreatic fistula after LDLT should be adopted because of its high recovery rate and low invasiveness.
| 1. | Freise CE, Gillespie BW, Koffron AJ, Lok AS, Pruett TL, Emond JC, Fair JH, Fisher RA, Olthoff KM, Trotter JF. Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL Retrospective Cohort Study. Am J Transplant. 2008;8:2569-2579. [PubMed] |
| 2. | Ho MC, Wu YM, Hu RH, Ko WJ, Ni YH, Chang MH, Yang PM, Lai MY, Lin MH, Lin HY. Surgical complications and outcome of living related liver transplantation. Transplant Proc. 2004;36:2249-2251. [PubMed] |
| 3. | Marsh JW, Gray E, Ness R, Starzl TE. Complications of right lobe living donor liver transplantation. J Hepatol. 2009;51:715-724. [PubMed] |
| 4. | Emiroglu R, Sevmis S, Moray G, Savas N, Haberal M. Living-donor liver transplantation: results of a single center. Transplant Proc. 2007;39:1149-1152. [PubMed] |
| 5. | Tanaka K, Miyashiro I, Yano M, Kishi K, Motoori M, Seki Y, Noura S, Ohue M, Yamada T, Ohigashi H. Accumulation of excess visceral fat is a risk factor for pancreatic fistula formation after total gastrectomy. Ann Surg Oncol. 2009;16:1520-1525. [PubMed] |
| 6. | Parr ZE, Sutherland FR, Bathe OF, Dixon E. Pancreatic fistulae: are we making progress? J Hepatobiliary Pancreat Surg. 2008;15:563-569. [PubMed] |
| 7. | Pratt WB, Maithel SK, Vanounou T, Huang ZS, Callery MP, Vollmer CM Jr. Clinical and economic validation of the International Study Group of Pancreatic Fistula (ISGPF) classification scheme. Ann Surg. 2007;245:443-451. [PubMed] |
| 9. | Tung BY, Kowdley KV, Kimmey MB. Pancreatic fistula without pancreatitis after endoscopic biliary stent placement for bile leak after orthotopic liver transplantation. Gastrointest Endosc. 1999;49:647-651. [PubMed] |
| 10. | Kozarek RA, Ball TJ, Patterson DJ, Freeny PC, Ryan JA, Traverso LW. Endoscopic transpapillary therapy for disrupted pancreatic duct and peripancreatic fluid collections. Gastroenterology. 1991;100:1362-1370. [PubMed] |
| 11. | Boerma D, Rauws EA, van Gulik TM, Huibregtse K, Obertop H, Gouma DJ. Endoscopic stent placement for pancreaticocutaneous fistula after surgical drainage of the pancreas. Br J Surg. 2000;87:1506-1509. [PubMed] |
| 12. | Bracher GA, Manocha AP, DeBanto JR, Gates LK Jr, Slivka A, Whitcomb DC, Bleau BL, Ulrich CD 2nd, Martin SP. Endoscopic pancreatic duct stenting to treat pancreatic ascites. Gastrointest Endosc. 1999;49:710-715. [PubMed] |
| 13. | Telford JJ, Farrell JJ, Saltzman JR, Shields SJ, Banks PA, Lichtenstein DR, Johannes RS, Kelsey PB, Carr-Locke DL. Pancreatic stent placement for duct disruption. Gastrointest Endosc. 2002;56:18-24. [PubMed] |
| 14. | Varadarajulu S, Noone TC, Tutuian R, Hawes RH, Cotton PB. Predictors of outcome in pancreatic duct disruption managed by endoscopic transpapillary stent placement. Gastrointest Endosc. 2005;61:568-575. [PubMed] |
| 15. | Saeed ZA, Ramirez FC, Hepps KS. Endoscopic stent placement for internal and external pancreatic fistulas. Gastroenterology. 1993;105:1213-1217. [PubMed] |
| 16. | Kozarek RA, Ball TJ, Patterson DJ, Raltz SL, Traverso LW, Ryan JA, Thirlby RC. Transpapillary stenting for pancreaticocutaneous fistulas. J Gastrointest Surg. 1997;1:357-361. [PubMed] |
| 17. | Costamagna G, Mutignani M, Ingrosso M, Vamvakousis V, Alevras P, Manta R, Perri V. Endoscopic treatment of postsurgical external pancreatic fistulas. Endoscopy. 2001;33:317-322. [PubMed] |
| 18. | Fischer A, Benz S, Baier P, Hopt UT. Endoscopic management of pancreatic fistulas secondary to intraabdominal operation. Surg Endosc. 2004;18:706-708. [PubMed] |
| 19. | Le Moine O, Matos C, Closset J, Devière J. Endoscopic management of pancreatic fistula after pancreatic and other abdominal surgery. Best Pract Res Clin Gastroenterol. 2004;18:957-975. [PubMed] |
| 20. | Goasguen N, Bourrier A, Ponsot P, Bastien L, Lesurtel M, Prat F, Dousset B, Sauvanet A. Endoscopic management of pancreatic fistula after distal pancreatectomy and enucleation. Am J Surg. 2009;197:715-720. [PubMed] |
| 21. | Romano A, Spaggiari M, Masetti M, Sassatelli R, Di Benedetto F, De Ruvo N, Montalti R, Guerrini GP, Ballarin R, De Blasiis MG. A new endoscopic treatment for pancreatic fistula after distal pancreatectomy: case report and review of the literature. Gastrointest Endosc. 2008;68:798-801. [PubMed] |
| 22. | Arvanitakis M, Delhaye M, Bali MA, Matos C, Le Moine O, Devière J. Endoscopic treatment of external pancreatic fistulas: when draining the main pancreatic duct is not enough. Am J Gastroenterol. 2007;102:516-524. [PubMed] |
Peer reviewer: Marco Vivarelli, MD, Assistant Professor, Department of Surgery and Transplantation, University of Bologna, S. Orsola Hospital, 40123 Bologna, Italy
S- Editor Tian L L- Editor O’Neill M E- Editor Ma WH