Published online Jul 28, 2011. doi: 10.3748/wjg.v17.i28.3310
Revised: December 19, 2010
Accepted: December 26, 2010
Published online: July 28, 2011
AIM: To examine the effect of doxycycline on the activity of matrix metalloproteinases (MMPs) and oxidative stress in gastric tissues of rats following gastric injury.
METHODS: Gastric ulcers were generated in rats by administration of 70% ethanol, and activity of doxycycline was tested by administration 30 min prior to ethanol. Similarly, the effect of doxycycline was tested in an indomethacin-induced gastric ulcer model. The activities and expression of MMPs were examined by zymography and Western blot analysis.
RESULTS: Gastric injury in rats as judged by elevated ulcer indices following exposure to ulcerogen, either indomethacin or ethanol, was reversed significantly by doxycycline. Indomethacin-induced ulcerated gastric tissues exhibited about 12-fold higher proMMP-9 activity and about 5-fold higher proMMP-3 activity as compared to control tissues. Similarly, ethanol induced about 22-fold and about 6-fold higher proMMP-9 and proMMP-3 activities, respectively, in rat gastric tissues. Both proMMP-9 and MMP-3 activities were markedly decreased by doxycycline in ulcerogen treated rat gastric tissues. In contrast, the reduced MMP-2 activity in ulcerated tissues was increased by doxycycline during ulcer prevention. On the other hand, doxycycline inhibited significantly proMMP-9, -2 and -3 activities in vitro. In addition, doxycycline reduced oxidative load in gastric tissues and scavenged H2O2in vitro. Our results suggest a novel regulatory role of doxycycline on MMP-2 activity in addition to inhibitory action on MMP-9 and MMP-3 during prevention of gastric ulcers.
CONCLUSION: This is the first demonstration of dual action of doxycycline, that is, regulation of MMP activity and reduction of oxidative stress in arresting gastric injury.
- Citation: Singh LP, Mishra A, Saha D, Swarnakar S. Doxycycline blocks gastric ulcer by regulating matrix metalloproteinase-2 activity and oxidative stress. World J Gastroenterol 2011; 17(28): 3310-3321
- URL: https://www.wjgnet.com/1007-9327/full/v17/i28/3310.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i28.3310
Matrix metalloproteinases (MMPs) are a class of zinc-dependent endopeptidases that degrade matrix macromolecules and numerous other components such as growth factors, proteinases, and plasma proteins[1,2]. MMPs are best known for their action on extracellular matrix (ECM) remodeling, wound healing and angiogenesis, and have been implicated in the pathogenesis of various diseases including gastric ulcers[3-6]. The endogenous inhibitors of MMPs [tissue inhibitors of metalloproteinases (TIMPs) and α-2 macroglobulin] play a major role in balancing protease-antiprotease action[7,8]. MMPs are synthesized and secreted by gastric epithelial cells, macrophages, and neutrophils[9,10]. Numerous exogenous factors including nonsteroidal anti-inflammatory drugs (NSAIDs), stress, alcohol and Helicobacter pylori infection have been implicated in the etiology of gastric ulceration[11-14]. We have previously demonstrated that MMP-9 increases significantly in indomethacin-induced as well as ethanol-induced gastric ulcers[3,4,12]. Overexpression of MMP-9 has been attributed to coronary artery disease, retinopathy, renal disease, chronic obstructive pulmonary disease and atherosclerosis[15-17]. Involvement of several MMPs including gelatinases and stromelysins in various pathological conditions has been documented[5]. However, downregulation of MMP-2 activity in retinal and renal vasculature leads to thickening of the basement membrane due to collagen deposition and contributes to diabetic retinopathy and nephropathy, respectively[18]. Research into the pathophysiology of gastric ulcers suggests a central role of reactive oxygen species (ROS) in the development of the disease. MMP can be activated by ROS in vitro, and thus, imbalance of MMP activation and inactivation occurs[19]. An imbalance of MMP activity can lead to release of growth factors, and therefore, inhibition of MMPs can be a potential therapy for various diseases[20]. Ulcer healing is a complicated process that requires interactions between ECM proteins including collagens, proteases, cytokines, and growth factors. Repair of gastric wounds encompasses a series of cell-matrix interactions that involve cellular proliferation, migration, and differentiation[3,4,12,21]. Doxycycline, which belongs to the tetracycline family, is a pluripotent drug that affects many cellular functions including cell proliferation, matrix remodeling and apoptosis, in addition to its use as an antibiotic[22,23].
Doxycycline as well as tetracycline are non-specific potent MMP inhibitors[23-27]. Doxycycline has been used at a dose of 100-300 mg/d for the protection of pancreatitis-associated lung injury and abdominal aneurysm[28,29]. Doxycycline treatment greatly potentiates vasoconstriction, which is attributed to inhibition of MMP-2 and MMP-9 and restoration of elastic fiber integrity during prevention of aortic aneurysm in mice[29]. However, the mechanism of its actions on gastric ulcers, especially at the MMP level, remains unknown and needs to be resolved. Considering a value of 56 for human/rat conversion coefficient, assuming rat and human weights of 200 and 70 kg, respectively, 5-10 mg/kg doxycycline is equal to the 2-3 mg/kg recommended human dose[30,31]. It has been widely available in the market as an antibiotic for over three decades and has become established for long-term use too. However, its use against inhibition of overexpression of MMPs has not been investigated to date in therapeutic trials for gastric ulcers.
This study was conducted to evaluate the ability of the broad-spectrum MMP inhibitor doxycycline to prevent gastric ulcers in rats. In this paper, we report that doxycycline inhibited MMP-3 and MMP-9 activity and expression during prevention of indomethacin- and ethanol-induced gastric ulcers. Remarkably, doxycycline upregulated MMP-2 activity and expression in vivo, whereas it downregulated the same activity in vitro. Finally, we suggest that doxycycline might exert its regulatory role in addition to its inhibitory role on MMPs in vivo, which may be associated with ROS scavenging activity. These results provide an insight into the gastroprotective mechanisms of doxycycline that underlie the dual actions, antioxidant and MMP modulatory role. Hence, establishment of use of doxycycline as an effective therapy will be a boon to gastric ulcer patients.
Gelatin from porcine skin, casein, Triton X-100 (TX), 1, 10 phenanthroline, catalase and protease inhibitor cocktail were obtained from Sigma Chemicals (St. Louis, MO, USA). MMP-9 and MMP-2 were purchased from Chemicon (UK). Polyclonal anti-MMP-2, anti-MMP-3, anti-MMP-9, anti-TIMP-1, anti-TIMP-2, and anti-β-tubulin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Doxycycline was purchased from Dr. Reddy’s Laboratory (India), and all other chemicals were purchased from Sisco Research Laboratory (Mumbai, India).
Sprague-Dawley rats (180-220 g) bred in-house with free access to food and water were used for all experiments, which were carried out following the guidelines of the animal ethics committee of the institute. Experiments were designed to minimize animal suffering and to use the minimum number associated with valid statistical evaluation. Animals were anesthetized by ketamine (12 mg/kg), followed by cervical dislocation for killing. Animals in control and experimental groups were fasted overnight with free access to water before each experiment.
Indomethacin (48 mg/kg) was dissolved in distilled water with a minimum amount of NaOH and was orally fed to rats to induce a maximum level of acute gastric ulcer. The control group received the vehicle only. After 4 h, the animals were sacrificed, gastric lesions in the fundic stomach were scored and expressed as ulcer index as follows: 0 = no pathology; 1 = a small pinhead ulcer spot; and 2-5 = a band-like lesion of 2-5 mm in length. The sum of the total scores divided by the number of animals was expressed as the mean ulcer index[3]. Again, rats were orally fed with 70% ethanol at 4 mL/kg to induce maximum level of acute gastric ulcer or 2 mL/kg to induce moderate ulcer, while the control group received sterile water only. Animals were sacrificed after 3 h, and stomachs were scored for ulcer index as described by Pradeepkumar Singh et al[12]. The sum of the total scores divided by the number of animals indicates the mean ulcer index. Doxycycline at a dose of 50 mg/kg was administered orally 30 min prior to indomethacin or ethanol treatment to test the gastroprotective effects.
A homogenate of stomach tissue without the connective tissue layer was made in 0.2 mol/L Tris, pH 8.2, which contained 20 mmol/L EDTA, and centrifuged at 12 000 r/min for 15 min at 4°C. An aliquot of 1.0 mL homogenate was precipitated with 10% TCA and centrifuged at 14 000 r/min for 3 min at 4°C. The supernatant (1 mL) was added to 2 mL of 0.8 mol/L Tris-HCl, pH 9.0, which contained 20 mmol/L EDTA and mixed with 0.1 mL 10 mmol/L 5,5’-dithiobis-2-nitrobenzoic acid (DTNB). The intense yellow color of nitromercaptobenzoate was read at 412 nm. For calibration, a standard curve was prepared treating varied concentrations of reduced glutathione with DTNB under identical conditions[3].
The mitochondrial membrane fraction from gastric tissue homogenate was used for measurement of lipid peroxide content as thiobarbituric acid reactive species (TBARS). Briefly, 1 mL of the membrane fraction was allowed to react with 2 mL of TCA/TBA/HCl (15% TCA, 0.375% TBA, 0.25N HCl) reagent, heated in a boiling water bath for 15 min, cooled and centrifuged at 1000 r/min for 10 min at 4°C. The absorbance of the supernatant was measured at 535 nm and nmol TBARS produced was determined from a standard curve using tetraethoxypropane as standard[3,12].
Protein oxidation was measured as carbonyl content in the low speed supernatant of the gastric tissue homogenate[3,12]. The stomach from control, indomethacin-treated and doxycycline (50 mg/kg) pretreated indomethacin-treated rats was homogenized in 50 mmol/L sodium phosphate buffer, pH 7.4, in a Potter-Elvehjem glass homogenizer for 2 min, to obtain 20% homogenate. After centrifugation at 600 g for 10 min, the proteins from 1.0 mL of the supernatant were precipitated with 10% TCA and allowed to react with 0.5 mL 10 mmol/L 2,4-dinitrophenylhydrazine for 1 h. After precipitation with 20% TCA, the protein was washed three times with a mixture of ethanol-ethyl acetate (1:1), dissolved in 1.0 mL of a solution that contained 6 mol/L guanidine HCl in 20 mmol/L potassium phosphate, adjusted to pH 2.3 with TCA, centrifuged at 1000 r/min for 5 min at 4°C, and the supernatant was read for carbonyl content at 362 nm (e = 22 000/M per centimeter).
The whole stomach (including fundic, body and pyloric parts) was washed with saline and used for extraction. Gastric tissues were suspended in 10 mmol/L phosphate buffered saline (PBS) containing protease inhibitors, minced, and incubated for 10 min at 4°C. After centrifugation at 12 000 g for 15 min, the supernatant was collected as PBS extracts. The pellet was then extracted in lysis buffer (10 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 1% TX and protease inhibitors) and centrifuged at 12 000 g for 15 min to obtain TX extracts. PBS and TX extracts were preserved at -70°C[3,12].
For assay of MMP-2 and MMP-9 activity, PBS and/or TX extracts were electrophoresed in 8% SDS-polyacrylamide gel containing 1 mg/mL gelatin under non-reducing conditions. For assay of MMP-3 activity, tissue extracts were electrophoresed in casein gel instead of gelatin. The gels were washed in 2.5% TX and incubated in calcium assay buffer (40 mmol/L Tris-HCl, pH 7.4, 0.2 mol/L NaCl, 10 mmol/L CaCl2) for 18 h at 37°C, and stained with 0.1% Coomassie Blue followed by destaining[3,12,13]. The zones of gelatinolytic or caseinolytic activity came as negative staining.
Equal amount of PBS extract from ulcerated tissue were incubated with two different concentrations of doxycycline (100 and 200 μmol/L) in calcium assay buffer (40 mmol/L Tris-HCl, pH 7.4, 0.2 mol/L NaCl, 10 mmol/L CaCl2) for 1.5 h at 37°C. Gelatin and casein zymography were performed from the samples as described previously[3,12,13]. Parallel assays were performed with the addition of phenanthroline (100 and 200 μmol/L in 10% DMSO) to show that inhibition could be attributed to MMP-2, MMP-3 and MMP-9 activity. PBS extract incubated with requisite volume of 10% DMSO was used as a control[32].
To determine the effect of H2O2, equal amounts of PBS extract from control stomach (60 μg) were incubated with 500 μmol/L concentration of H2O2 at 37°C for 1 h. To examine the protective effect of doxycycline on H2O2 mediated changes in MMP-2 activity, equal amounts of PBS extract were pretreated with doxycycline (200 μmol/L) prior to incubation with 500 μmol/L H2O2. Pretreatment with catalase followed by incubation with H2O2 was used as a positive control. Gelatin zymography was performed with the samples as described previously[19].
PBS extracts (100 μg/lane) were resolved by 8% reducing SDS-PAGE and processed for Western blotting[13]. Proteins were transferred to membranes, blocked in 3% BSA solution in 20 mmol/L Tris-HCl, pH 7.4, containing 150 mmol/L NaCl and 0.02% Tween 20 (TBST), and incubated at 4°C in 1:200 dilutions of the respective primary antibodies in TBST containing 0.2% BSA. The membranes were then washed with TBST, incubated with alkaline-phosphatase-conjugated secondary antibody, and bands were visualized using 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium substrate solution.
Ulcer index data were fitted using Sigma plot software. Data were presented as the mean ± SE. Statistical analysis was performed using Student-Newman-Keuls test (ANOVA). P < 0.05 was considered statistically significant.
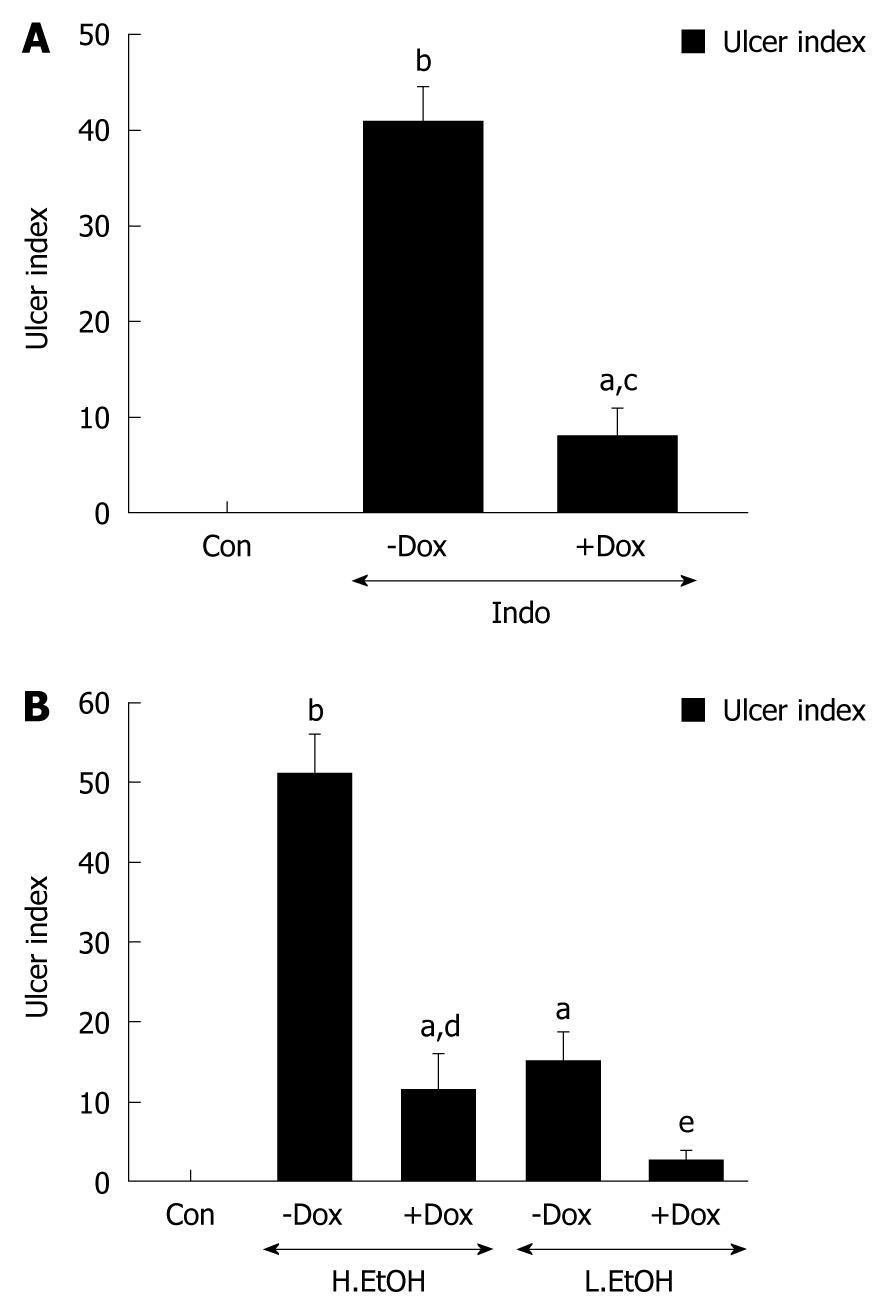
Indomethacin- and ethanol-induced gastric ulcer models in rat were used to test the protective effect of doxycycline against gastric damage. Doxycycline, a tetracycline analogue, has a number of non-antibiotic properties including inhibition of connective tissue breakdown. The action of doxycycline treatment on gastric damage was evaluated in rats by measuring ulcer index. One single dose of doxycycline (50 mg/kg) was selected for our study. Gastric ulceration was not detected in doxycycline-pretreated, indomethacin-treated rat gastric tissues (Supplementary Figure 1). Figure 1A illustrates the effect of doxycycline on significant reduction in ulcer indices that occurred due to indomethacin-induced ulceration. In addition, doxycycline caused a significant decrease in ulcer index score from 50 to 15 when administered to high-dose ethanol-treated rats. Similarly, ulcer index score was reduced by less than 5 from 20 in low-dose ethanol-treated rats (Figure 1B). Thus, doxycycline arrested gastric damage by > 70% in rats with severe or moderate ulceration.
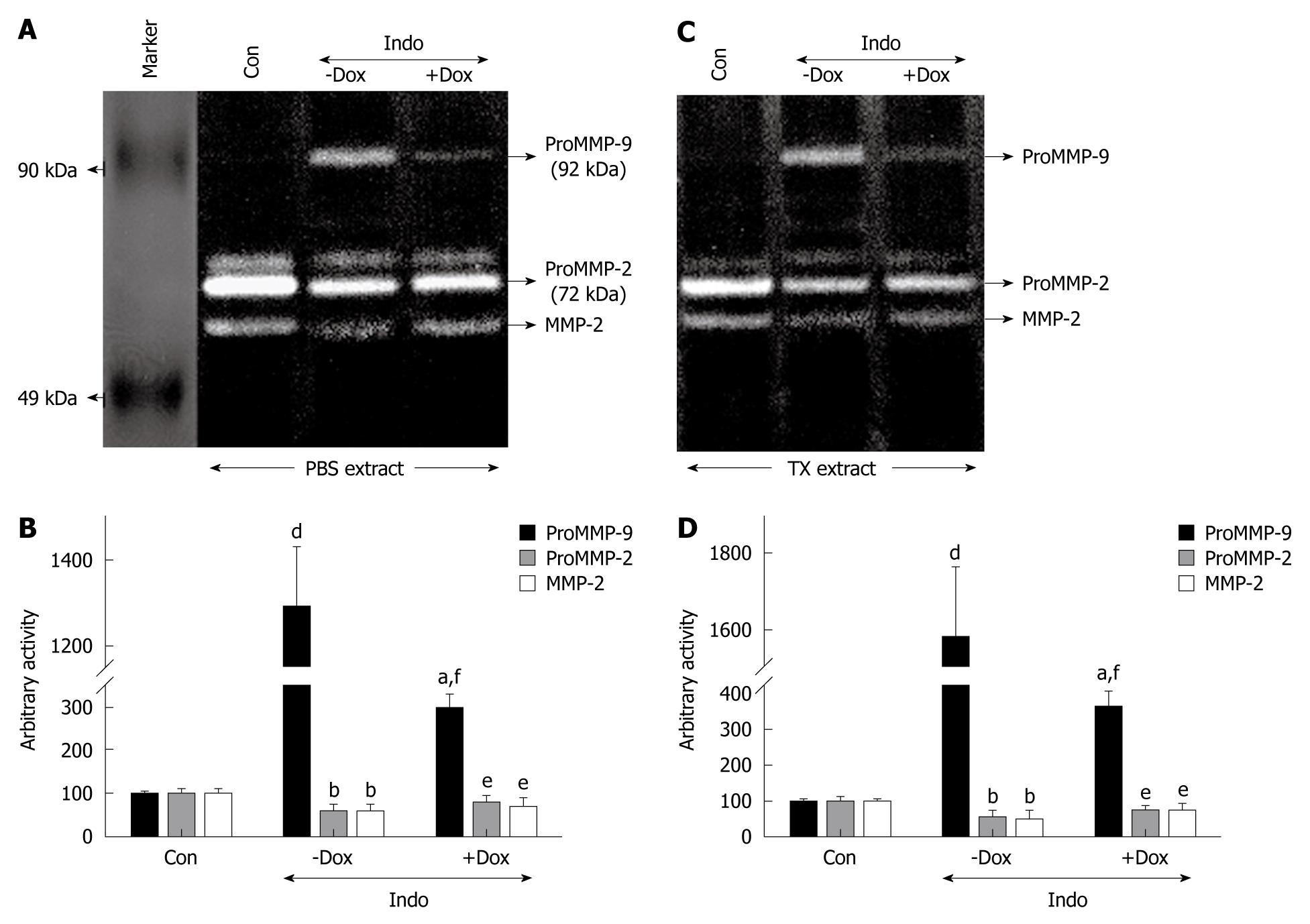
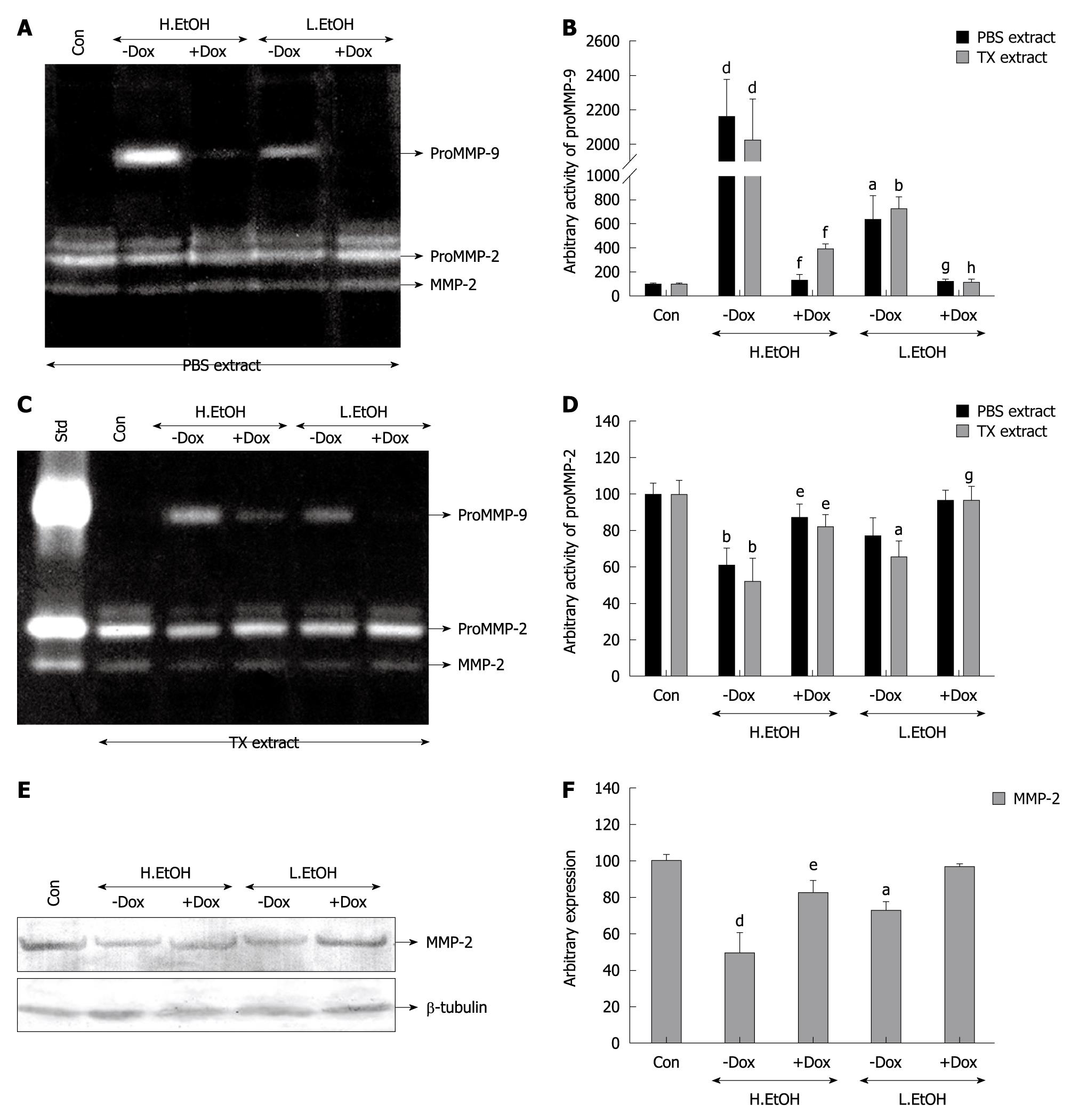
The gastroprotective action of doxycycline and association with MMP-2 and MMP-9 activities were examined in rat gastric tissues. Figure 2A and 2C shows that doxycycline inhibited proMMP-9 activity in PBS- and TX-extracted gastric tissues during protection against indomethacin-induced ulcer. PBS and TX extracts represented secreted and synthesized proMMP-9, respectively. ProMMP-9 activity is an inflammatory indicator of gastric damage and it increased with increasing gastric indices. Secreted and synthesized proMMP-9 activity was increased by about 12-fold and about 16-fold in indomethacin-ulcerated gastric tissues as compared to the controls, and doxycycline reduced the activity by 80% and 70%, respectively (Figure 2B and 2D). In contrast, doxycycline upregulated proMMP-2 activity at the level of secretion and synthesis, while protecting against indomethacin-induced gastric ulcer (Figure 2A and 2C). Indomethacin treatment decreased proMMP-2 activity by about 50% and docycycline pretreatment reversed the activity almost to control levels for both secretion and synthesis (Figure 2B and 2D). Doxycycline significantly reduced proMMP-9 activity in gastric tissues both at the level of secretion and synthesis in ethanol-induced ulcerated rats that were doxycycline treated, compared to untreated controls (Figure 3A and 3C). Doxycycline caused a reduction in secreted proMMP-9 activity to about 5-fold and about 1.5-fold, which was increased about 20-fold and about 7-fold during ulceration by high and low doses of ethanol, respectively (Figure 3B). Similarly, there was a decrease in synthesized proMMP-9 activity by about 18-fold and about 7-fold by doxycycline during protection of ethanol-induced ulceration by low and high doses (Figure 3B). In contrast, doxycycline-pretreated, ethanol-treated rats showed an increase in MMP-2 activity in gastric tissues at the level of secretion and synthesis (Figure 3A and 3C). Upregulation of MMP-2 activity by doxycycline was more prominent with low-dose compared to high-dose ethanol-induced ulceration (Figure 3D). Thus, MMP-9 activity was downregulated while proMMP-2 activity was upregulated in rat gastric tissues during gastroprotection by doxycycline. Expression of MMP-2 was also examined by Western blot analysis (Figure 3E) in doxycycline-pretreated, ethanol-treated gastric tissues. Figure 3F reveals that upregulation of proMMP-2 reached control levels in doxycycline-pretreated, ethanol-treated (low dose) rat gastric tissues. Similarly, upregulated expression of MMP-2 was also reflected in doxycycline-pretreated, ethanol-treated (high dose) rat gastric tissues. β-tubulin was run in parallel to confirm equal protein loading.
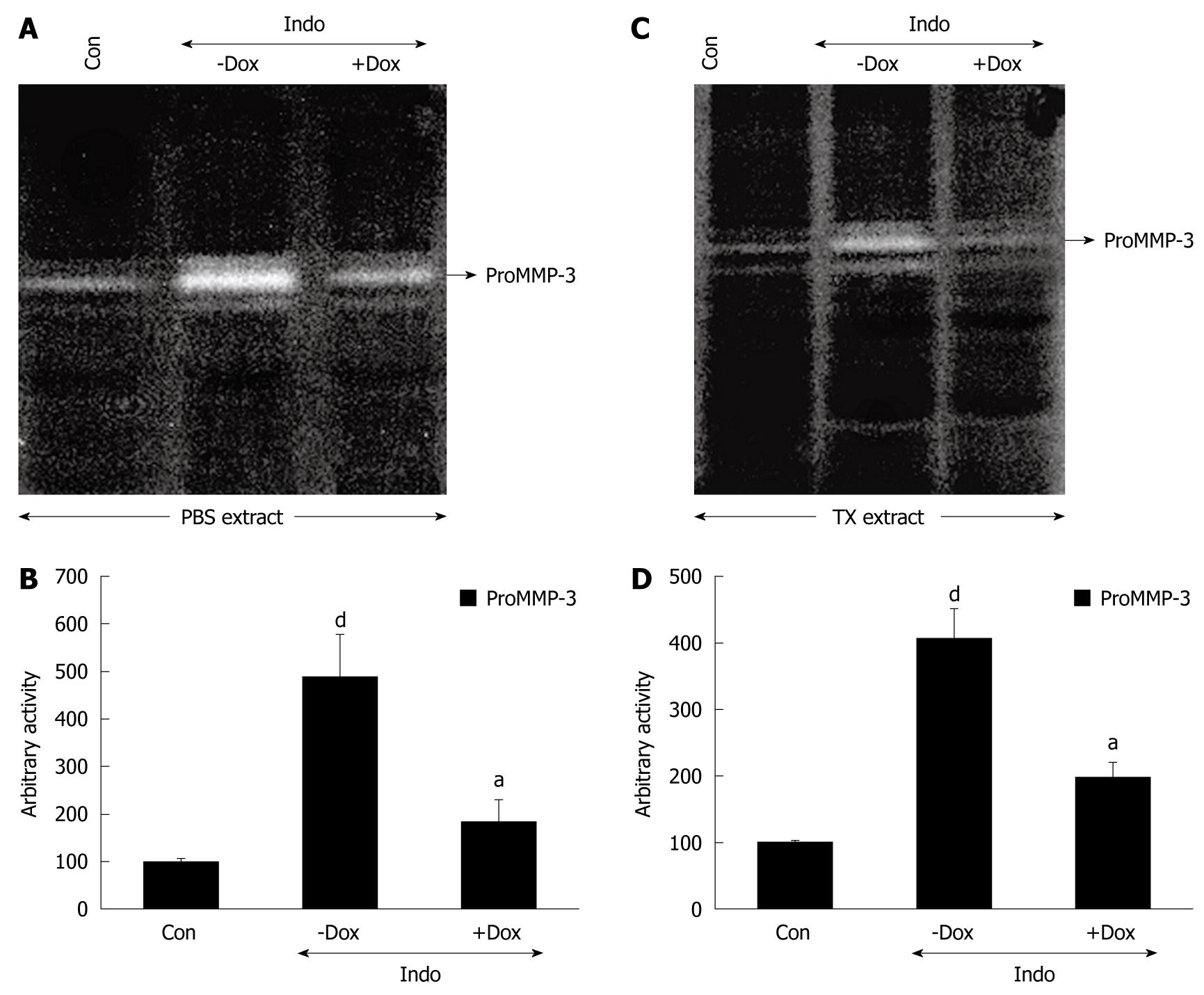
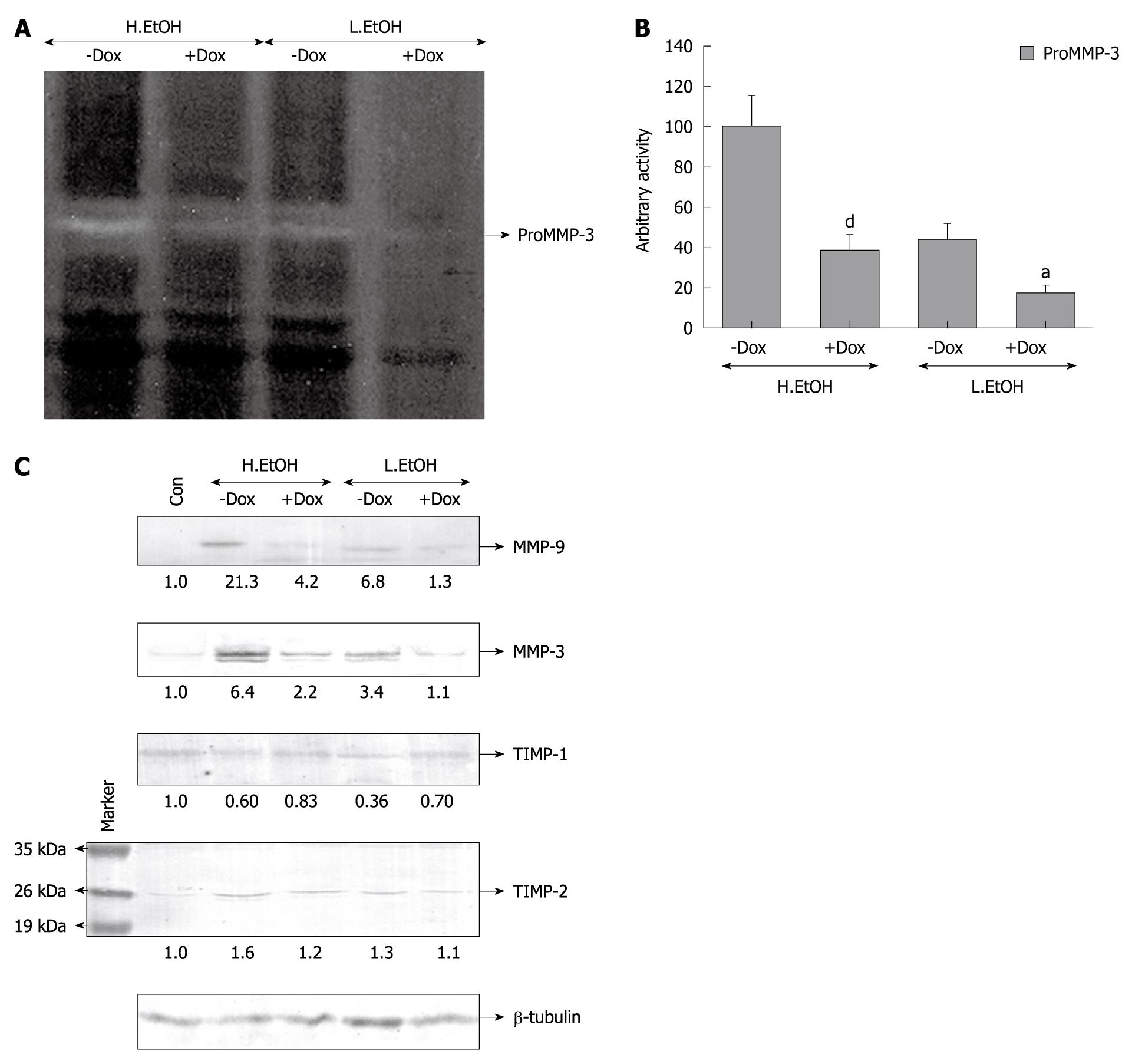
To investigate the ability of doxycycline to regulate MMP-3 during prevention of ulcers, indomethacin- and ethanol-induced ulceration were both examined. Figure 4 suggests that indomethacin increased proMMP-3 activity significantly in ulcerated gastric tissues. Figure 4A and C shows that doxycycline inhibited proMMP-3 activity very significantly in PBS- and TX-extracted gastric tissues during protection against indomethacin-induced ulcer. ProMMP-3 inhibitory activity of doxycycline was at a magnitude of about 75% and about 66% for secreted and synthesized protein, respectively, during protection of indomethacin-induced gastric ulcer (Figure 4B and 4D). Doxycycline inhibited the activity of secreted proMMP-3 while preventing both severe and moderate gastric ulceration induced by ethanol (Figure 5A). Doxycycline downregulated the activity by about 2-fold while protecting ethanol-treated gastric tissues, and the efficacy was greater for low-dose ethanol-treated tissues (Figure 5B). In other words, doxycycline exhibited an inhibitory effect on proMMP-3 activity by about 50% and about 70% for high-dose and low-dose ethanol-treated gastric tissues, respectively, as depicted in Figure 5B. The effect of doxycycline on MMP-9 and MMP-3 expression in rats was analyzed by Western blotting (Figure 5C). Both high and low doses of ethanol increased the expression of MMP-9 by about 21-fold and about 7-fold, respectively, in rat gastric tissues compared to controls, while treatment with doxycycline inhibited MMP-9 expression by 95% and 85%, respectively. Similarly, MMP-3 expression increased by about 6.5-fold and about 3.5-fold for high- and low-dose ethanol treatment, respectively, and doxycycline reduced their expression by 60% and 80%, respectively (Figure 5C). Moreover, the effect of doxycycline was more pronounced when ulcer was moderate rather than severe. To ascertain the regulatory role of TIMPs in ethanol-induced gastric ulcer, TIMP-1 and TIMP-2 expression was checked by Western blot analysis. TIMP-1 expression was reduced by 40% and 60% for high- and lose-dose ethanol, respectively, and doxycyline reversed TIMP-1 expression almost to control values. On the other hand, TIMP-2 expression followed an opposite trend. It increased in rat gastric tissue after ethanol treatment. TIMP-2 expression induced by 1.6-fold and 1.3-fold, respectively, and doxycyline reduced TIMP-2 expression near to control levels. β-tubulin was run in parallel to confirm equal protein loading.
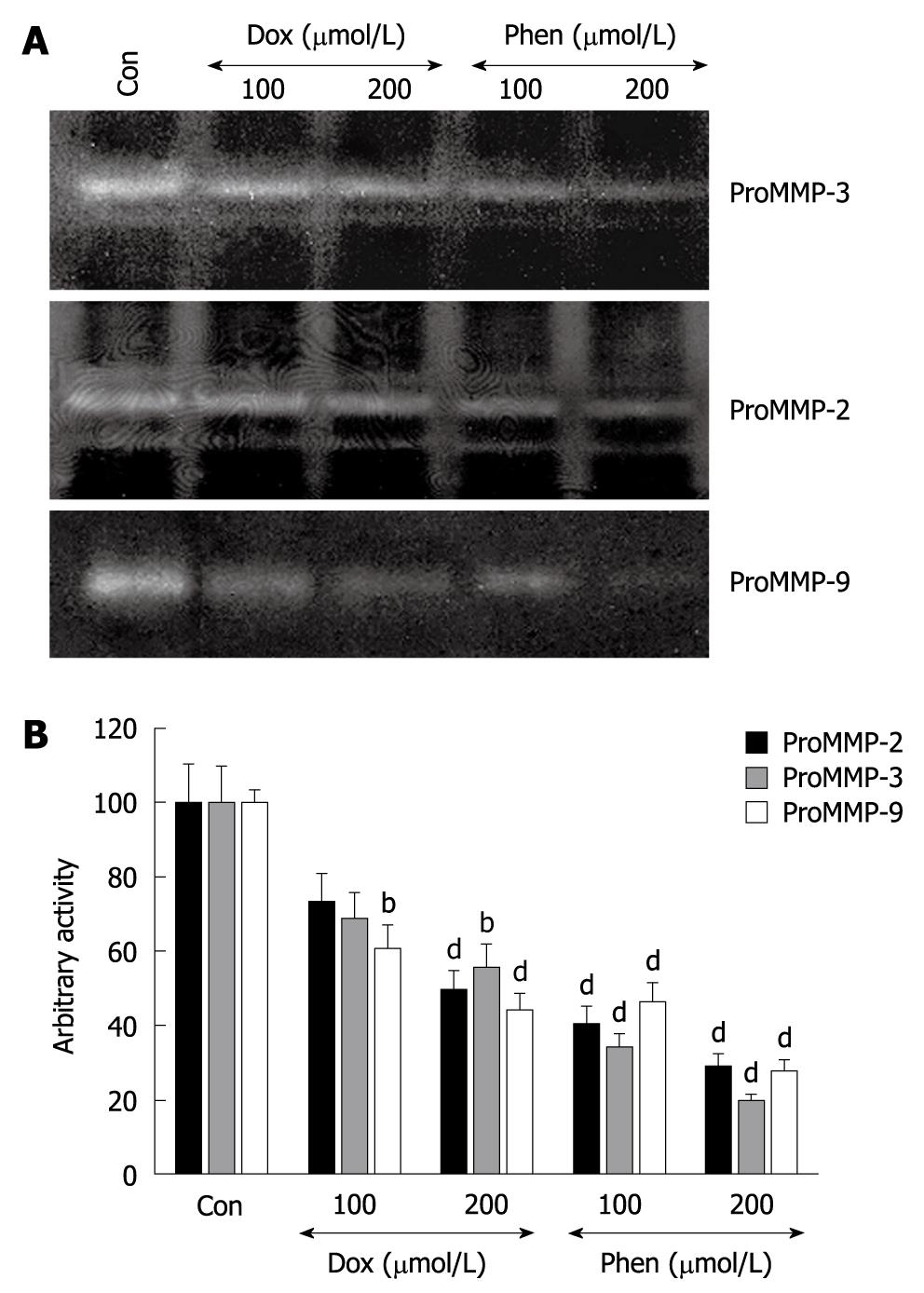
The effect of doxycycline on MMP activity in vitro was evaluated by casein as well as gelatin zymography. Gastric tissue extracts with detectable MMP-2, MMP-3 and MMP-9 activities were used as controls for the inhibition experiment. Doxycycline inhibited MMP-2, MMP-3 and MMP-9 activities dose dependently, as judged by zymography (Figure 6A). Doxycycline at a dose of 100 μmol/L and 200 μmol/L inhibited MMP-9 activity by 30% and 50%, respectively, while MMP-2 and MMP-3 activities were inhibited by 65% in each case. In vitro inhibition studies of MMP activity were also performed in parallel using zinc chelator (1, 10 phenanthroline) to confirm the metalloprotease property. Phenanthroline at a dose of 100 μmol/L inhibited MMP-2, MMP-3 and MMP-9 by about 60%, about 62% and about 55%, respectively. In addition, the activity of MMP-2, MMP-3 and MMP-9 were further decreased by 10-15% more at high doses of phenanthroline (200 μmol/L) in each case (Figure 6B).
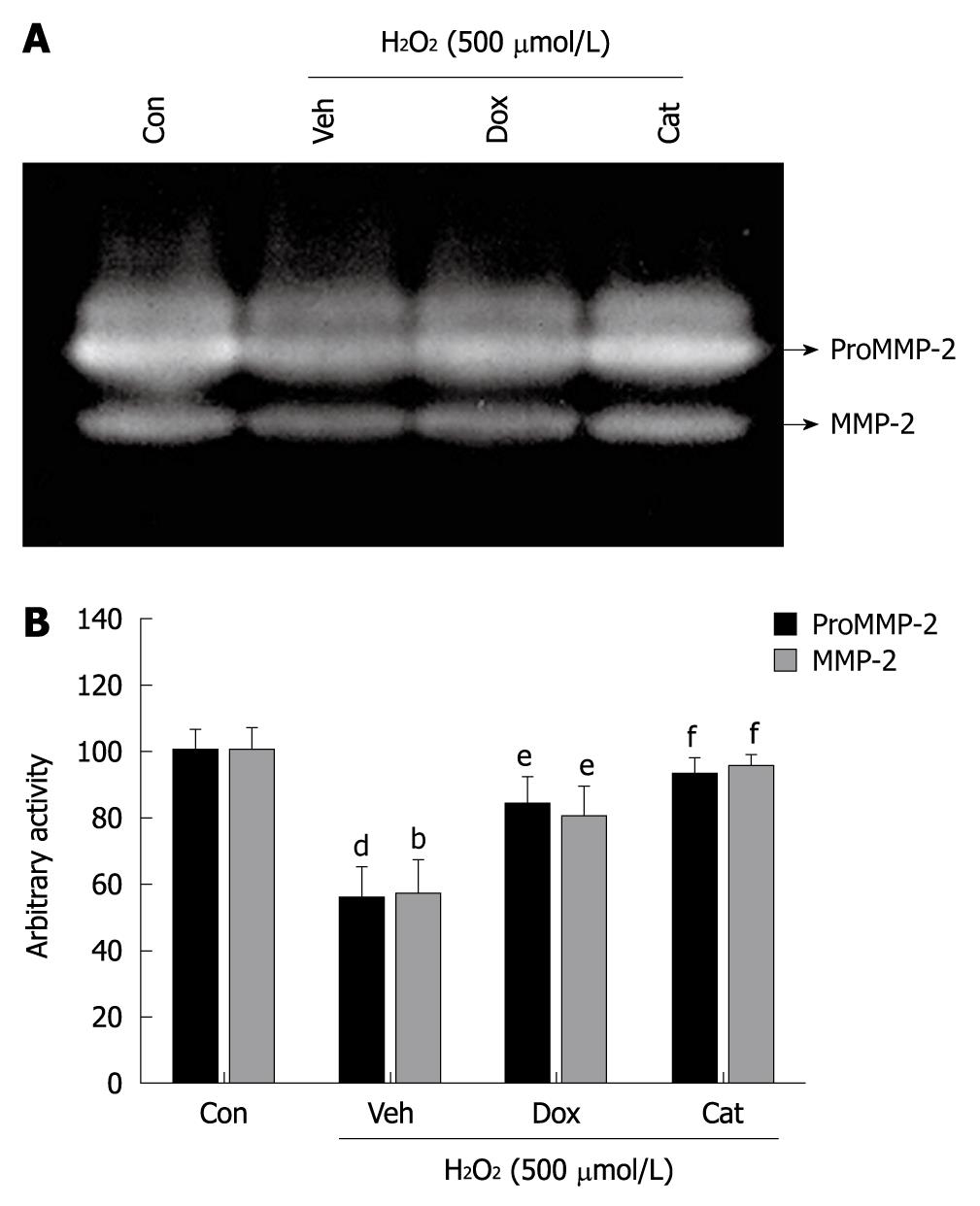
Oxidative damage of cellular lipid and protein is directly associated with gastric ulceration. To determine the effect of doxycycline on oxidative damage of gastric tissues due to indomethacin, we measured protein oxidation and lipid peroxidation in control, indomethacin-treated, and indomethacin-treated doxycycline-pretreated gastric tissue homogenates. Indomethacin caused excessive oxidative damage that was associated with a about 2.5-fold and about 2-fold increase in lipid peroxidation and protein oxidation, respectively, while doxycycline treatment reduced them by about 80% and about 93%, respectively (Table 1). Moreover, the antioxidant, glutathione was reduced by about 36% compared to controls, and doxycycline recovered the glutathione content up to 92% during gastroprotection. In order to assess the possible antiulcer mechanism of doxycycline, we examined its protective effect on H2O2-mediated suppression of proMMP-2 activity in vitro. PBS-extracted gastric tissues from control rats were incubated with 200 μmol/L doxycycline followed by H2O2 treatment for 1 h at 37°C, and gelatin zymography was performed. The activity of MMP-2 in the zymogram (Figure 7A) was decreased by about 45% compared to the controls by H2O2, and doxycycline rescued H2O2-mediated suppression of MMP-2 activity by about 80% (Figure 7B). As expected when H2O2 treated samples were incubated with catalase, it failed to suppress MMP-2 activity. Indomethacin-induced ulceration is associated with generation of H2O2, which in turn, damages tissues by interacting with protein and lipid. Thus, neutralizing H2O2-mediated damage by doxycycline suggests the antioxidant property of doxycycline.
NSAIDs, along with other aggressive factors like ethanol, Helicobacter pylori infection, stress and steroids, increases the risk of gastric ulcer formation[3,4,11-13,19]. Increased production of ROS and inflammatory cytokines, inhibition of cell proliferation, infiltration of inflammatory cells, reduced production of mucus, growth factors, and prostaglandins are factors involved in the pathogenesis of gastric ulcers[9,11]. Research has been focused recently on the importance of ECM and its turnover in the ulcerated margins of gastric tissue. Increased epithelial cell turnover is associated with the repair process without altering the composition and organization of the gastric ECM. Matrix proteins are secreted by different cell types including fibroblasts and epithelial and endothelial cells and assemble into an ECM network in the spaces surrounding the cells[5,9,10]. Recent studies have described the involvement of MMPs in cleaving and remodeling of ECM. MMPs have been implicated in various processes including tumor growth metastasis, chronic ulceration, fetal tissue development, and bone resorption. MMPs capable of degrading ECM proteins are believed to play a significant role during the pathogenesis and healing of gastric ulcer[3,4,12]. These enzymes are synthesized as latent proenzymes and their activity is tightly regulated at several levels including the post-translational level[33]. The coordinate action of proteases, in particular the MMPs and antiproteases, including the TIMPs, is thought to play an important role in connective tissue degradation[5]. In the present study, the authors intended to assess the relationship between MMP-2, MMP-3 and MMP-9 activity due to induction of gastric injury. Herein, doxycycline has been used to examine its gastroprotective potential.
Doxycycline, a widely prescribed antibiotic, is also a non-specific MMP inhibitor. It also possesses antiangiogenic and anti-inflammatory and neuroprotective properties, and is a convenient drug with low cost, and clinical tolerability without serious side effects[34]. Ryan et al[35] have reported divalent chelating activity of doxycycline. The beneficial effects of doxycycline are mainly mediated by its inhibition of MMP-9 and MMP-2. Doxycycline is safe in treating certain diseases because it reduces the level of pathophysiologically excessive MMPs but not to the level below normal physiological conditions. The effective dose of doxycycline is 100-200 mg/d for adult humans[36].
In the present study, we primarily showed that doxycycline potentially inhibited MMP-9 and MMP-3 activity while arresting gastric damage in rats. It is noteworthy that doxycycline upregulated MMP-2 activity and expression during gastroprotection. Our results suggest that doxycycline acts through mechanisms distinctly different from its inhibitory effect on MMPs. Thus, doxycycline has a different action on MMP-2 activity in gastric damage, and indicates its regulatory role in addition to its inhibitory effect on MMPs. The increased MMP-2 activity may be required for preservation of matrix integrity, because function of the gastric epithelial and mucosal layer could be modulated by ECM components in gastric tissues[3,19]. Herein, an interesting feature of doxycycline is that it reduced MMP-2 activity in vitro and increased it in vivo. One interpretation is that doxycycline binds to Ca2+/Zn2+ at catalytic sites of MMP in vitro and inhibits the enzymatic activity of MMPs[22,35]. Another interpretation may be that doxycycline perturbs the signaling pathways related to mitogen-activated protein kinase or Smad[27], and subsequently leads to beneficial systemic changes. In vivo upregulation of MMP-2 activity could be explained as an anti-apoptotic role of doxycycline during gastroprotection[34]. An alternative interpretation is that doxycycline inhibits oxidative stress and upregulates expression of MMP-2, or decreases antiprotease expression, thus shifting the protease/antiprotease balance towards high enzymatic activity. The beneficial effect on gastric damage may not be solely attributed to MMP-9 and MMP-3 inhibition. Other proteases and antiproteases may play a critical role, and pleiotropic effects of doxycycline may offer a better approach. This study provided convincing data to suggest a new pharmacological approach for the drug doxycycline for prevention of NSAID- or ethanol-induced gastric ulcers.
While the exact mechanism of gastric ulceration is still not completely understood, various cellular stresses including oxidative stress play a central role in the progression of gastric ulcers. Inflammatory reactions induced by indomethacin are a significant source of ROS, for example, H2O2 and the superoxide radical, which play a causative role in gastric injury[19]. ROS generally modulate MMP activity either indirectly through redox-dependent regulation of MMP gene transcription or directly through modification of MMP structure[2]. However, the direct association of ROS with the regulation of MMPs during gastric ulceration is not well studied. Several in vivo and in vitro cell culture studies have hypothesized activation of gelatinases through ROS or ROS generators when applied time and dose dependently. Herein, incubation of pro-MMP-2 in an ex vivo system with H2O2 resulted in suppression of enzyme activity, which suggests that ROS play a significant role in modulating MMP-2 activity. We also showed that doxycycline possessed inhibitory activity against H2O2-mediated suppression of MMP-2 activity, which indicated the antioxidant action of doxycycline. Doxycycline offered gastroprotection by preventing oxidative damage caused by lipid peroxidation and protein oxidation. Ulcerated samples exhibited reduced thiol content, whereas that was almost completely restored to control levels by doxycycline during gastroprotection. Although doxycycline has been reported as a potent inhibitor of MMPs, the antioxidant activity is worth mentioning in arresting gastric injury.
The major observations of the present study highlight that: (1) gastric injury is associated with upregulation of proMMP-3 and -9 activity, as well as expression in rat gastric tissues; (2) doxycycline is very effective in preventing gastric damage, and the mechanism of gastroprotection is attributable to its inhibitory effect on proMMP-3 and -9, while enhancing MMP-2 in vivo; and (3) doxycycline has a modulatory role with TIMP-1 and TIMP-2 during protection against gastric ulcers. The regulatory role of proMMP-2 activity may be dependent on the antioxidant action of doxycyline. We showed that doxycycline provides protection against gastric damage via inhibition of protein oxidation, lipid peroxidation and glutathione depletion. Moreover, suppression of MMP-2 activity by oxidative stress is rescued by doxycycline. Overall, this study is believed to be the first to demonstrate effectiveness of doxycycline in prevention of gastric damage via regulation of MMPs. Doxycycline therefore, may be considered as a candidate towards a therapeutic strategy for gastric ulcers. Also, proMMP-3 and -9 overexpression in gastric injury may be exploited as therapeutic targets, and doxycycline may be used as target-based therapy.
A class of zinc-dependent enzyme named matrix metalloproteinases (MMPs) is capable of degrading proteins of cellular and extracellular origin of various tissues. Their function is regulated by endogenous inhibitors such as tissue inhibitor of metalloproteinases and α-2 macroglobulin, which helps to maintain the protease-antiprotease balance. Imbalance in MMPs action is implicated in different diseases including gastric ulcer. Various factors mainly alcohol, nonsteroidal anti-inflammatory drugs and infection with Helicobacter pylori cause gastric injury. In recent years, there has been a paradigm shift in understanding the mechanism of gastric ulcers, which includes regulation of MMPs and remodeling of the matrix surrounding the cells. Healing of gastric injury is a complicated process that comprises a series of cell-matrix interactions, and cell-cell signaling. Existing drugs against gastric injury have some limitation and side effects. Researchers are looking for novel drugs that have fewer side effects, easy availability, and low cost. A research group in India has examined the effect of doxycycline on gastric injury via regulation of MMPs.
Doxycycline (derivative of tetracycline) is used commonly as an antibiotic. However, the antiulcer mechanism of doxycyline has not yet been explored fully. This study is believed to be the first to investigate the regulation of MMP-2 expression by doxycycline while preventing gastric injury.
The gastroprotective effect of doxycycline was tested against ethanol- or indomethacin-induced gastric mucosal injury in rats. Doxycycline prevented oxidative damage caused by lipid peroxidation and protein oxidation. Ulcerated samples exhibited reduced glutathione content, and it was almost completely restored to control levels by doxycycline during gastroprotection. Injured tissues exhibited significantly higher MMP-3 and MMP-9 activities, while doxycycline treatment markedly decreased both enzymes during gastroprotection. In contrast, the reduced MMP-2 activity in injured tissues was reversed by doxycycline during ulcer prevention. In addition, in vitro, doxycycline inhibited significantly all MMPs and proMMP-2, -3 and -9. Swarnakar et al have demonstrated a novel role of doxycycline in the upregulation of MMP-2 activity and expression during protection against gastric ulcers.
Altogether, the work established the use of doxycycline as an antiulcer drug that may lead to effective therapy for gastric ulcer. Combination therapy of doxycycline along with other existing antiulcer drugs such as famotidine, ranitidine or omeprazole may be better for treatment of gastric inflammation.
This is believed to be the first demonstration of the dual action of doxycycline, namely, upregulation of MMP-2 activity and reduction of oxidative stress, in arresting gastric injury. Long-term use of doxycycline may provide therapeutic intervention for chronic gastric ulceration that poses a risk of progression to carcinoma.
| 1. | Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161-174. |
| 2. | Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med. 2004;37:768-784. |
| 3. | Swarnakar S, Ganguly K, Kundu P, Banerjee A, Maity P, Sharma AV. Curcumin regulates expression and activity of matrix metalloproteinases 9 and 2 during prevention and healing of indomethacin-induced gastric ulcer. J Biol Chem. 2005;280:9409-9415. |
| 4. | Swarnakar S, Mishra A, Ganguly K, Sharma AV. Matrix metalloproteinase-9 activity and expression is reduced by melatonin during prevention of ethanol-induced gastric ulcer in mice. J Pineal Res. 2007;43:56-64. |
| 5. | Parks WC, Mecham RP. Matrix metalloproteinases. San Diego: Academic Press; 1998; . |
| 6. | McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534-540. |
| 7. | Gaggar A, Li Y, Weathington N, Winkler M, Kong M, Jackson P, Blalock JE, Clancy JP. Matrix metalloprotease-9 dysregulation in lower airway secretions of cystic fibrosis patients. Am J Physiol Lung Cell Mol Physiol. 2007;293:L96-L104. |
| 8. | Nie J, Pei J, Blumenthal M, Pei D. Complete restoration of cell surface activity of transmembrane-truncated MT1-MMP by a glycosylphosphatidylinositol anchor. Implications for MT1-MMP-mediated prommp2 activation and collagenolysis in three-dimensions. J Biol Chem. 2007;282:6438-6443. |
| 9. | Shahin M, Konturek JW, Pohle T, Schuppan D, Herbst H, Domschke W. Remodeling of extracellular matrix in gastric ulceration. Microsc Res Tech. 2001;53:396-408. |
| 10. | Ohmiya N, Saga S, Ohbayashi M, Kozaki K, Miyaishi O, Kobayashi M, Kasuya S, Arisawa T, Goto H, Hayakawa T. Kinetics and collagenolytic role of eosinophils in chronic gastric ulcer in the rat. Histochem Cell Biol. 1997;108:27-34. |
| 11. | Biswas K, Bandyopadhyay U, Chattopadhyay I, Varadaraj A, Ali E, Banerjee RK. A novel antioxidant and antiapoptotic role of omeprazole to block gastric ulcer through scavenging of hydroxyl radical. J Biol Chem. 2003;278:10993-11001. |
| 12. | Pradeepkumar Singh L, Kundu P, Ganguly K, Mishra A, Swarnakar S. Novel role of famotidine in downregulation of matrix metalloproteinase-9 during protection of ethanol-induced acute gastric ulcer. Free Radic Biol Med. 2007;43:289-299. |
| 13. | Kundu P, Mukhopadhyay AK, Patra R, Banerjee A, Berg DE, Swarnakar S. Cag pathogenicity island-independent up-regulation of matrix metalloproteinases-9 and -2 secretion and expression in mice by Helicobacter pylori infection. J Biol Chem. 2006;281:34651-34662. |
| 14. | Pillinger MH, Marjanovic N, Kim SY, Lee YC, Scher JU, Roper J, Abeles AM, Izmirly PI, Axelrod M, Pillinger MY. Helicobacter pylori stimulates gastric epithelial cell MMP-1 secretion via CagA-dependent and -independent ERK activation. J Biol Chem. 2007;282:18722-18731. |
| 15. | Camp TM, Tyagi SC, Senior RM, Hayden MR, Tyagi SC. Gelatinase B(MMP-9) an apoptotic factor in diabetic transgenic mice. Diabetologia. 2003;46:1438-1445. |
| 16. | Jin M, Kashiwagi K, Iizuka Y, Tanaka Y, Imai M, Tsukahara S. Matrix metalloproteinases in human diabetic and nondiabetic vitreous. Retina. 2001;21:28-33. |
| 17. | Maxwell PR, Timms PM, Chandran S, Gordon D. Peripheral blood level alterations of TIMP-1, MMP-2 and MMP-9 in patients with type 1 diabetes. Diabet Med. 2001;18:777-780. |
| 18. | Zhang SX, Wang JJ, Lu K, Mott R, Longeras R, Ma JX. Therapeutic potential of angiostatin in diabetic nephropathy. J Am Soc Nephrol. 2006;17:475-486. |
| 19. | Ganguly K, Kundu P, Banerjee A, Reiter RJ, Swarnakar S. Hydrogen peroxide-mediated downregulation of matrix metalloprotease-2 in indomethacin-induced acute gastric ulceration is blocked by melatonin and other antioxidants. Free Radic Biol Med. 2006;41:911-925. |
| 20. | Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene. 2000;19:6642-6650. |
| 21. | Tomita M, Ando T, Minami M, Watanabe O, Ishiguro K, Hasegawa M, Miyake N, Kondo S, Kato T, Miyahara R. Potential role for matrix metalloproteinase-3 in gastric ulcer healing. Digestion. 2009;79:23-29. |
| 22. | Bendeck MP, Conte M, Zhang M, Nili N, Strauss BH, Farwell SM. Doxycycline modulates smooth muscle cell growth, migration, and matrix remodeling after arterial injury. Am J Pathol. 2002;160:1089-1095. |
| 23. | Lee CZ, Yao JS, Huang Y, Zhai W, Liu W, Guglielmo BJ, Lin E, Yang GY, Young WL. Dose-response effect of tetracyclines on cerebral matrix metalloproteinase-9 after vascular endothelial growth factor hyperstimulation. J Cereb Blood Flow Metab. 2006;26:1157-1164. |
| 24. | Golub LM, McNamara TF, Ryan ME, Kohut B, Blieden T, Payonk G, Sipos T, Baron HJ. Adjunctive treatment with subantimicrobial doses of doxycycline: effects on gingival fluid collagenase activity and attachment loss in adult periodontitis. J Clin Periodontol. 2001;28:146-156. |
| 25. | Axisa B, Loftus IM, Naylor AR, Goodall S, Jones L, Bell PR, Thompson MM. Prospective, randomized, double-blind trial investigating the effect of doxycycline on matrix metalloproteinase expression within atherosclerotic carotid plaques. Stroke. 2002;33:2858-2864. |
| 26. | Choi DH, Moon IS, Choi BK, Paik JW, Kim YS, Choi SH, Kim CK. Effects of sub-antimicrobial dose doxycycline therapy on crevicular fluid MMP-8, and gingival tissue MMP-9, TIMP-1 and IL-6 levels in chronic periodontitis. J Periodontal Res. 2004;39:20-26. |
| 27. | Lee HM, Ciancio SG, Tüter G, Ryan ME, Komaroff E, Golub LM. Subantimicrobial dose doxycycline efficacy as a matrix metalloproteinase inhibitor in chronic periodontitis patients is enhanced when combined with a non-steroidal anti-inflammatory drug. J Periodontol. 2004;75:453-463. |
| 28. | Sochor M, Richter S, Schmidt A, Hempel S, Hopt UT, Keck T. Inhibition of matrix metalloproteinase-9 with doxycycline reduces pancreatitis-associated lung injury. Digestion. 2009;80:65-73. |
| 29. | Abdul-Hussien H, Hanemaaijer R, Verheijen JH, van Bockel JH, Geelkerken RH, Lindeman JH. Doxycycline therapy for abdominal aneurysm: Improved proteolytic balance through reduced neutrophil content. J Vasc Surg. 2009;49:741-749. |
| 30. | Akkaya P, Onalan G, Haberal N, Bayraktar N, Mülayim B, Zeyneloglu HB. Doxycycline causes regression of endometriotic implants: a rat model. Hum Reprod. 2009;24:1900-1908. |
| 31. | Prall AK, Longo GM, Mayhan WG, Waltke EA, Fleckten B, Thompson RW, Baxter BT. Doxycycline in patients with abdominal aortic aneurysms and in mice: comparison of serum levels and effect on aneurysm growth in mice. J Vasc Surg. 2002;35:923-929. |
| 32. | Kwan JA, Schulze CJ, Wang W, Leon H, Sariahmetoglu M, Sung M, Sawicka J, Sims DE, Sawicki G, Schulz R. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J. 2004;18:690-692. |
| 33. | Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827-839. |
| 34. | Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258-265. |
| 35. | Ryan ME, Ramamurthy S, Golub LM. Matrix metalloproteinases and their inhibition in periodontal treatment. Curr Opin Periodontol. 1996;3:85-96. |
| 36. | Ramamurthy NS, Schroeder KL, McNamara TF, Gwinnett AJ, Evans RT, Bosko C, Golub LM. Root-surface caries in rats and humans: inhibition by a non-antimicrobial property of tetracyclines. Adv Dent Res. 1998;12:43-50. |
Peer reviewer: Fabrizio Montecucco, MD, Assistant, Division of Cardiology, Department of Internal Medicine, University of Geneva, Avenue de la Roseraie 64, 1211 Geneva, Switzerland
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM